Abstract
Background:
The actions of adipocytokines may be a connective factor between obesity and polycystic ovary syndrome (PCOS). It is suggested that irisin, as recently described cytokine secreted by skeletal muscles and glucose-dependent insulinotropic polypeptide (GIP) as an incretin hormone that induces cytokine expression, may play key roles in favoring obesity in these women.Objectives:
We aimed to evaluate the association between body mass index (BMI) and serum concentrations of irisin/GIP in women with and without PCOS in the linear and non-linear models.Methods:
This cross-sectional study was conducted among 159 PCOS and 82 healthy eumenorrheic non-hirsute women aged 20 - 50 years. The fractional-polynomial model was used to develop a model of continuous risk factors, which evaluates non-linear associations between irisin/GIP and BMI among women with and without PCOS.Results:
Women with PCOS were significantly younger (28.2 ± 5.8 vs. 33.0 ± 7.8 years, P < 0.001) and had a greater BMI (26.6 ± 5.2 vs. 25.2 ± 4.8 kg/m2, P = 0.04) than the healthy counterparts. There were no significant linear and non-linear associations between serum concentration of irisin/GIP and BMI in both groups. The analysis of pair-wise age and BMI matching of women with PCOS and controls confirmed these findings.Conclusions:
This study showed that irisin and GIP have no association with BMI in women with or without PCOS. This finding could help to better understand the underlying pathophysiological status of PCOS, insulin resistance, and obesity-related disorders. Further large cohort studies are needed to confirm these findings.Keywords
Adipocytokine Body Mass Index Insulin Resistance Polycystic Ovary Syndrome
1. Background
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among women of reproductive age (1). The syndrome is highly associated with reproductive and metabolic disturbance (2, 3). Women with PCOS present with greater risk for hyperandrogenism, hyperinsulinemia, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM) (4, 5). While the underlying pathogenesis of PCOS is still unclear, the role of insulin resistance (IR) and impaired adipocytokines actions secreted by adipose tissue have recently been explored (6-8).
Adipose tissue is identified as an active endocrine organ that could regulate insulin sensitivity by adipocytokines secretion via hormonal signals (9) and regulation of thermogenesis (10).
Irisin, as a novel adipokine/myokine hormone, mainly produced from myokine after physical exercise, could promote adipose tissue energy expenditure (11). Recent studies indicated that irisin is associated with obesity, T2DM, and gestational T2DM (12-14). Moreover, it is suggested that irisin metabolism is abnormal in patients with PCOS, which may result in susceptibility to PCOS development (15, 16).
In addition, studies have shown that glucose-dependent insulinotropic peptide (GIP), as the stimulator of insulin release after nutrients intake, is impaired in many patients with IR and obesity (16-19). This peptide could regulate the deposition of some lipids in the muscle or liver and insulin-mediated uptake of glucose into white adipose tissues (20).
However, there have been some debates about the role of obesity in the association of irisin/GIP and PCOS. The over-expression or their reduced productions have been reported in obese patients with PCOS (17, 21). Seemingly, these cytokines may be a connective factor between obesity, IR, and PCOS.
2. Objectives
This study aimed to investigate the linear and non-linear association between body mass index (BMI) and serum concentration of irisin/GIP in women with and without PCOS.
3. Methods
The study proposal was approved by the Ethics Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Iran. A written informed consent was obtained from all women who willingly accepted to participate in this study.
3.1. Study Population
This cross-sectional case-control study was conducted among 159 women aged 20 - 50 years who were known cases of PCOS referred from outpatient endocrinology clinic in Tehran from March 2015 to March 2018. Also, 82 healthy eumenorrheic non-hirsute volunteer women aged 20 - 50 years were recruited as healthy controls. Exclusion criteria were history of endocrine disorders, including thyroid diseases, hyperprolactinemia, and known hyperandrogenic disorders, as well as any medication that may interfere with the normal function of the adipokines, including anti-androgen and anti-lipid therapies, anti-diabetic agents, glucocorticoids, and any hormones. Moreover, menopausal or pregnant women and those with menstrual irregularities or hyperandrogenism were excluded.
3.2. Clinical and Biochemical Measurements
The weight and height were measured with light clothing without shoes by standard devices to the nearest 0.1 kg and 0.5 cm, respectively. The waist circumference (WC) was measured at the superior border of the iliac crest to the nearest 0.5 cm. The hip circumference (HC) was measured at the levels of buttocks’ maximum extension to the nearest 0.5 cm. BMI was computed as dividing weight in kilograms by height in meters squared. The waist-to-hip ratio (WHR) was computed as the WC divided by the HC. Blood pressure (BP) was measured in the sitting position after a 15-min rest using a standard mercury sphygmomanometer, which was calibrated by the Iranian Institute of Standards and Industrial Research.
For biochemical measurements, a blood sample was taken after 12 to 14 h of overnight fasting between 07:00 and 09:00 a.m., and then plasma was separated in a refrigerated centrifuge at 3000 rpm for ten minutes, and sera were stored at -80ºC until tested. Sex hormone binding globulin was evaluated by enzyme immunometric assay (Mercodia, Uppsala, Sweden). Androstenedione and total testosterone (TT) were evaluated using enzyme immunoassay (Diagnostic Biochem Canada). All enzyme-linked immunosorbent assay (ELISA) tests were performed using the Sunrise ELISA reader (Tecan Co. Salzburg, Austria). All samples were analyzed when internal quality control met the acceptable criteria. The intra-assay and inter-assay coefficients of variation (CV) for TT were 3.6% and 6.0%, for sex hormone-binding globulin 1.1% and 4.1%, and for androstenedione 2.2% and 3.5%, respectively. The irisin and GIP were measured using a sandwich ELISA method (ZellBio GmbH, Germany). The sensitivity and intra-assay CV of studied adipokines were 0.09 ng/ mL, 5.8%, and 6.7% for irisin and 10 pg/ml, 6.9%, and 77.5% for GIP, respectively.
3.3. Definition of Terms
The PCOS was diagnosed based on the US National Institutes of Health (NIH) criteria, including presence of the two following criteria: chronic oligo-anovulation and hyperandrogenism (clinical or biological) after exclusion of other related disorders (22).
Oligo-anovulation was defined menstruation interval of more than 37 days or a history of less than eight menstrual cycles in a year (23). The clinical hyperandrogenism included hirsutism (according to modified Ferriman-Gallwey scale score less than 8), androgenic alopecia, or acne vulgaris. Biochemical hyperandrogenism was defined as elevated serum concentration of one or more androgens based on the 95th percentile of healthy women (24).
3.5. Statistical Analysis
The sample size was calculated by using a two-sided two-sample equal-variance t-test with the following parameters: significance level = alpha 5%; power = 82.9%; and mean difference (standard deviation) = 25.0 (65.0).
All variables were presented as mean (standard deviation) or median with inter-quartile range (IQ25-75) as appropriated. T-test and the Mann-Whitney U test were used to compare the basic characteristics of healthy and PCOS groups. To detect the relationship between irisin/GIP and BMI, scatter plot matrix was drawn. We explored a more exact association by fitting a fractional polynomial (FP) regression model, which is a flexible parametric approach to model continuous risk factors and evaluates the potential non-linear associations more precisely (25). We also prepared data for pair-wise (1:1) age-BMI matching. Spearman’s correlation coefficient was used to determine the relationship between the variables. The software package STATA (version 14; STATA Inc., College station, TX, USA) was used to perform all statistical analysis; the significance level was set at P < 0.05.
4. Results
The characteristics of the women in both groups are shown in Table 1. The women with PCOS were significantly younger (28.2 ± 5.8 vs. 33.0 ± 7.8 years, P < 0.001), had a greater BMI (26.6 ± 5.2 vs. 25.2 ± 4.8 kg/m2, P = 0.04), and higher values for WC (88.9 ± 13.2 vs. 83.4 ± 11.3 cm, P = 0.001) and WHR (0.85 ± 0.08 vs. 0.82 ± 0.07, P = 0.002) than the healthy women.
The Anthropometric, Clinical, and Biochemical Features of the Groupsa
| Characteristics | PCOS (N =159) | Healthy (N= 82) | P-Value |
|---|---|---|---|
| Age (y) | 28.20 (5.81) | 33.02 (7.83) | < 0.001 |
| BMI (kg/m2) | 26.60 (5.24) | 25.16 (4.82) | 0.04 |
| WC (cm) | 88.93 (13.20) | 83.40 (11.27) | 0.001 |
| WHR | 0.85 (0.08) | 0.82 (0.07) | 0.002 |
| SBP (mmHg) | 105.19 (17.09) | 101.19 (9.30) | 0.007 |
| DBP (mmHg) | 72.37 (13.55) | 65.54 (8.03) | < 0.001 |
| TT (ng/mL) | 0.47 (0.28 - 0.90) | 0.49 (0.39 - 0.68) | 0.8 |
| SHBG (nmol/L) | 43.4 (33.6 - 53.7) | 93 (69.0 - 121.0) | < 0.001 |
| Androstenedione (ng/mL) | 1.20 (0.80 - 2.10) | 1.36 (1.0 - 1.81) | 0.6 |
| Irisin (ng/mL) | 404.65 (150.13) | 372.46 (115.21) | 0.1 |
| GIP (pg/mL) | 3.74 (3.15 - 4.76) | 3.56 (2.62 - 4.51) | 0.7 |
Figure 1 shows the scatter plot matrix of irisin and GIP levels against BMI, indicating no association between irisin/GIP and BMI in both PCOS cases and healthy controls. After adjusting for age, the results of FP analysis showed that there were no significant linear nor non-linear associations between serum concentration of irisin/GIP and BMI in both groups.
Scatter plot matrix of (A) irisin and (B) glucose-dependent insulinotropic polypeptide levels against body mass index
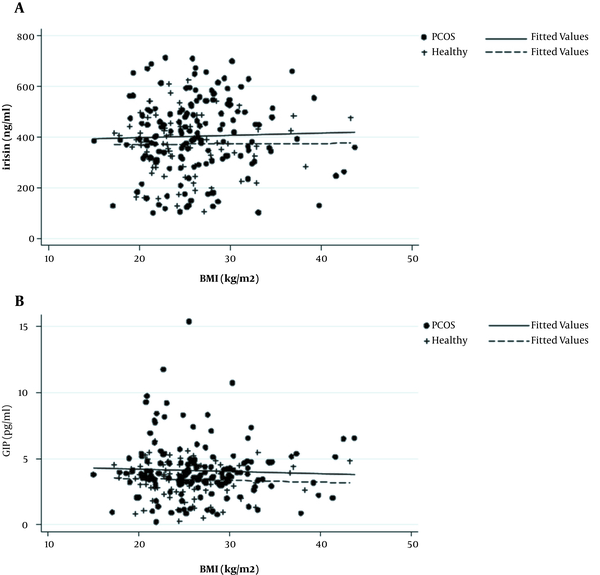
In addition, the association of irisin/GIP and BMI in pair-wise age and BMI matching of women with PCOS (n = 68) and controls (n = 68) showed that there were no associations between irisin/GIP and BMI in both PCOS (r = 0.02, P = 0.8 for irisin and r = -0.03, P = 0.9 for GIP) and healthy groups (r = 0.08, P = 0.5 for irisin and r = 0.01, P = 0.9 for GIP).
5. Discussion
Results of the study demonstrated that there was no association between irisin/GIP and BMI independent of the age in women with and without PCOS using both linear and non-linear methods. In addition, no correlation was found in pair-wise age and BMI matching comparison between irisin/GIP and BMI in both PCOS and healthy groups.
The underlying etiology of PCOS, as a heterogeneous disorder, is complex and multifactorial. It is suggested that adiposity and related metabolic alterations play an important role in the pathogenesis of PCOS (6). Several muscle-derived factors are responsible for the modulation of insulin sensitivity. Irisin, as a muscle-derived brown adipose differentiation factor, is identified as a cleaved and secreted product of the Fndc5 protein (26). Irisin could induce the translocation of glucose transporter type 4 (GLUT-4) to the plasma membrane and stimulate glucose uptake in differentiated skeletal muscles (27). Higher serum irisin concentration is related to a greater energy expenditure and diet-induced IR improvement (28). However, emerging literature showed that in aggravated IR status such as metabolic syndrome, pre-diabetes, and PCOS, irisin concentrations compensatory increase to promote the insulin sensitivity (15, 29, 30) In this respect, obesity, as an important IR risk factor, may have a major effect in relation to irisin and PCOS status.
There are limited studies with controversial results assessing the correlation between BMI and irisin in both PCOS and non-PCOS women. While some studies reported that serum concentration of irisin in PCOS was negatively associated with BMI (15), some investigations showed the positive correlation (21, 31) or even no association between circulating irisin levels and BMI (32, 33). Wang et al. evaluated the serum irisin in 40 women with PCOS and 30 infertile women without PCOS. They showed that serum concentration of irisin was negatively associated with BMI (R = -0.762 , P = 0.000), and irisin level in obese PCOS patients was significantly lower than that in non-obese PCOS patients (15). On the contrary, Bostanci et al. evaluated 35 PCOS patients and reported that serum irisin was positively associated with BMI in the overall population, but not for PCOS group alone (32). However, in agreement with our study, Abali et al. (33) and Timmons et al. (34) reported that irisin levels had no correlation with BMI.
The discrepancies in the above-mentioned studies may be related to various PCOS population characteristics such as different age, BMI, physical activity, ethnicity, and phenotypes of PCOS, which may influence plasma irisin levels (35-37). Moreover, some studies suggested that pathological state of POCS may influence the irisin level. In this respect, it is suggested that irisin level in cases with the newly diagnosed and well-regulated impaired insulin sensitivity tend to be increased to compensate for the IR (36, 37). In contrast, in a fully developed IR situation, physiological irisin cannot maintain the balance of IR, and thus irisin levels may be remained in normal or even lower than normal levels (35-37). This finding suggests the relationship between this hormone and the disease process (35). Supporting this hypothesis, a lower concentration of irisin in the presence of complications of T2DM compared to newly diagnosed and well-regulated T2DM has been shown (36, 37). Zhang et al. (2014) showed a significantly reduced serum concentrations of irisin among patients with T2DM with macro/peripheral vascular complications and cardiovascular diseases compared to patients without such complications (36). Accordingly, based on the findings of these studies, it is hypothesized that the positive correlation of circulating irisin with obesity may be nullified by other factors in the pathological states of obesity.
However, studies of human gut derived incretins, including GIP, have shown that these hormones play a role in the regulation of the triglycerides and insulin mediated uptake of glucose (30-33). There are limited studies investigating the association between GIP and BMI in PCOS subjects. Pontikis et al. investigated the secretion of GIP between age matched obese and lean patients with PCOS after oral glucose tolerance tests (OGTT). In agreement with our findings, the GIP values did not differ between the groups. However, after OGTT test, obese PCOS patients demonstrated lower GIP level in response to OGTT compared to the controls. The authors argued that although the serum concentrations of GIP in different states of obesity were similar, its response to the oral glucose tolerance test and IR may be attenuated. This paper suggested that the activity of the entero-insular axis may be impaired in PCOS patients (38). However, many factors have been associated with GIP responses, including age and gender (38), BMI (17, 39), inflammatory cytokines (40), and IR and metformin treatment, which may have an important effect on the findings. Further well-designed studies with an appropriate sample size are warranted to explore these associations.
Measurement of relatively new laboratory markers and analysis by both linear and non-linear statistical models could be considered as the strengths of this study. However, there are some limitations that need to be considered when interpreting the results of this study. The clinical-based nature of this study might present severe phenotypes of PCOS women referred for treatment. Therefore, it could not be representative of all phenotypes of PCOS. In addition, the current study was conducted among women who referred to a tertiary clinic in Tehran, and the findings need to be interpreted in other ethnicities with caution.
Moreover, lack of adequate samples did not allow to perform the subgroup analyses based on various phenotypes of PCOS. In addition, we did not assess other adiposity indices, IR, OGTT, as well as IR related factors, which may help to clarify the findings. Furthermore, the cross-sectional design of this study and lack of specific data on irisin protein levels in muscles restricted finding the causal relationships between the studied variables. Finally, since irisin is a kind of myokine which is secreted by exercise, the evaluation of irisin level without including the exercise information may confound the findings.
5.1. Conclusions
This study showed that irisin and GIP have no association with BMI in women with or without PCOS. This finding could help to better understand the underlying pathophysiological status of PCOS, IR, and obesity-related disorders. Further large cohort studies are needed to confirm these results.
References
-
1.
Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351-8. [PubMed ID: 29221211]. [PubMed Central ID: PMC5707105]. https://doi.org/10.18632/oncotarget.19180.
-
2.
Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Noroozzadeh M, Farahmand M, Rostami Dovom M, et al. The risk of metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2018;88(2):169-84. [PubMed ID: 28930378]. https://doi.org/10.1111/cen.13477.
-
3.
Behboudi-Gandevani S, Ramezani Tehrani F, Hosseinpanah F, Khalili D, Cheraghi L, Kazemijaliseh H, et al. Cardiometabolic risks in polycystic ovary syndrome: long-term population-based follow-up study. Fertil Steril. 2018;110(7):1377-86. [PubMed ID: 30503137]. https://doi.org/10.1016/j.fertnstert.2018.08.046.
-
4.
Dimitriadis GK, Kyrou I, Randeva HS. Polycystic ovary syndrome as a proinflammatory state: The role of adipokines. Curr Pharm Des. 2016;22(36):5535-46. [PubMed ID: 27464726]. https://doi.org/10.2174/1381612822666160726103133.
-
5.
Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, et al. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril. 2017;108(6):1078-84. [PubMed ID: 29202960]. https://doi.org/10.1016/j.fertnstert.2017.09.004.
-
6.
Behboudi-Gandevani S, Ramezani Tehrani F, Rostami Dovom M, Farahmand M, Bahri Khomami M, Noroozzadeh M, et al. Insulin resistance in obesity and polycystic ovary syndrome: systematic review and meta-analysis of observational studies. Gynecol Endocrinol. 2016;32(5):343-53. [PubMed ID: 27052492]. https://doi.org/10.3109/09513590.2015.1117069.
-
7.
Behboudi-Gandevani S, Ramezani Tehrani F, Bidhendi Yarandi R, Noroozzadeh M, Hedayati M, Azizi F. The association between polycystic ovary syndrome, obesity, and the serum concentration of adipokines. J Endocrinol Invest. 2017;40(8):859-66. [PubMed ID: 28332170]. https://doi.org/10.1007/s40618-017-0650-x.
-
8.
Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017;40(1):1-8. [PubMed ID: 27473078]. [PubMed Central ID: PMC5206255]. https://doi.org/10.1007/s40618-016-0523-8.
-
9.
Cinti S. Between brown and white: Novel aspects of adipocyte differentiation. Ann Med. 2011;43(2):104-15. [PubMed ID: 21254898]. https://doi.org/10.3109/07853890.2010.535557.
-
10.
Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131(2):242-56. [PubMed ID: 17956727]. https://doi.org/10.1016/j.cell.2007.10.004.
-
11.
Rana KS, Pararasa C, Afzal I, Nagel DA, Hill EJ, Bailey CJ, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16(1):147. [PubMed ID: 29121940]. [PubMed Central ID: PMC5680831]. https://doi.org/10.1186/s12933-017-0627-2.
-
12.
Gizaw M, Anandakumar P, Debela T. A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture. 2017;20(4):235-42. [PubMed ID: 30151293]. [PubMed Central ID: PMC6104716]. https://doi.org/10.3831/KPI.2017.20.029.
-
13.
Ural UM, Sahin SB, Tekin YB, Cure MC, Sezgin H. Alteration of maternal serum irisin levels in gestational diabetes mellitus. Ginekol Pol. 2016;87(5):395-8. [PubMed ID: 27304658]. https://doi.org/10.5603/GP.2016.0013.
-
14.
Sahin-Efe A, Upadhyay J, Ko BJ, Dincer F, Park KH, Migdal A, et al. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism. 2018;79:24-32. [PubMed ID: 29108900]. https://doi.org/10.1016/j.metabol.2017.10.011.
-
15.
Wang C, Zhang XY, Sun Y, Hou XG, Chen L. Higher circulating irisin levels in patients with polycystic ovary syndrome:a meta-analysis. Gynecol Endocrinol. 2018;34(4):290-3. [PubMed ID: 29069945]. https://doi.org/10.1080/09513590.2017.1393065.
-
16.
Chang CL, Huang SY, Soong YK, Cheng PJ, Wang CJ, Liang IT. Circulating irisin and glucose-dependent insulinotropic peptide are associated with the development of polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(12):E2539-48. [PubMed ID: 25029417]. https://doi.org/10.1210/jc.2014-1180.
-
17.
Svendsen PF, Nilas L, Madsbad S, Holst JJ. Incretin hormone secretion in women with polycystic ovary syndrome: roles of obesity, insulin sensitivity, and treatment with metformin. Metabolism. 2009;58(5):586-93. [PubMed ID: 19375579]. https://doi.org/10.1016/j.metabol.2008.11.009.
-
18.
Suh S, Kim. M.Y, Kim. S.K, et al. Glucose-dependent insulinotropic peptide level is associated with the development of type 2 diabetes mellitus. Endocrinol Metab. Metabolism. 2016;31(5):134-41. [PubMed ID: 19375579].
-
19.
Calanna S, Urbano F, Piro S, Zagami RM, Di Pino A, Spadaro L, et al. Elevated plasma glucose-dependent insulinotropic polypeptide associates with hyperinsulinemia in metabolic syndrome. Eur J Endocrinol. 2012;166(5):917-22. [PubMed ID: 22391044]. https://doi.org/10.1530/EJE-11-0765.
-
20.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131-57. [PubMed ID: 17498508]. https://doi.org/10.1053/j.gastro.2007.03.054.
-
21.
hang CL, Huang SY, Hsu YC, et al. The serum level of irisin, but not asprosin, is abnormal in polycystic ovary syndrome patients. Sci Rep. 2019;9(6):6447. [PubMed ID: 17498508]. https://doi.org/10.1053/j.gastro.2007.03.054.
-
22.
Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(4):272-7. [PubMed ID: 29084464]. https://doi.org/10.1080/09513590.2017.1395841.
-
23.
Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-90. [PubMed ID: 22065325]. https://doi.org/10.1055/s-0031-1287662.
-
24.
Hashemi S, Ramezani Tehrani F, Noroozzadeh M, Azizi F. Normal cut-off values for hyperandrogenaemia in Iranian women of reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;172:51-5. [PubMed ID: 24220143]. https://doi.org/10.1016/j.ejogrb.2013.09.029.
-
25.
Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. Applied Statistics. 1994;43(3). https://doi.org/10.2307/2986270.
-
26.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-8. [PubMed ID: 22237023]. [PubMed Central ID: PMC3522098]. https://doi.org/10.1038/nature10777.
-
27.
Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol. 2015;29(6):873-81. [PubMed ID: 25826445]. [PubMed Central ID: PMC5414737]. https://doi.org/10.1210/me.2014-1353.
-
28.
Martinez Munoz IY, Camarillo Romero EDS, Garduno Garcia JJ. Irisin a novel metabolic biomarker: Present knowledge and future directions. Int J Endocrinol. 2018;2018:7816806. [PubMed ID: 30402097]. [PubMed Central ID: PMC6198573]. https://doi.org/10.1155/2018/7816806.
-
29.
Chen X, Jia X, Qiao J, et al. Adipokines in reproductive function: A link between obesity and polycystic ovary syndrome. J Mol Endocrinol. 2013;50:21-37.
-
30.
Sartori C, Lazzeroni P, Merli S, Patianna VD, Viaroli F, Cirillo F, et al. From Placenta to Polycystic Ovarian Syndrome: The Role of Adipokines. Mediators Inflamm. 2016;2016:4981916. [PubMed ID: 27746590]. [PubMed Central ID: PMC5056282]. https://doi.org/10.1155/2016/4981916.
-
31.
Li M, Yang M, Zhou X, Fang X, Hu W, Zhu W, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(4):1485-93. [PubMed ID: 25675380]. https://doi.org/10.1210/jc.2014-2544.
-
32.
Bostanci MS, Akdemir N, Cinemre B, Cevrioglu AS, Ozden S, Unal O. Serum irisin levels in patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2015;19(23):4462-8. [PubMed ID: 26698239].
-
33.
Abali R, Temel Yuksel I, Yuksel MA, Bulut B, Imamoglu M, Emirdar V, et al. Implications of circulating irisin and Fabp4 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2016;36(7):897-901. [PubMed ID: 27184575]. https://doi.org/10.3109/01443615.2016.1174200.
-
34.
Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488(7413):E9-10. discussion E10-1. [PubMed ID: 22932392]. https://doi.org/10.1038/nature11364.
-
35.
Gao S, Cheng Y, Zhao L, Chen Y, Liu Y. The relationships of irisin with bone mineral density and body composition in PCOS patients. Diabetes Metab Res Rev. 2016;32(4):421-8. [PubMed ID: 26589554]. https://doi.org/10.1002/dmrr.2767.
-
36.
Zhang L, Fang X, Li L, Liu R, Zhang C, Liu H, et al. The association between circulating irisin levels and different phenotypes of polycystic ovary syndrome. J Endocrinol Invest. 2018;41(12):1401-7. [PubMed ID: 29785700]. https://doi.org/10.1007/s40618-018-0902-4.
-
37.
Liu JJ, Liu S, Wong MD, Tan CS, Tavintharan S, Sum CF, et al. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J Diabetes Complications. 2014;28(2):208-13. [PubMed ID: 24332937]. https://doi.org/10.1016/j.jdiacomp.2013.09.011.
-
38.
Pontikis C, Yavropoulou MP, Toulis KA, Kotsa K, Kazakos K, Papazisi A, et al. The incretin effect and secretion in obese and lean women with polycystic ovary syndrome: a pilot study. J Womens Health (Larchmt). 2011;20(6):971-6. [PubMed ID: 21671782]. https://doi.org/10.1089/jwh.2010.2272.
-
39.
Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57(5):1340-8. [PubMed ID: 18162504]. https://doi.org/10.2337/db07-1315.
-
40.
Kahles F, Meyer C, Diebold S, Foldenauer AC, Stohr R, Mollmann J, et al. Glucose-dependent insulinotropic peptide secretion is induced by inflammatory stimuli in an interleukin-1-dependent manner in mice. Diabetes Obes Metab. 2016;18(11):1147-51. [PubMed ID: 27350651]. https://doi.org/10.1111/dom.12711.


