Abstract
Background:
Selecting a host system for the expression of recombinant proteins should be carefully evaluated prior to the initiation of any bio therapeutic development programs. Since different hosts express proteins with various efficiencies and with different posttranslational modifications, changing hosts may impact the expected activity of the protein. The main expression systems have members of the mammalian cell family, however, there are remarkable differences between the species. The most generally used mammalian hosts for the production of recombinant proteins are CHO and HEK293 cells.Objectives:
In order to compare the differences between HEK versus CHO cells, the human coagulation factor IX in a transient and stable expression was examined.Methods:
After transfection of CHO and HEK cells with an hFIX-expressing mammalian plasmid, pcDNA-hFIX, transient expression was analyzed on the culture supernatant during 72 hours. The stable clones were also recovered after 10 weeks of geneticin selection. The expression and activity of the hFIX was evaluated by performing enzyme-linked immunosorbent assay (ELISA) as well as a coagulation test, respectively. The γ-carboxylation of the hFIX was confirmed by evaluation of the expressed protein, after being precipitated with barium citrate.Results:
Although the stable CHO clones secreted hFIX protein 30% higher than HEK cells, the transient HEK cell line proved to be superior in the production of total hFIX protein (%42 increased) and functional hFIX (%29 higher) relative to the CHO cell line. Moreover, specific activity and the fully γ-carboxylated hFIX are almost constant in transient and stable expression, indicating that γ-carboxylation efficiency in both CHO and HEK cell lines is almost equal.Conclusions:
In conclusion, transient transfection studies with HEK cells provide a simple and strong method for a high production of recombinant proteins in weeks, a process which is more time consuming in the CHO cell line.Keywords
HEK Cells CHO Cells Transient and Stable Expression Coagulation Factor IX
1. Background
The production of proteins in adequate quantity and quality is a necessary demand of the present time. Before we begin any bio therapeutic development program, we must pay close attention in choosing a suitable host system for the expression of recombinant proteins. Since a gene can be expressed in multiple systems and different hosts, and it can express proteins with different efficiencies as well as variant posttranslational modifications, determining the best system for expression of recombinant proteins is one of the most important issues in biotechnology. A recombinant protein expression system must be able to produce the biological material with the highest biological activity and safety while it has the lowest cost. Scientists must also consider the expression system’s productivity, consistency, and the current regulatory environment (1).
There are numerous available expression systems for large scale production of recombinant proteins including Escherichia coli (E. coli), yeast, insect, and several mammalian based systems. Each of these has its own relevant advantages depending on cost, simplicity, and their post-translational modification profiles (2). Now, some famous companies are using eukaryotic systems to produce recombinant proteins. Among them, mammalian expression systems are the main expression systems for the production of recombinant human proteins. Mammalian cell expression systems have several advantages over other eukaryotic expression systems (3). Mammalian expression systems are able to fulfill the appropriate post-translational modifications, which are important for protein folding, product assemblies, and biological activity. However, it should be noted that there are differences between species. A large number of mammalian cells including myeloma (NS0 and SP2/0) cells, Chinese hamster ovary (CHO) cells, human retina-derived cells (PerC6), COS and Vero (both green African monkey kidney), Human cervical cancer (HeLa), human embryonic kidney (HEK) cells, as well as baby hamster kidney (BHK) cells have been successfully used to express recombinant proteins (2).
Although CHO cells are the first candidate cell lines for vitro cultivation and recombinant protein production, HEK 293 cell line has been extensively used as an expression tool for production of mammalian and non-mammalian recombinant proteins, both by transient transfection and by the formation of stable cell lines (4, 5). So far, the main focus of expression approaches has been on employing members of the CHO cell family, however, little has been published notably on side by-side comparisons of CHO and HEK293 cell. In addition to transfection efficiency comparison of both cells with reagent-FreeStyleTM MAX (6), polyethyleneimine (PEI) reagents (7), or in a suspension culture (8), multiple studies have reported differences in the glycosylation patterns of proteins when produced in CHO versus HEK cells (9, 10). Furthermore, a few single publications report differences in expression levels of recombinant proteins in HEK and CHO cells (11, 12). It has been showed that the expression of bispecific T cell engager (BiTE) antibody in transient HEK cells is higher than CHO cells (11). A recent review by Durocher and Butler (12) report differences in expression levels as well as biological activity. In vitro and in vivo linked to the capacity of individual host cell lines for secondary modifications such as glycosylation patterns based on stable transfected cell lines, however, similar differences may be observed in transient expression of proteins. It has been shown that high glycosylation heterogeneity for HEK293 and mono- to tetrasialylated glycans for CHO-derived material. Furthermore, sialic acid content of N-glycans was 25 % lower for HEK293- than for CHO recombinant factor IX (10).
Since the current treatment for hemophilia B patients consists of replacement therapy with recombinant factor IX (FIX) (13), selecting a host system for the expression of recombinant proteins should be carefully evaluated. On the other hand, the production of cost-effective and high-quality recombinant human FIX would have a remarkable impact on the treatment of hemophilia B patients worldwide. Moreover, to quickly evaluate proteins, it is better to use faster approaches such as transient gene expression technology (TGE) in the production of recombinant proteins. Therefore, the choice of an appropriate host cell system for a production of novel protein target is currently a question of availability of systems and experimental testing. Furthermore, it is unlikely that CHO cell and their derivatives systems will be capable of expressing all target proteins. Therefore usage of other established cell lines with different cellular origin may be extremely beneficial for production of research proteins (4).
2. Objectives
Our aim in this work is the comparison of 2 different mammalian cell lines that are used for transfection, which can finally lead to gene expression of human factor IX.
3. Methods
3.1. Materials
All cell culture reagents were provided from the thermo fisher scientific, except for penicillin G as well as streptomycin (Sigma-Aldrich). Geneticin (G418) and protease inhibitors were purchased from Roche.
3.2. Construction of Recombinant Plasmids
The cDNA of hFIX (1.4 kb) was PCR-amplified from a human liver cDNA library with pfu-DNA polymerase, using oligonucleotide pairs, hFIX-KpnI (5GGGGTACCGCCACCATGCAGCGCGTGAAC3) and hFIX-XhoI (5 CCGCTCGAGATCCATCTTTCATTAAGTGAGC3) as forward/reverse primers. Following subcloning into T-vector, the KpnI-XhoI fragments were inserted into the pcDNA3 vector, 3’ to the CMV (cytomegalovirus) promoter. The resulting plasmid was designated pcDNA3-hFIX (Figure 1). The identity of the fragments was confirmed using restriction digestion followed by nucleotide sequence analysis.
Plasmid Construction Diagram
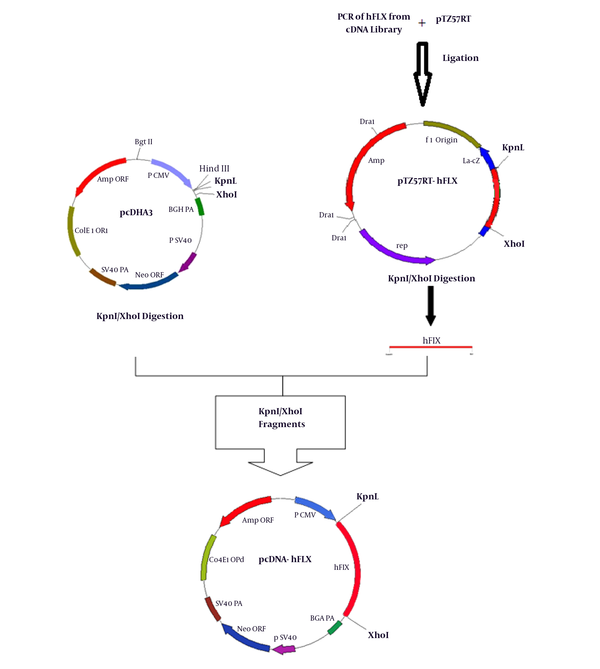
3.3. Transfection and Preparation of Stable Clones
CHO and HEK cells (a kindly gift of Dr. Zomorodipour, NIGEB, Iran) were grown in a 5% CO2 and 37°C, subcultured at a density of 2 × 105 cells in a volume of 2 mL in 6-well plates (13), and transfected with 2 µg pcDNA3-hFIX using the calcium phosphate method. Individual clones were expanded in the presence of 450 µg/mL Geneticin for 2 months. The expression media containing 6 µg/mL vitamin K1 and 10% (v/v) FBS were added to ~70% confluent cells, upon which the individual clones were screened for FIX production during 3 days.
3.4. RNA Preparation and RT-PCR Analysis
Total RNA from 106 cells expressing human FIX was extracted according to the protocol provided by the manufacturer (Tripure kit). First-strand cDNA synthesis was performed using random primers and M-MuLV reverse transcriptase (Sinaclone, Iran). The generated fragments were subsequently used as a template for the PCR-amplification of the double stranded cDNA, corresponding to the hFIX coding sequence.
3.5. Quantitative and Qualitative Analysis of Human Factor IX
Human FIX was quantified in a conditioned media employing an ELISA (Asserachrom IX:Ag, Stago, France) following the procedure provided by the manufacturer. In addition, intracellular accumulated FIX was assessed, for which, the cells were centrifuged at 100 g for 5 minutes, upon which pellet were suspended in 500 µL of ice-cold lysis buffer (100 mM KCl, 2 mM MgCl2, 10 mM, HEPES pH 7.5, 0.5% Triton X100) containing an antiprotease mix (complete Protease Inhibitor, Roche). Subsequently, the lysate was centrifuged at 12,000 g for 10 minutes at 4°C, upon which the supernatant was assessed for FIX. The aPTT assay was also used to examine functional activity of FIX (13). Human plasma immuno-depleted of FIX (100 µL; Stago) was mixed with conditioned media (100 µL) and aPTT reagent (100 µL; BioMerieux). After 3 minutes of incubation at 37°C, 100 µL of a prewarmed CaCl2 solution (25 mM) was added to the mixture, and the clotting time was recorded. Normal human plasma (prepared from the Iranian blood transfusion organization) was used as a reference and 1 unit of FIX activity corresponded to the amount of FIX in 1 mL of normal plasma (~ 5 μg/mL).
3.6. Quantification of γ-carboxylated Factor IX
The barium citrate precipitation of γ-carboxylated human FIX was adapted from previously described methods (14). Briefly, sodium citrate (14 mg) and 1 M barium chloride (95 µL) were added to 2 mL of conditioned media, which were incubated for 1 hour at 4°C with gentle mixing. The mixture was centrifuged at 100 g for 5 minutes and the supernatant was kept for the analysis of unabsorbed FIX lacking γ-carboxyglutamic residues. The pelleted γ-carboxylated FIX was washed with ice-cold 5 mM BaC12, subsequently centrifuged at 100 g for 5 minutes, suspended again in 0.1 M sodium citrate (1 mL), and adjusted to 10% ammonium sulphate to dissolve the adsorbed hFIX. After 30 minutes, at 0°C, and subsequent centrifugation at 100 g for 5 minutes, after which the barium sulphate pellet was discarded, FIX was determined in the supernatant by ELISA as described.
3.7. Statistical Analysis
The ANOVA program for analysis of variance followed by a Tukey post-hoc test was performed for the evaluation; P < 0.05 was considered statistically significant. All statistical analyses were carried out with SPSS 16 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. RNA Expression Analyze
Factor IX (FIX) expression by the CHO and HEK cell lines was further confirmed employing RT-PCR, which demonstrated the presence of FIX mRNA in both cell types (Figure 2).
Gel Electrophoresis of RT-PCR Products of hFIX from CHO and HEK Cell Lines
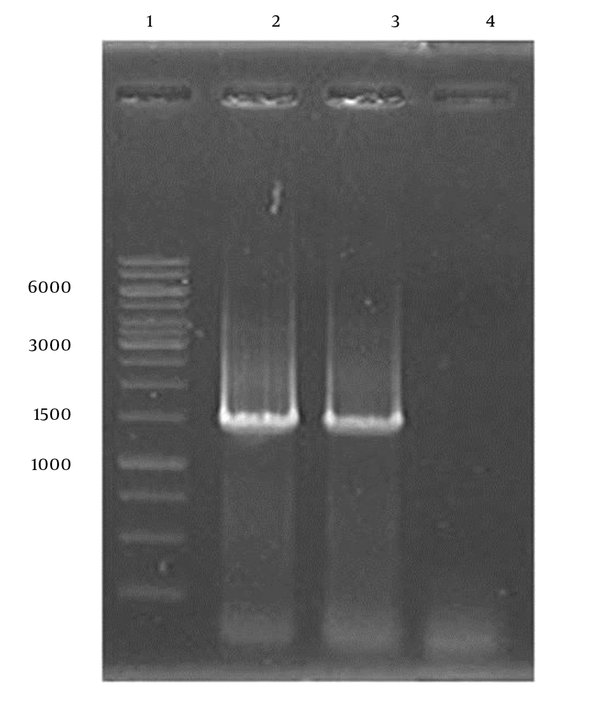
4.2. Selection of Stable Clones Expressing Factor IX
The selection of single cell-derived CHO clones that stably express human FIX was performed in a period of 2 months and resulted in the selection of 12 high-producer clones. Selection of an hFIX-expressing cell line derived from stable transfected HEK cells was performed in the time span of 10 weeks, and the numbers of stable transformed HEK cell lines, which express rhFIX (4 clones), were lower than stable CHO cells. This suggests that efficiency of delivery to the target region of genome, loss of the plasmid, and resistance to antibiotic may contribute to differences in these cellular vehicles.
4.3. Characterization of rhFIX Secreted from Transiently Transfected HEK and CHO Cell Lines
To evaluate the transient expression of rhFIX in the HEK and CHO cells, both conditioned media and cell lysates were examined for FIX antigen levels. While the CHO cell line secreted up to 96 ng/mL/106 cells FIX after 72 hours, FIX expression time in the media by the HEK cell line was increased up to 42% and accumulated to 137 ng/mL/106 cells (Table 1). Consistent with these findings, the HEK cell line expressed up to a 29% higher FIX activity as compared to that of the CHO cells (58 vs. 45 mU/mL/106 cells). However, the CHO-expressed FIX displayed a 20% enhanced specific activity relative to that expressed by the HEK cell line (0.48 vs. 0.4 U/mg). Taken together, these findings show that the transiently transfected HEK cell line produces higher levels of functional rhFIX as compared to the CHO cell line.
Factor IX expression by transfected HEK and CHO cells. Factor IX expression was determined in conditioned media after 72 hours of FIX expression. The data are the mean ± SD of 3 similar experiments.
| Cell Type | FIX Antigen in median, /mL/106 Cell | FIX Activity, mU/mL/106 Cell | FIX Specific Activity, U/mg | Recovery% |
|---|---|---|---|---|
| CHO | 96 ± 4 | 45 ± 1 | 0.46 | 80 |
| HEK | 136 ± 5 | 58 ± 3 | 0.40 | 78 |
4.4. Expression of rhFIX from Stable HEK and CHO Cell Lines
After a selection of single cell clones that stably express rhFIX, comparison of the rhFIX expression following expansion of these clones in a 25 cm2 flask to 95% confluence revealed that the stable CHO cells are capable of expressing up to 30% more of human FIX proteins as compared to HEK cells (148 vs. 113 ng/mL/106 cells, Table 2). Consistent with the findings obtained on the FIX antigen levels, the activity levels of CHO-expressed FIX were also increased by 40% in comparison with those of the stable expressing HEK cells. Specific activity of rhFIX in both cells was 0.3 U/mg.
Factor IX expression by stable HEK and CHO cells. Factor IX expression was determined in conditioned media after 72 hours of FIX expression. The data are the mean ± SD. of 3 similar experiments.
| Cell Type | FIX Antigen, ng/mL/106 Cell | FIX Activity, mU/mL 106 Cell | FIX Specific Activity, U/mg | Recovery% |
|---|---|---|---|---|
| CHO | 148 ± 3 | 47 ± 2 | 0.3 | 82 |
| HEK | 113 ± 4 | 33 ± 2 | 0.3 | 79 |
4.5. Recovery
In order to assess the FIX recovery, the general characteristics of vitamin K-dependent (VKD) proteins, which can be absorbed to barium citrate via it’s γ-carboxylated, glutamic residue was used to find out whether the recombinant cells were capable of the proper γ-carboxylation of human FIX (15). During this process, neither poorly γ-carboxylated molecules nor non- γ-carboxylated were trapped by the barium action and were remained in the soluble fraction; the barium-bound hFIX can be subsequently eluted from the precipitate (15). Following barium citrate precipitation, the supernatant obtained from the HEK and CHO cell lines was enriched for FIX. Quantification of FIX recovered from the barium citrate precipitation showed that around 80% of the CHO and HEK cell-produced FIX was recovered employing barium citrate adsorption (Table 2), and therefore was most likely fully γ-carboxylated. Moreover, the amount of fully γ-carboxylated rhFIX is similar in CHO cells rather than HEK cells.
4.6. Secretion Efficiency
As defined, the secretion efficiency of a protein is the secreted fraction ratio to its total amount (16). On the basis of the data obtained, the secretion efficiency of FIX in transient HEK and CHO cells were 68% and 81%, respectively. The secretion efficiency of human FIX by both of the stable HEK and CHO cells reached up to 66% and 78%, whereas the intracellular FIX content remained minimal all through the experiment.
5. Discussion
Over the past decades, the production of r-proteins has been successfully undertaken in a variety of mammalian cell lines, however, there are still some concerns regarding the expression levels and long periods of clonal selection when moving forward toward a large-scale production for therapeutic purposes. The most commonly used mammalian hosts for the production of r-proteins are CHO and HEK293 cells, although other mammalian cell types have also been used. We generated a clonal, FIX-expressing HEK cell line, which required up to 10 weeks of selection. In contrast, using the CHO cell system we were able to generate a clonal cell line in the short time period of 7 weeks. Moreover, the number of stable clones was small in the case of HEK cells. This was surprising because during the time transfectability in HEK cells was higher comparing to that of CHO cells, the time of stable clones preparation and the number of high producing clones were less. It has been shown that the transfectability of HEK cells is 90% versus 40% of CHO cells, when using the calcium phosphate transfection method (17). Gonçalves has also indicated that the transfection of HEK293T cells in a suspension is more efficient than transfection of CHO cells in terms of the number of transfected cells (8). Liu et al. has also shown that transfectability of HEK293 cells is extremely high and able to tolerate a wider range of cultural conditions during transfection, although transient transfection efficiency with cationic lipid-based transfection reagent-FreeStyleTM MAX is similar in both 293-F and CHO-S cells (6). Suen et al. indicated that although transfection of HEK cells is generally performed using inexpensive transfection reagents such as polyethyleneimine (PEI), the CHO cells are commonly transfected with more expensive lipofection reagents (7). Therefore, although HEK cells are generally more likely to be transfected than CHO cells, the generation of high-producing stable HEK cell clones is more time-consuming than CHO cells. This may be due to these cellular vectors, which are usually derived from different species and tissue source and are likely to differ in their efficiency of delivery to the target region, loss of the plasmid, as well as resistance to antibiotics.
We also noted that although the stable CHO clones secreted slightly higher levels of hFIX protein than HEK cells (30% higher), transient transfection of CHO cells leaded to a very poor protein production (42% lower). In contrast, the transient expression of hFIX in HEK cells resulted in a quick production of secreted protein and more important, the use of the same plasmid vector as for the stable CHO cell clone generation. In this regard, Diepenbruck, C. et al. noted that although stable CHO clones secreted slightly higher levels of r-protein than HEK cells (17% higher), transient HEK cells have higher expression r-protein than CHO cells (11). They also showed the CHO cells are not well suited to deliver reasonable amounts of protein following transient transfection (11). Suen et al. has also indicated that the protein expression yield is generally 2 - 5 times higher for PEI-transfected HEK vs. CHO cells (7). Other studies has also indicated that using a CHO-based transient production of proteins was limited by poor transfection efficiencies, viabilities, and production of insufficient quantities of recombinant proteins so that transient production of recombinant scFv-Fc antibodies in HEK293 is efficient and robust (18). The productivity of transient transfections depends on various factors such as the amount of cell population transfected, the gene transcription and translation efficiency in the host cells, innate property of producer cells related to protein folding and the extent of post-translational processing, as well as modification required for optimal protein function (6). It has also been shown that mRNA stability is very important for the transgene expression levels and mRNA stability in transient HEK cells is more than CHO cells (19, 20). Liu et al. showed that the EPO specific mRNA expressed in transient HEK cells remained constant, whereas the EPO mRNA level has been dramatically reduced in CHO cells after 4 days post-transfection (6). On the other hand, although a threefold elevation of EPO mRNA level was expressed in HEK cells compared to CHO cells, the maximal accumulations of EPO were greater in FreeStyleTM CHO, being sevenfold higher than the maximum accumulations obtained from FreeStyleTM HEK cells (6). It could be due to the lower secretion efficiency of HEK cells rather than that of CHO cells. We also indicated that the secretion efficiency in CHO cells was 20% more than HEK cells, which may be due to provision of different intracellular signaling pathways such as protein glycosylation and folding (5).
The hFIX as a vitamin K dependent protein (VKD) undergoes multiple post translational modifications (PTMs), among them, γ-carboxylation has a considerable effect on its biological activity (21). Our findings suggest that the FIX produced in both CHO and HEK cell lines was properly γ-carboxylated, since up to 80% of both cell-produced FIX was recovered employing barium citrate adsorption, indicating that the majority of the FIX produced was γ-carboxylated. It was also interesting that the specific activity and the fully γ-carboxylated FIX were almost constant in both cell lines in transient and stable expression, indicating that the carboxylation efficiency in both CHO and HEK cell lines has been almost equal.
High functional FIX expression levels are another requirement for a successful therapeutic protein production. In our expression systems, the transient HEK cell line proved to be superior in the production of total FIX protein (42% increased) and functional FIX (29% higher) relative to the CHO cell line. It is important to note that due to the increasing demand for the fast and efficient production of proteins in expression systems, many industrial laboratories prefer to use the host cell line in transient gene expression (TGE) system to produce r-proteins for pre-clinical purposes. Although the best method for large-scale production of recombinant proteins in mammalian cells is to establish stable clones, this approach can be laborious, expensive, and time-consuming. As an alternative, the large-scale transient transfection of mammalian cells can produce remarkable amounts of recombinant protein in a very short period of time (5, 22). The ideal mammalian cell line for the transient production of r-proteins is highly susceptible to transfection, naturally inclined to have a high cell specific productivity, and easy to maintain on a large-scale (23). Although, for the stable expression of recombinant proteins, CHO cells are still prior to HEK293, however, the HEK293 is the preferred cell line for its transient expression. This is attributable to the fact that the large-scale transfection of CHO cells has low efficiency and low productivity when cost-effective transfection reagents are used. On the other hand, CHO cells, which are widely used for stable transfection, are not well suited to deliver adequate amounts of protein following transient transfection (11). HEK cells have generated the highest volumetric yields in TGE systems, and are therefore the most widely used (23). For many applications, the use of transient HEK, rather than CHO cells, appears to be superior, since HEK cells are generally more easily transfected (7).
In conclusion, our findings suggest that the large-scale transient HEK cell system has the potential of reducing the cell development time significantly, while maintaining FIX function. More studies and extensive efforts need to be carried out on this expression system in order to maximize protein production in transient expression systems by the optimization of host cell lines, vector systems, and cell culture conditions. Overall, these observations support that the HEK cell system is a respectable candidate for the efficient production of recombinant FIX.
Acknowledgements
References
-
1.
Kim KR, Kim YK, Cha HJ. Recombinant baculovirus-based multiple protein expression platform for Drosophila S2 cell culture. J Biotechnol. 2008;133(1):116-22. [PubMed ID: 17963934]. https://doi.org/10.1016/j.jbiotec.2007.09.010.
-
2.
Khan KH. Gene expression in Mammalian cells and its applications. Adv Pharm Bull. 2013;3(2):257-63. [PubMed ID: 24312845]. https://doi.org/10.5681/apb.2013.042.
-
3.
Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127(3):335-47. [PubMed ID: 16959350]. https://doi.org/10.1016/j.jbiotec.2006.07.012.
-
4.
Geisse S, Voedisch B. Transient expression technologies: past, present, and future. Methods Mol Biol. 2012;899:203-19. [PubMed ID: 22735955]. https://doi.org/10.1007/978-1-61779-921-1_13.
-
5.
Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51(3):187-200. [PubMed ID: 15862464]. https://doi.org/10.1016/j.vascn.2004.08.014.
-
6.
Liu C, Dalby B, Chen W, Kilzer JM, Chiou HC. Transient transfection factors for high-level recombinant protein production in suspension cultured mammalian cells. Mol Biotechnol. 2008;39(2):141-53. [PubMed ID: 18327552]. https://doi.org/10.1007/s12033-008-9051-x.
-
7.
Suen KF, Turner MS, Gao F, Liu B, Althage A, Slavin A, et al. Transient expression of an IL-23R extracellular domain Fc fusion protein in CHO vs. HEK cells results in improved plasma exposure. Protein Expr Purif. 2010;71(1):96-102. [PubMed ID: 20045465]. https://doi.org/10.1016/j.pep.2009.12.015.
-
8.
Goncalves C, Gross F, Guegan P, Cheradame H, Midou P. A robust transfection reagent for the transfection of CHO and HEK293 cells and production of recombinant proteins and lentiviral particles - PTG1. Biotechnol J. 2014;9(11):1380-8. [PubMed ID: 25215936]. https://doi.org/10.1002/biot.201400324.
-
9.
Krista S, James B, Meg D, Karen D. Literature Review: CHO versus HEK Cell Glycosylation. MaxCyte; 2015.
-
10.
Bohm E, Dockal M, Graninger M, Hasslacher M, Kaliwoda M, Konetschny C, et al. Expression of recombinant human coagulation factors VII (rFVII) and IX (rFIX) in various cell types, glycosylation analysis, and pharmacokinetic comparison. BMC Proc. 2011;5 Suppl 8:P23. [PubMed ID: 22373195]. https://doi.org/10.1186/1753-6561-5-S8-P23.
-
11.
Diepenbruck C, Klinger M, Urbig T, Baeuerle P, Neef R. Productivity and quality of recombinant proteins produced by stable CHO cell clones can be predicted by transient expression in HEK cells. Mol Biotechnol. 2013;54(2):497-503. [PubMed ID: 22915356]. https://doi.org/10.1007/s12033-012-9590-z.
-
12.
Durocher Y, Butler M. Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol. 2009;20(6):700-7. [PubMed ID: 19889531]. https://doi.org/10.1016/j.copbio.2009.10.008.
-
13.
Vatandoost J, Zomorodipour A, Sadeghizadeh M, Aliyari R, Bos MH, Ataei F. Expression of biologically active human clotting factor IX in Drosophila S2 cells: gamma-carboxylation of a human vitamin K-dependent protein by the insect enzyme. Biotechnol Prog. 2012;28(1):45-51. [PubMed ID: 22012919]. https://doi.org/10.1002/btpr.723.
-
14.
Vatandoost J, Bos MH. Efficient expression of functional human coagulation factor IX in stably-transfected Drosophila melanogaster S2 cells; comparison with the mammalian CHO system. Biotechnol Lett. 2016;38(10):1691-8. [PubMed ID: 27565667]. https://doi.org/10.1007/s10529-016-2156-6.
-
15.
Jallat S, Perraud F, Dalemans W, Balland A, Dieterle A, Faure T, et al. Characterization of recombinant human factor IX expressed in transgenic mice and in derived trans-immortalized hepatic cell lines. EMBO J. 1990;9(10):3295-301. [PubMed ID: 2209546].
-
16.
Kim YK, Shin HS, Tomiya N, Lee YC, Betenbaugh MJ, Cha HJ. Production and N-glycan analysis of secreted human erythropoietin glycoprotein in stably transfected Drosophila S2 cells. Biotechnol Bioeng. 2005;92(4):452-61. [PubMed ID: 16025538]. https://doi.org/10.1002/bit.20605.
-
17.
Preuss AK, Connor JA, Vogel H. Transient transfection induces different intracellular calcium signaling in CHO K1 versus HEK 293 cells. Cytotechnology. 2000;33(1-3):139-45. [PubMed ID: 19002821]. https://doi.org/10.1023/A:1008150402616.
-
18.
Jager V, Bussow K, Wagner A, Weber S, Hust M, Frenzel A, et al. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol. 2013;13:52. [PubMed ID: 23802841]. https://doi.org/10.1186/1472-6750-13-52.
-
19.
Tienen LM. Transient gene expression in mammalian cells for the production of recombinant protein therapeutics. Amsterdam; 2012.
-
20.
Sarah W. Transient recombinant protein expression in mammalian cells: The role of mrna level and stability. Swiss; 2009.
-
21.
Tie JK, Zheng MY, Pope RM, Straight DL, Stafford DW. Identification of the N-linked glycosylation sites of vitamin K-dependent carboxylase and effect of glycosylation on carboxylase function. Biochemistry. 2006;45(49):14755-63. [PubMed ID: 17144668]. https://doi.org/10.1021/bi0618518.
-
22.
Li R. Transient transfection of CHO cells using linear polyethylenimine is a simple and effective means of producing rainbow trout recombinant IFN-gamma protein. Cytotechnology. 2015;67(6):987-93. [PubMed ID: 24897997]. https://doi.org/10.1007/s10616-014-9737-9.
-
23.
Tienen LM. Transiant gene expression in mammilian cells for the production of recombinant protein therapeutics. Amsterdam, the Netherlands: Amsterdam; 2012.
reply