Abstract
Background:
The use of herbal supplements and exercise to treat chronic diseases and metabolic disorders is a common practice.Objectives:
This study aimed to investigate the effect of eight weeks of grape seed nanoparticle extract and resistance training on SOD, MDA, and NRF-1 expression in the myocardial infarction rat model.Methods:
In this experimental trial, 25 Wistar rats were randomly divided into five groups: (1) control, (2) MI, (3) RT + MI, (4) GS + MI, and (5) RT + MI + GS. Resistance training was performed for eight weeks and five sessions per week. Also, 150 mg of grape seed extract was gavage daily. One-way analysis of variance and the Tukey test was used to compare groups at the P ≤ 0.05.Results:
Resistance training with grape seed significantly increased NRF-1 gene expression (P = 0.001) and SOD (P = 0.01) compared to MI grope. While resistance training with grape seed significantly reduced MDA compared to the MI group (P = 0.01).Conclusions:
It seems that resistance training with grape seed consumption has a more positive effect on increasing NRF-1 gene expression and improving the antioxidant system in the heart tissues of rats with myocardial infarction rather than using each alone.Keywords
Resistance Training Grape Seed NRF-1 MDA SOD Myocardial Infarction
1. Background
Myocardial infarction (MI) is pathologically defined as myocardial cell death due to prolonged ischemia, which is the most severe manifestation of coronary artery disease (CAD). Moreover, it causes over seven million deaths globally every year. Increased oxidative stress due to ischemia and myocardial hypoxia causes the death of large numbers of heart cells, cardiac fibrosis, and heart failure (1).
Mitochondria are important as cellular power plants for providing the energy needed by eukaryotic cells. However, mitochondrial function is not limited to cell energy supply but includes calcium storage, apoptosis, and intracellular signaling. NRFs, particularly NRF-1, are strong stimuli for the expression of nuclear genes needed for mitochondrial respiration. NRF-1 can directly regulate the expression of several nuclear genes that are necessary for the expression, assembly, and function of the respiratory chain, whereas indirectly regulate cytochrome C oxidase genes by activating TFAM. Therefore, a broader concept is considered for NRF-1 to organize mitochondrial biogenesis events (2). Biogenesis, mitochondrial function, and the maintenance of structural integrity are essential for cells, as mitochondrial dysfunction can impair energy metabolism, intensify ROS synthesis, and trigger apoptotic cell death mechanisms (3).
Under these conditions, when there is no balance between the production of free radicals and antioxidant defenses in the body, it can lead to oxidative stress, which plays a prominent role in the pathogenesis of various diseases. On the other hand, oxidative stress due to increased free radicals, deficiency of antioxidant enzymes, and inflammation are important risk factors for cardiovascular diseases (4). Malondialdehyde is a reactive dialdehyde made by the peroxidation of unsaturated fatty acids in the human body. The rate of lipid peroxidation is obtained by measuring MDA levels in various biological samples to be utilized as an indicator of oxidative stress. MDA affects the function of molecules and other cells and produces carcinogenic, mutagenic, and atherogenic effects by reacting with purines (5).
Also, the superoxide dismutase system is a major antioxidant mechanism that catalyzes the conversion of superoxide anions to hydrogen peroxide. Experimental studies have shown that increased oxidative stress and decreasing antioxidant status affect heart structure and function (6). In this regard, Short et al. (7) showed that 16 weeks of aerobic exercise significantly increased NRF-1 and PGC-1α expression.
Grape seed proanthocyanins are a new generation antioxidant that removes excess ROS produced in the disease process and acts as a signaling molecule. Recent research has shown that grape seed proanthocyanins improve myocardial ischemic reperfusion injury by reducing ROS in the myocardium (8, 9). Pataki et al. (10) showed that grape seed proanthocyanins had cardioprotective effects against reperfusion injury by reducing or removing direct or indirect free radicals in the myocardium after ischemia. Despite reports on the benefits of grape seed on MI, our understanding of the mechanisms to explain how grape seed improves mitochondrial biogenesis and antioxidant capacity is to be elucidated. Furthermore, the combined effect of resistance training and grape seed on proteins involved in antioxidant system function and NRF-1 expression in cardiomyocytes is not investigated as yet.
2. Objectives
Owing to the increasing prevalence of MI and the need for effective treatment methods to decrease the complications of MI and heart damage in the patients, conducting basic experimental studies in this field may shed light on nutrition and sport. Therefore, this study aimed to investigate the effect of eight weeks of grape seed nanoparticle extract and resistance training on SOD, MDA, and NRF-1 expression in the myocardial infarction rat model.
3. Methods
3.1. Animals
In this experimental study, twenty-five Wistar rats were purchased from the Pasteur Institute of Iran and kept in the laboratory for one week for adaptation. The present study maintained the animals in standard conditions, including free access to water and food, 55% relative humidity, a 12-hour dark-light cycle, and 22 ± 3°C. Also, all procedures performed on human participants should be in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The present study was approved by the Islamic Azad University of Isfahan (Khorasgan) Branch, Iran (code: IR.IAU.KHUISF.REC.1400.289).
3.2. Modeling Method and Induction of Myocardial Infarction
Isoproterenol was subcutaneously injected at 85 mg/kg as a normal saline solution (1 mg/mL) for two consecutive days with 24 hours of intervals. This method developed ischemia in mice 24 hours after the second injection (11). One day after injection, four animals lost their lives and were replaced immediately. Thus, the induction of myocardial infarction was directed based on the standard protocols. Finally, 25 rats were included in the study. These 25 rats with myocardial infarction were randomly assigned to five groups, including (1) control, (2) MI, (3) MI + training, (4) MI + supplement, and (5) MI + training + supplement.
3.3. Preparation of Nanoparticles of Grape Seed
The active ingredient of grape seed extract was purchased, purified from Sigma Company, and dissolved in DMSO. Based on our data, DLS results indicated that nanoparticle sizes vary from 200 to 720 nm and the most significant number of nanoparticles generated is a mean intensity of 394.26 nm. After preparation of the supplement in the form of nano, 150 mg daily was gavaged to the supplement groups (12).
3.4. Exercise Training
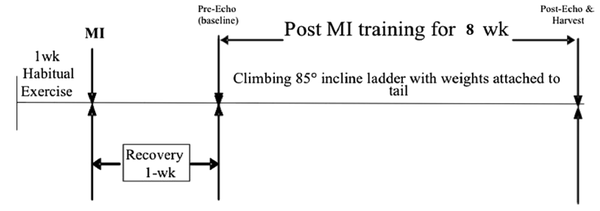
Each test included 85-degree ladder ascent using 50 to 130 percent g/body weight (BM), with 20% weight gain per ascent. Also, 1 RM was used to schedule the exercises. The training program was started with a starting climb weight of 50 percent 1RM, incremental weight increase of five percent per ascent, 8 - 10 ascent per session, with two min rest between ascent (13) (Figure 1).
Experiment flow chart
3.5. Molecular Analysis of Myocardial Tissue by Real Time PCR
Here, 200 µL Qiazol was added to the samples and incubated for 24 hours at -80°C. The plaque inside the cryotube grinned in semi-freezing state, and 100 µL chloroform was added to the samples for one minute to slip the samples. Then, cDNA was synthesized according to the manufacturer’s protocol (Fermentas, USA) after exploitation of high purity and concentration RNA from all samples, and then the synthesized cDNA was utilized for reverse transcription reaction. The expression level of NRF-1 gene in heart tissue was measured by real time-PCR. NRF-1 expression of cardiac tissue was performed by real quantitative time-PCR (Table 1). Moreover, gene expression was assessed according to the 2-ΔΔCT method, and the reference gene expression level was considered glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Moreover, MDA was measured by Ochiyama method with a spectrophotometer. MDA produces a color complex with thiobarbituric acid that absorbs light at a wavelength of 532 nm. Tetramotoxy propane was used as the standard material. Furthermore, SOD activity was measured by inhibition of nitroblue tetrazolium reduction by xanthine-xanthine oxidase system as superoxidase-producing superoxidase. Optical absorption of each sample was read at 550 nm for five minutes every 30 seconds.
The Primer Sequences of Genes
| Gene Name | Oligo Sequence | Lengths (bp) | Tm (°C) | Accession No. |
|---|---|---|---|---|
| NRF-1 | 98 | 61 | NM_001100708.1 | |
| F | TGGCTGAAGCCACCTTACAA | |||
| R | ATGAACTCCATCTGGGCCATT | |||
| GAPDH | 121 | 60 | NM_017008.4 | |
| F | GTGCTACAGAGGGGTGGAGGG | |||
| R | GCTTGAGAGGGAGAGGGTTAGG |
3.6. Statistical Analysis
Mean and standard deviation (SD) were used for descriptive data. Shapiro-Wilkes test was used for data normality. For comparison between groups, one-way ANOVA (analysis of variance) and pairwise comparison of Tukey's test was used. The data were collected and analyzed with SPSS version 22 at P ≤ 0.05.
4. Results
The results of the analysis of variance indicated that eight weeks of RT with GS nanoparticle use had a significant effect on rising NRF-1 gene expression and SOD. Also, it had a significant effect on decreasing MDA in myocardial infarction rat model.
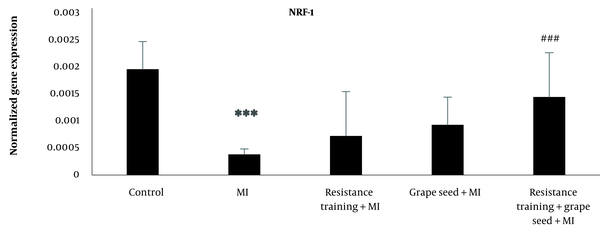
The results of Tukey’s test indicated that NRF-1 mRNA expression in the myocardial infraction group was significantly reduced compared to the control group (P = 0.001). While other myocardial infraction groups with RT and GS did not show significant changes in comparison to the MI group (P > 0.05). Compared to the MI group, only the MI + RT + GS group showed a significant rise in NRF-1 (P = 0.001) (Figure 2).
NRF-1 expression in five research groups (***Significant decrease compared to the control group P ≤ 0.001; ###Significant increase compared to the MI group P ≤ 0.001).
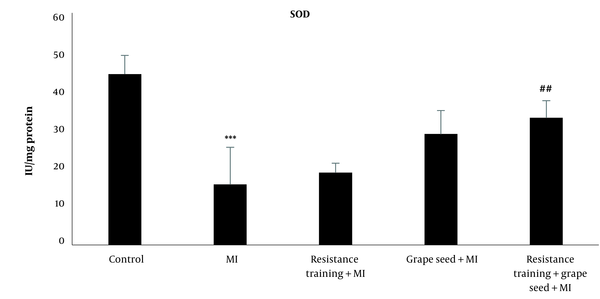
The results of Tukey's test showed that SOD in the myocardial infraction group was significantly reduced compared to the control group (P = 0.001). While other myocardial infraction groups with RT and GS did not show significant changes in comparison to the MI group (P > 0.05). Compared to the MI group, only the MI + RT + GS group showed a significant rise in SOD (P = 0.01) (Figure 3).
SOD levels in five research groups (***Significant decrease compared to the control group P ≤ 0.001; ##Significant increase compared to the MI group P ≤ 0.001).
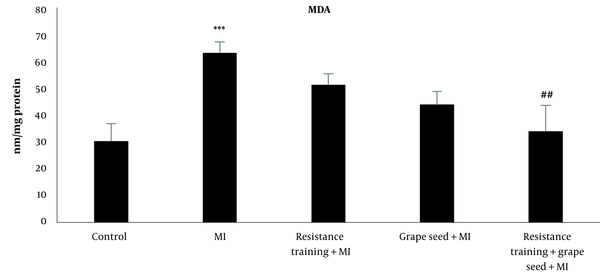
The results of Tukey's test indicated that MDA in the MI group significantly increased compared to the control group (P = 0.001). While other MI groups with RT and GS did not indicate significant changes in comparison to the MI group (P > 0.05). Compared to the MI group, only the MI + RT + GS group showed a significant decrease in MDA (P = 0.01) (Figure 4).
MDA levels in five research groups (***Significant increase compared to the control group P ≤ 0.001; ##Significant decrease compared to the MI group P ≤ 0.001).
5. Discussion
The results of the present research indicated that the expression level of NRF1 was downregulated compared to the control groups immediately after the MI. We found a marked elevation in NRF-1 expression in MI + training + grape seed group compared with MI. Myocardial infarction can interfere with protein synthesis in mitochondrial biogenesis or other related processes (14). Low regulation of NRF1 protein is also included in the inefficient synthesis of complex mitochondrial respiratory subunits, leading to decreased mitochondrial respiratory function. Thus, by reducing PGC-1 and downstream transcription agents, NRF proteins help to a lack of myocardial oxidative capacity and energy generation (15).
The findings of the present study are consistent with a previous study that indicated a significant reduction in NRF-1 expression in the muscles of heart patients. Therefore, due to mitochondrial dysfunction and decreased regulation of NRF-1 expression, increasing biogenesis and restoration of mitochondrial function may help heart patients improve their metabolic status. Robinson et al. showed that 12 weeks of resistance training increased mitochondrial protein levels through proteomics (16). Also, Barbosa et al. showed that 12 weeks of resistance training increased the expression of PGC1-α, NRF-1, TFAM, p-AMPK, and p-CREB genes, which are involved in skeletal muscle mitochondrial biogenesis and oxidative metabolism (17). The results of the present research are consistent with the results of the heroic Gahramani and Karbalaeifar showed that eight weeks of HIIT rise mitochondrial biogenesis in slow muscle of MI rats with an effect on NRF-1, NRF-2, and Tfam genes (18).
Mitochondrial biogenesis needs the coordinated regulation of transcription of nuclear and mitochondrial genes. PGC-1α is the main regulator of mitochondrial biogenesis. PGC-1α activates NRF1 and increases nuclear transcription of mitochondrial genes. Despite the important role of PGC-1α, NRF1, and TFAM in mitochondrial biogenesis, an acute increase in NRF1 protein levels was observed. Wright et al. also showed that the expression of mitochondrial biogenesis proteins (NRF-1, cytochrome C, NRF-2, and cytochrome oxidase type 4 promoters) increased earlier than the increase in PGC1α, possibly inducing early-stage PGC1α activation. Increasing acceptance/adaptation to exercise in muscle mitochondria and subsequent rise in PGC1α improves proteins in rising mitochondrial biogenesis (19).
Ogborn et al. examined the adaption of PGC-1α, NRF1, and TFAM mRNA following RT. The results indicated no significant change in NRF1 mRNA and no initial increase in PGC-1α and Tfam (20). Nikoozadeh et al. indicated that training and curcumin supplementation alone and in combination with each other could increase the gene expression of some factors that stimulate mitochondrial biogenesis in cardiomyocytes of male rats with myocardial infarction (21). Elevated NRF-1 indicates that exercise can improve energy metabolism by increasing mitochondrial biogenesis regulators and myocardial infarction caused by myocardial infarction. In this regard, various factors such as exercise and phytochemicals affect the expression of many genes. Therefore, resistance training can improve energy metabolism by increasing mitochondrial PGC-1α and NRF-1 and improve myocardial injury due to myocardial infarction. Also, resistance training as a strong stimulant probably dilates blood vessels and increases myocardial blood flow and calcium, thus activating matrix dehydrogenases. Resistance training activates PGC-1α, thereby binding to the transcription factor and regulating the expression of mitochondrial genes located in the nucleus, as well as activating NRF-1,2 and Tfam (19).
Other results of the present study showed that MDA levels were upregulated compared to the control groups immediately after the MI. Also, SOD levels were downregulated compared to the control groups immediately after the MI. Indeed, MI increases the production of free radicals during mitochondrial electron transfer chain dysfunction. Electron transfer chain dysfunction can overproduce ROS over antioxidant function. It can be concluded that exercise increases mitochondrial function in the skeletal and heart muscles, resulting in rose SOD and reduced the generation of free radicals and oxidative stress, which all contribute to decreased risk of heart disease (22). The results of the present research are in line with the results of Tayebi et al. (23) the results of this study indicated that resistance training can increase GSH and TAC and thus reduce MDA, and this is independent of the intensity of training.
Bagheri et al. showed that exercise reduces heart damage in myocardial infarction, and this reduction occurs independent of changes in oxidative and antioxidant factors (24). Ristic et al. indicated that moderate-intensity physical training of sufficient duration alone or in combination with PDE-1 inhibitors leads to beneficial adaptations, manifested as decreased oxidative stress and an improved antioxidant defense system (25). Resistance training increases complex IV activity and mitochondrial creatine kinase content without altering total mitochondrial mass (23). Mitochondrial creatine kinase is an important component of the phosphocreatine shuttle. Free creatine re-phosphorylation is known as an aerobic system, and the rate of re-phosphorylation reflects the function of the electron transfer chain. Therefore, this may lead to a decrease in oxidative pressure and an increase in electron transfer current, resulting in less oxidation of glutathione. In another study, resistance training increased the activity of antioxidant enzymes and decreased the production of reactive oxygen species. However, in one study, resistance training had no effect on the activity of antioxidant enzymes (26).
The polyphenols in grape seed extract include flavonoids, gallic and dimeric, monomeric, and polymeric proanthocyanidins. Proanthocyanidin dimer in GS is the most effective antioxidant (9). Studies have shown that grape seed extract has an important role in eliminating free radicals and inhibiting oxidative stress, and in myocardial infarction and tissue regeneration, has an inhibitory role against oxidative stress. Moradi et al. (27) showed that eight weeks of aerobic exercise alone or in combination with grape seed extract and consumption of grape seed extract alone reduced cardiovascular risk factors in young men. The ability of procyanidin has been confirmed by increasing the expression of NRF-1 and SOD. Lack of control over calorie intake and calorie intake in rat during the study period and the use of various measurement methods such as Western blotting was one of the limitations of the present research. It is suggested that future studies use apoptotic markers and Tunnel method to identify structural damage to heart tissue.
5.1. Conclusions
The results of the present research generally indicate that grape seed nanoparticle, along with exercise training, especially resistance training is effective in controlling the damage to the heart muscle by positively regulating NRF-1 and increasing antioxidant capacity in the heart tissue.
Acknowledgements
References
-
1.
Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology. 2007;230(2-3):178-88. [PubMed ID: 17188415]. https://doi.org/10.1016/j.tox.2006.11.053.
-
2.
Seyydi SM, Tofighi A, Rahmati M, Tolouei Azar J. Exercise and Urtica Dioica extract ameliorate mitochondrial function and the expression of cardiac muscle Nuclear Respiratory Factor 2 and Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha in STZ-induced diabetic rats. Gene. 2022;822:146351. [PubMed ID: 35189251]. https://doi.org/10.1016/j.gene.2022.146351.
-
3.
DEVRİM A, BİLGİÇ P. Does Exercise Effect on Mitochondrial Dysfunction and Insulin Sensitivity? Hacettepe University Faculty of Health Sciences Journal. 2017;4(3):31-7. https://doi.org/10.21020/husbfd.341817.
-
4.
Radak Z, Ishihara K, Tekus E, Varga C, Posa A, Balogh L, et al. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285-90. [PubMed ID: 28285189]. [PubMed Central ID: PMC5345970]. https://doi.org/10.1016/j.redox.2017.02.015.
-
5.
Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15(5):251-8. [PubMed ID: 15238821]. https://doi.org/10.1097/01.mca.0000131573.31966.34.
-
6.
Rinaldi B, Corbi G, Boccuti S, Filippelli W, Rengo G, Leosco D, et al. Exercise training affects age-induced changes in SOD and heat shock protein expression in rat heart. Exp Gerontol. 2006;41(8):764-70. [PubMed ID: 16822632]. https://doi.org/10.1016/j.exger.2006.05.008.
-
7.
Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888-96. [PubMed ID: 12882902]. https://doi.org/10.2337/diabetes.52.8.1888.
-
8.
Zhang FL, Gao HQ, Wu JM, Ma YB, You BA, Li BY, et al. Selective inhibition by grape seed proanthocyanidin extracts of cell adhesion molecule expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol. 2006;48(2):47-53. [PubMed ID: 16954821]. https://doi.org/10.1097/01.fjc.0000242058.72471.0c.
-
9.
Du Y, Guo H, Lou H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J Agric Food Chem. 2007;55(5):1695-701. [PubMed ID: 17295515]. https://doi.org/10.1021/jf063071b.
-
10.
Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75(5):894-9. [PubMed ID: 11976164]. https://doi.org/10.1093/ajcn/75.5.894.
-
11.
Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225(1-):75-83. [PubMed ID: 11716367]. https://doi.org/10.1023/a:1012220908636.
-
12.
Llopiz N, Puiggros F, Cespedes E, Arola L, Ardevol A, Blade C, et al. Antigenotoxic effect of grape seed procyanidin extract in Fao cells submitted to oxidative stress. J Agric Food Chem. 2004;52(5):1083-7. [PubMed ID: 14995102]. https://doi.org/10.1021/jf0350313.
-
13.
Garza MA, Wason EA, Cruger JR, Chung E, Zhang JQ. Strength training attenuates post-infarct cardiac dysfunction and remodeling. J Physiol Sci. 2019;69(3):523-30. [PubMed ID: 30911900]. https://doi.org/10.1007/s12576-019-00672-x.
-
14.
Liu M, Zhou B, Xia ZY, Zhao B, Lei SQ, Yang QJ, et al. Hyperglycemia-induced inhibition of DJ-1 expression compromised the effectiveness of ischemic postconditioning cardioprotection in rats. Oxid Med Cell Longev. 2013;2013:564902. [PubMed ID: 24303086]. [PubMed Central ID: PMC3835206]. https://doi.org/10.1155/2013/564902.
-
15.
Lim WC, Shin EJ, Lim TG, Choi JW, Song NE, Hong HD, et al. Ginsenoside Rf Enhances Exercise Endurance by Stimulating Myoblast Differentiation and Mitochondrial Biogenesis in C2C12 Myotubes and ICR Mice. Foods. 2022;11(12). [PubMed ID: 35741909]. [PubMed Central ID: PMC9222511]. https://doi.org/10.3390/foods11121709.
-
16.
Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017;25(3):581-92. [PubMed ID: 28273480]. [PubMed Central ID: PMC5423095]. https://doi.org/10.1016/j.cmet.2017.02.009.
-
17.
Barbosa MR, Shiguemoto GE, Tomaz LM, Ferreira FC, Rodrigues MF, Domingues MM, et al. Resistance Training and Ovariectomy: Antagonic Effects in Mitochondrial Biogenesis Markers in Rat Skeletal Muscle. Int J Sports Med. 2016;37(11):841-8. [PubMed ID: 27428645]. https://doi.org/10.1055/s-0042-107247.
-
18.
Gahramani M, Karbalaeifar S. [Effect of eight weeks high intensity interval training on NRF-1, 2 and Tfam gene expressione levels in ST muscles in rats with myocardial infarction]. Med J Tabriz Uni Med Sciences Health Services. 2020;41(6):75-82. Persian. https://doi.org/10.34172/mj.2020.009.
-
19.
Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282(1):194-9. [PubMed ID: 17099248]. https://doi.org/10.1074/jbc.M606116200.
-
20.
Ogborn DI, McKay BR, Crane JD, Safdar A, Akhtar M, Parise G, et al. Effects of age and unaccustomed resistance exercise on mitochondrial transcript and protein abundance in skeletal muscle of men. Am J Physiol Regul Integr Comp Physiol. 2015;308(8):R734-41. [PubMed ID: 25695287]. https://doi.org/10.1152/ajpregu.00005.2014.
-
21.
Nikoozadeh A, Pouzesh Jadidi R, Nourazar MA, Asgharpour Arshad M, Bashiri J. [Interactive Effect of Aerobic Training and Curcumin Supplementation on Mitochondrial Cardiomyocyte Nrf1 and Nrf2 Gene Expression in Myocardial infraction Male Rat Model]. Applied Health Studies in Sport Physiology. 2021;8(2):44-50. Persin. https://doi.org/10.22049/JAHSSP.2021.27321.1370.
-
22.
Park SY, Kwak YS. Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes. J Exerc Rehabil. 2016;12(2):113-7. [PubMed ID: 27162773]. [PubMed Central ID: PMC4849490]. https://doi.org/10.12965/jer.1632598.299.
-
23.
Tayebi SM, Khalili F, Saeidi A. [Effects of Eight Weeks Resistance Training with Two Different Intensities on Oxidative Stress Markers of Young Men]. Sport Physiology. 2017;9(33):187-200. Persian. https://doi.org/10.22089/spj.2017.2288.1308.
-
24.
Bagheri A, Hematfar A, Rozbehani M, Behpour N. [The effect of exercise pre-conditioning with intense intermittent training on MDA, SOD and GPX of cardiac tissue following induction of acute myocardial infarction in male rats]. Yafteh. 2021;22(4):146-61. Persian.
-
25.
Ristic J, Folic M, Radonjic K, Rosic MI, Bolevich S, Alisultanovich OI, et al. Preconditioning with PDE1 Inhibitors and Moderate-Intensity Training Positively Affect Systemic Redox State of Rats. Oxid Med Cell Longev. 2020;2020:6361703. [PubMed ID: 32104536]. [PubMed Central ID: PMC7035562]. https://doi.org/10.1155/2020/6361703.
-
26.
Ji LL, Zhang Y. Antioxidant and anti-inflammatory effects of exercise: role of redox signaling. Free Radic Res. 2014;48(1):3-11. [PubMed ID: 24083482]. https://doi.org/10.3109/10715762.2013.844341.
-
27.
Moradi H, Nikbakht H, Ebrahim K, Abed Natanzi H. [The effects of aerobic training and grape seed extract on some cardiovascular risk indexes in young men]. Iranian J Nutr Sci Food Technol. 2017;12(2):27-34. Persian.