Abstract
Introduction:
Trichorhinophalangeal syndrome (TRPS) is a sporadic autosomal dominant disorder with approximately 200 reported cases worldwide. We aimed to report a 15-year-old girl with TRPS type 1 (TRPS1) and the second reported case with a rare non-ossifying fibroma (NOF) in the distal part of her left femur.Case Presentation:
We introduce a 15-year-old girl who presented to the outpatient rheumatology clinic at 17 Shahrivar Children's Hospital, Rasht, Iran, with the chief complaint of osteoarticular pain and bone deformities. She had sparse hair, a recession of the fronto-temporal hairline, and unusually thick eyebrows at the medial and abnormal sparseness of the lateral margins. Physical examination of the limbs revealed short fingers and toes with proximal interphalangeal (PIP) ulnar deviation of the second and third fingers in both hands. Shortness of the fourth fingers, especially in the right hand, and the swelling of the PIP joints of both hands were prominent. Genetic analysis showed deletion mutation in the TRPS1 gene in chromosome 8q24 compatible with TRPS1.Conclusions:
Several symptoms and signs, including distinctive craniofacial features and ectodermal and skeletal abnormalities, are used for proper TRPS diagnosis. A correct and on-time diagnosis is essential to perform supportive care for the patient to prevent morbidities. Bone lesions, such as NOF1, can also be presented in TRPS1 patients and may be correlated with TRPS1 mutation. Further investigations are required on the association of the TRPS gene with NOF bone lesions.Keywords
1. Introduction
Trichorhinophalangeal syndrome (TRPS) is a sporadic autosomal dominant (AD) disorder with approximately 200 reported cases over the world (1). It is caused by heterozygote pathogenic defects in the TRPS1 gene, located on the long arm (q) of the eighth chromosome, which encodes a transcription factor composed of 1294 amino acids encoded in seven exons (2). It produces a protein in the cell nucleus responsible for regulating bone growth by adjusting the proliferation and differentiation of chondrocytes, hair follicles, and other tissues (3-5). This syndrome is characterized by a triad of distinctive craniofacial features, ectodermal problems, and skeletal abnormalities (3). Three subtypes of TRPS have been described in the literature, including subtypes 1, 2, and 3. All these three types are inherited as AD diseases with high penetrance and various clinical signs and symptoms depending on the pattern of mutation in the TRPS1 gene (1, 6). Craniofacial features entail a thin upper lip with a horizontal smile, the bulbous or round tip of the nose, flat philtrum, maxillary prognathism and micrognathia, as well as large and protruding ears (7, 8). Ectodermal abnormalities in these patients present as sparse and slow-growing hairs, especially in the temporal or occipital areas resembling androgenic alopecia, and the recession of the frontotemporal or occasionally occipital hairline (7, 9). Male patients may become completely bald soon after puberty. Eyebrows are commonly thick at medial and thin at lateral margins. Brittle, dystrophic, thin, or racket nails are other common presenting signs (7, 8). Loose skin is another problem that improves over time (1). Dental abnormalities may be observed, such as oligodontia, supernumerary teeth, and malocclusion (1, 3). Small breasts are also reported in some cases (4). Skeletal involvement includes short stature, brachydactyly, shortness in metacarpals or metatarses of fingers and toes, ulnar and radial deviation of fingers, clinodactyly, and growth plate abnormalities (i.e., cone shape epiphyses). Joints have a limited range of motion, especially in the hip or upper extremities. Perthes-like changes of the hip joint, hip dysplasia, pes planus, multiple exostoses (in TRPS type II), and in some cases, osteoporosis can be found (10-12). There may be joint hypermobility early in the disease, which improves over time (1). Pectus carinatum, "wing-like" scapula, scoliosis, lordosis, and subsequent recurrent respiratory infections might also occur. Body weight is normal for their height. Various neurologic, renal, cardiac, and growth abnormalities in the literature have been associated with this syndrome (4, 13, 14). Development is normal in TRPS type 1 (TRPS1), while acquiring certain motor skills may not occur due to hip joint involvement (4). Mild to moderate intellectual disability, microcephaly, retarded bone age, and multiple exostoses of long bones and around the scapula (sessile or pedunculated) are associated with TRPS type II, known as Langer-Giedion Syndrome. This syndrome results from a microdeletion in EXT1 which seems to be a regulator of longitudinal bone growth (6, 8). Microcephaly and redundant skin may be present in TRPS II. These findings are not found in types I and III (7). Type III, also known as Sugio-Kajii Syndrome, is similar to type I with more prominent symptoms, more marked growth delay, and more severe shortness of fingers and toes. Therefore, it is considered a severe form of TRPS (2, 8). In this study, we report a 15-year-old girl with TRPS1 and the second reported case with a rare non-ossifying fibroma (NOF) in the distal part of her left femur.
2. Case Presentation
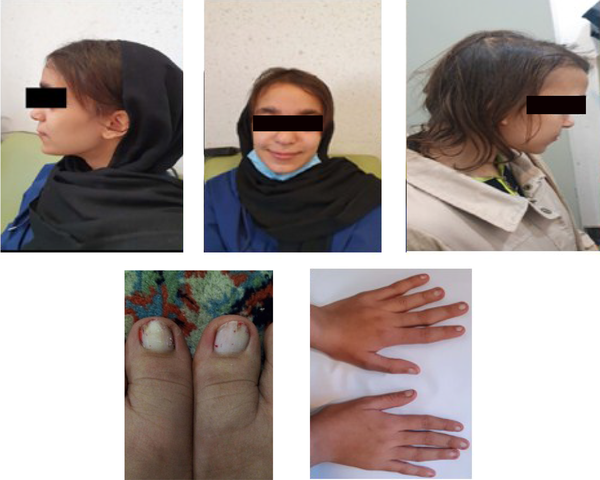
A 15-year-old girl was admitted to the outpatient rheumatology clinic at 17 Shahrivar Children's Hospital, Rasht, Iran, in January 2021, with the chief complaint of osteoarticular pain (especially in the lower part of the left femur) and deformities of the fingers and toes. The pain started about 2 - 3 years ago without any specific diagnosis. She was the first child of a non-consanguineous marriage. Her younger sister and parents were healthy. Her weight and height were 48 kg (percentile 35) and 161 cm (percentile 50), respectively. She had characteristic facies with a broad forehead, bulbous nose, thin upper lip, and an abnormally long philtrum. She had very thin and sparse hair, a recession of the fronto-temporal hairline, and unusually thick eyebrows at the medial and abnormal sparseness at the lateral margins. In the oral cavity, malocclusion without supernumerary teeth was observed. The nails were thin and hypoplastic (onychodystrophy). Physical examination of the limbs revealed short fingers and toes with proximal interphalangeal (PIP) ulnar deviation of the second and third fingers in both hands. Shortness of the fourth fingers, especially in the right hand, and swelling of the PIP joints of both hands were prominent (Figure 1). Breast, puberty stage, chest, and abdominal examinations were all normal.
Thin upper lip, abnormal long philtrum, the bulbous tip of the nose, thin and sparse hair, and thin and hypoplastic nails on hand and foot
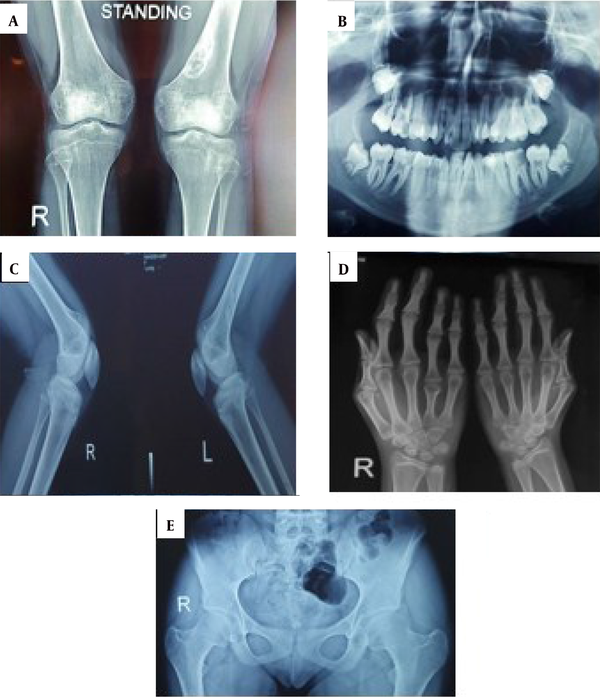
She had no intellectual disability. All laboratory tests, including complete blood antibody, calcium, inorganic phosphate, alkaline phosphatase, free T4, thyroid-stimulating hormone, parathormone, serum electrolytes, and urinalysis, were within normal limits. There was no specific diagnostic clue in multiple consultations. The radiological features were mostly consistent with skeletal dysplasia (Figure 2). Analgesics and calcium and vitamin D supplements were prescribed for the patient to relieve bone pain. Orthopedic counseling was requested for her bone lesion, and surgery was scheduled to remove her NOF lesion due to causing pain and discomfort even at rest and probable pathologic fracture. Avoiding trauma around the bone lesion was emphasized until planning for surgery. The patient was referred to the dentist for treating malocclusion. The endocrinologist did not request a growth hormone (GH) test due to the normal height percentile. Regular and annual height control examinations by endocrinologists were recommended.
2.1. Genetic Analysis
Twenty GTG banded chromosomal metaphases were investigated in phytohaemagglutinin (PHA)-stimulated blood culture and at a resolution of 400 band level revealing 46 chromosomes with no aberration. A chromosomal study showed normal female chromosomal complement (46xx). Data analysis in whole exome sequencing (WES) demonstrated one novel heterozygous frame shift deletion mutation in the TRPS1 gene in chromosome 8q24. This mutation leads to TRPS1 (TRPS1: NM-001282902exon 3c1152-1153delpN 384fs).
Radiologic bone changes. A, The lytic lesion with a well-defined sclerotic rim was observed in the distal metaphysis of the left femur, mostly suggestive of a non-ossifying fibroma; B, Oral cavity malocclusion without supernumerary teeth; C, There was no evidence of exostoses; D, Shortening of fourth and fifth metacarpal bones in both hands (more prominent on the right side) and the middle phalangeal bones of fingers combined with cone-shaped epiphyses; E, There was no hip involvement.
3. Discussion
TRPS is a rare genetic disorder characterized by distinctive craniofacial features and ectodermal and skeletal abnormalities (3). This syndrome may remain undiagnosed due to various expressions until more affected patients seek medical consults (6). In this study, we presented a 15-year-old girl with the diagnosis of TRPS1 and the presence of NOF in the distal part of her left femur. In the present case, despite recurrent referrals, no effective and serious evaluation was performed until severe typical presentations appeared. As a result, she was diagnosed with a delay of 15 years, much longer than many other reports. However, Jeyhun reported a case at the age of 39 (5). The diagnosis may be easily missed in individuals with mild symptoms. Therefore, the true frequency of this syndrome in the general population may be higher than reports in the literature. Consequently, dermatologists should consider TRPS1 a connective tissue disorder in patients who complain of thin and sparse hair or onychodystrophy, especially if they are associated with characteristic facies and subtle structural involvement (9). Management is principally supportive. Treatment requires a team consisting of a pediatrician, dentist, pathologist, orthopedic surgeon, dermatologist, and psychiatrist. Analgesics for joint pain (non-steroidal anti-inflammatory drugs or other non-opiates), hair care or wearing wig, extraction of supernumerary teeth if present, orthopedic surgery, physiotherapy, exercise, occupational therapy, and bisphosphonates must be considered as supportive treatments for these patients (4, 11). Almost all reported cases with TRPS1 exhibit growth retardation and some degrees of short stature. Consequently, GH therapy might be considered if there are subnormal results after GH stimulation tests (14). However, GH therapy was associated with inconsistent results in the literature (4, 10, 15, 16). In the present case, the height was normal, which might reflect her parents' tall stature (16, 17). Dental anomalies, scoliosis, lordosis, and kyphosis might be less frequently observed. This patient did not have any of the aforementioned anomalies except malocclusion. Other skeletal abnormalities, especially hip involvement, may frequently affect the outcome (18). The present case had neither abnormal skeletal changes nor radiologic lesions in the femoral head.
In this patient, NOF was reported in the distal part of femur radiography. NOF is a non-neoplastic, benign lesion characterized by the proliferation of fibroblasts admixed with the osteoclastic type of giant cells. The etiology of NOF is unknown, and it can be diagnosed by bone radiography (2, 19). NOF seems to be due to disturbance or dystrophic calcification rather than a neoplasm (2). The TRPS gene involves bone formation/mineralization and chondrocyte proliferation/differentiation. It seems that more studies are required about the association of the TRPS gene with NOF bone lesions. In children and adolescents, NOF is most frequently observed in the distal metaphysis of the femur and the proximal and distal regions of the tibia. It is found relatively infrequently in the fibula in approximately 9.09% of cases (20). Although NOF is usually a self-limiting lesion and may often disappear at the age of 20 - 25, it is a risk factor for pathologic bone fracture if it becomes too large. NOF is a benign lesion and does not predispose patients to develop cancer. Moreover, some investigations reported osteosarcoma in patients with TRPS (21). According to the previous reports, the present study is the second one to report a rare occurrence of NOF in a case with TRPS1 (2). Su et al. reported a 12-year-old girl by TRPS1 with fibula NOF and pathologic fracture caused by minor trauma (2).
Several reports described an association between TRPS1 and different endocrine disturbances, such as idiopathic hypoglycemia, diabetes mellitus, and hypothyroidism (14). Three subtypes of TRPS have been described in the literature, including I, II, and III (10, 22). This patient was most compatible with type I TRPS because symptoms were not so severe to be considered type III. Moreover, she had no intellectual disability, and there were not any signs of multiple exostoses, which is commonly observed in TRPSII. This syndrome is caused by disrupting at least one allele from the TRPS gene and sometimes in the form of a new mutation (1). In the present case, other family members (parents and the younger sister) were completely healthy. It seemed that she had a new mutation compatible with WES results. Considering that TRPS is a rare genetic disease, physicians avoid diagnostic mistakes and so many subsequent useless treatment approaches. Although TRPS is a rare genetic disorder with no conventional treatment, genetic evaluation of suspicious cases might lead to avoiding misdiagnosis and so many useless therapeutic approaches. Genetic counseling may be necessary for detecting affected family members.
3.1. Limitations
The lack of the cooperation of parents in performing genetic testing for themselves and the other child due to high cost was the most important limitation of the current study.
3.2. Recommendations
Several symptoms and signs, including distinctive craniofacial features and ectodermal and skeletal abnormalities, are used for proper TRPS diagnosis. The best way to decrease the misdiagnosis of TRPS is to notice the presence of the above three signs together. These signs and symptoms may help physicians gain insight into correct management. In addition, a physician should know that the lack of attention to these symptoms and signs not only increases the treatment cost and unprovoked referrals but also leads to delayed diagnosis and management (4, 15, 18). Further investigations are required on the association of the TRPS gene with NOF bone lesions.
3.3. Conclusions
Detailed history and clinical examination, along with the knowledge of the various signs and symptoms of the TRPS (triad of distinctive craniofacial features and ectodermal and skeletal abnormalities), are essential for diagnosis. However, a genetic test is necessary to confirm the diagnosis in suspicious cases. A correct and on-time diagnosis is essential to perform supportive care for the patient to prevent morbidities. Bone lesions, such as NOF1, can also be present in TRPS1 patients and may be correlated with TRPS1 mutation. The TRPS gene involves bone formation/mineralization and chondrocyte proliferation/differentiation. It seems that further investigations are required on the association of the TRPS gene with NOF bone lesions.
Acknowledgements
References
-
1.
Trippella G, Lionetti P, Naldini S, Peluso F, Monica MD, Stagi S. An early diagnosis of trichorhinophalangeal syndrome type 1: a case report and a review of literature. Ital J Pediatr. 2018;44(1):1-7. [PubMed ID: 30458885]. [PubMed Central ID: PMC6245908]. https://doi.org/10.1186/s13052-018-0580-z.
-
2.
Su W, Shi X, Lin M, Huang C, Wang L, Song H, et al. Non-ossifying fibroma with a pathologic fracture in a 12-year-old girl with tricho-rhino-phalangeal syndrome: a case report. BMC Med Genet. 2018;19(1):1-7. [PubMed ID: 30541476]. [PubMed Central ID: PMC6292130]. https://doi.org/10.1186/s12881-018-0732-4.
-
3.
Kunotai W, Ananpornruedee P, Lubinsky M, Pruksametanan A, Kantaputra PN. Making extra teeth: Lessons from a TRPS1 mutation. Am J Med Genet A. 2017;173(1):99-107. [PubMed ID: 27706911]. https://doi.org/10.1002/ajmg.a.37967.
-
4.
Curatola SL, Briuglia S, Capra AP, Novelli A, Aversa T, Wasniewska M. Tricho-rhino-phalangeal syndrome: a rare case of disharmonious short stature. Atti della Accademia Peloritana dei Pericolanti-Classe di Scienze Medico-Biologiche. 2021;109(2):1-5.
-
5.
Jeon J, Kim JH, Oh CH. Trichorhinophalangeal syndrome type I--clinical, microscopic, and molecular features. Indian J Dermatol Venereol Leprol. 2014;80(1):54-7. [PubMed ID: 24448126]. https://doi.org/10.4103/0378-6323.125515.
-
6.
Xu S, Lian Q, Wu J, Li L, Song J. Dual molecular diagnosis of tricho-rhino-phalangeal syndrome type I and Okur-Chung neurodevelopmental syndrome in one Chinese patient: a case report. BMC Med Genet. 2020;21(1):1-6. [PubMed ID: 32746809]. [PubMed Central ID: PMC7398275]. https://doi.org/10.1186/s12881-020-01096-w.
-
7.
Choi MS, Park MJ, Park M, Nam CH, Hong SP, Kim MH, et al. Treatment of Hair Loss in the Trichorhinophalangeal Syndrome. Ann Dermatol. 2018;30(3):382-3. [PubMed ID: 29853764]. [PubMed Central ID: PMC5929967]. https://doi.org/10.5021/ad.2018.30.3.382.
-
8.
Garcia-Garcia SC, Herz-Ruelas ME, Gomez-Flores M, Vazquez-Herrera NE, Misciali C, Tosti A, et al. Association of Trichorhinophalangeal Syndrome and Loose Anagen Syndrome: A Case Report. Skin Appendage Disord. 2020;6(3):162-7. [PubMed ID: 32656236]. [PubMed Central ID: PMC7325219]. https://doi.org/10.1159/000506524.
-
9.
Ahmed A, Almohanna H, Griggs J, Tosti A. Genetic Hair Disorders: A Review. Dermatol Ther (Heidelb). 2019;9(3):421-48. [PubMed ID: 31332722]. [PubMed Central ID: PMC6704196]. https://doi.org/10.1007/s13555-019-0313-2.
-
10.
Fang X, Yang Q. A Missense Mutation in TRPS1 in a Family with Trichorhinophalangeal Syndrome Type III Accompanied by Ankylosing Spondylitis. Ann Dermatol. 2022;34(2):139-43. [PubMed ID: 35450306]. [PubMed Central ID: PMC8989904]. https://doi.org/10.5021/ad.2022.34.2.139.
-
11.
Simonetti O, Radi G, Molinelli E, Diotallevi F, Offidani A. Trichorhinophalangeal syndrome: a case report and brief literature review. Acta Dermatovenerologica Alpina Pannonica et Adriatica. 2022;31(1):43-6. https://doi.org/10.15570/actaapa.2022.6.
-
12.
Shao C, Tian J, Shi DH, Yu CX, Xu C, Wang LC, et al. A novel mutation in TPRS1 gene caused tricho-rhino-phalangeal syndrome in a Chinese patient with severe osteoporosis. Chin Med J (Engl). 2011;124(10):1583-5. [PubMed ID: 21740822].
-
13.
Sen G, Barendt E, Sinha M. Cardiac arrest in a patient with trichorhinophalangeal syndrome and dilated cardiomyopathy. BMJ Case Rep. 2021;14(2). [PubMed ID: 33542011]. [PubMed Central ID: PMC7868179]. https://doi.org/10.1136/bcr-2020-237604.
-
14.
Nicolescu CR, Kasongo L, Rausin L. Dysmorphic Short Stature: Radiological Diagnosis of Trichorhinophalangeal Syndrome. Case Rep Pediatr. 2018;2018:5189062. [PubMed ID: 30584486]. [PubMed Central ID: PMC6280227]. https://doi.org/10.1155/2018/5189062.
-
15.
Forys-Dworniczak E, Zajdel-Cwynar O, Kalina-Faska B, Malecka-Tendera E, Matusik P. Trichorhinophalangeal syndrome as a diagnostic and therapeutic challenge for paediatric endocrinologists. Pediatr Endocrinol Diabetes Metab. 2019;25(1):41-7. [PubMed ID: 31343132]. https://doi.org/10.5114/pedm.2019.84708.
-
16.
Levy-Shraga Y, Modan-Moses D, Wientroub S, Ovadia D, Zeitlin L. The effect of growth hormone treatment in a child with tricho-rhino-phalangeal syndrome: A case report and review of the literature. Eur J Med Genet. 2020;63(4):103830. [PubMed ID: 31884116]. https://doi.org/10.1016/j.ejmg.2019.103830.
-
17.
Koomanaee S, Tabrizi M, Naderi N, Hassanzadeh Rad A, Boloky Moghaddam K, Dalili S. Parental Anthropometric Indices and Obesity in Children. Acta Med Iran. 2016;54(4):270-5. [PubMed ID: 27309269].
-
18.
de Barros GM, Kakehasi AM. Skeletal abnormalities of tricho-rhino-phalangeal syndrome type I. Rev Bras Reumatol Engl Ed. 2016;56(1):86-9. [PubMed ID: 27267340]. https://doi.org/10.1016/j.rbre.2014.08.017.
-
19.
Bovee JV, Hogendoorn PC. Non-ossifying fibroma: A RAS-MAPK driven benign bone neoplasm. J Pathol. 2019;248(2):127-30. [PubMed ID: 30809793]. [PubMed Central ID: PMC6593856]. https://doi.org/10.1002/path.5259.
-
20.
Sakamoto A, Arai R, Okamoto T, Matsuda S. Non-ossifying fibromas: Case series, including in uncommon upper extremity sites. World J Orthop. 2017;8(7):561-6. [PubMed ID: 28808627]. [PubMed Central ID: PMC5534405]. https://doi.org/10.5312/wjo.v8.i7.561.
-
21.
Evans S, Brewer P, Vaiyapuri S, Grimer R. High grade osteosarcoma on a background of trichorhinophalangeal syndrome: A family perspective. J Bone Oncol. 2013;2(2):92-3. [PubMed ID: 26909277]. [PubMed Central ID: PMC4723365]. https://doi.org/10.1016/j.jbo.2013.04.002.
-
22.
Li S, Chen Z, Yang Y. Novel mutation of TRPS1 in a patient with tricho-rhino-phalangeal syndrome. Clin Exp Dermatol. 2021;46(3):557-9. [PubMed ID: 32844440]. https://doi.org/10.1111/ced.14430.