Abstract
Background:
Exercise order affects repetition performance and acute hormonal responses to resistance training (RT) programs.Objectives:
The purpose of this study was to compare the acute effects of two different resistance exercise orders (REO) on number of repetitions and serum Insulin-like Growth Factor-1 (IGF-1), testosterone and cortisol levels in normal-weight and obese men.Materials and Methods:
25 untrained college-aged men were assigned to either obese (n = 11) or normal-weight (n = 15) groups. Subjects performed two REO protocols in 2 exercise groups. In the first group subjects began with large-muscle group and progressed to small-muscle group (Protocol A), while in the other group subjects performed the same exercise but in reverse sequence (Protocol B). Each activity was performed in 3 consecutive sets of 10 repetitions maximum to near fatigue.Results:
REOs did not affect number of repetitions in none of the groups. The average rating of perceived exertion was higher for protocol B in both groups. IGF-1 and testosterone increased immediately post exercise for both protocols and in both groups, however immediately post exercise increase in IGF-1 and testosterone were lower in obese group. Cortisol response to REO was weaker in obese group.Conclusions:
Performing large muscle group exercises first in RE training and progressing to small muscle group produced greater anabolic hormonal response relative to reverse sequence in normal-weight young adult men. Anabolic hormonal response to REOs was blunted in the obese group.Keywords
Acute Exercise Hormone Responses in Exercise Strength Training Obese
1. Background
Resistance exercise (RE) through signalling pathways, hormone response and neural adaptations has many beneficial health outcomes. Resistance training (RT) programs have many variables including intensity, volume, load, rest period between sets and exercises that can be manipulated to reach specific training goals (1, 2). One of the most influential components that has not been studied much yet is the sequence of exercise performed during a training session, called resistance exercise order (REO), which affects both acute and chronic adaptations to RT programs (2, 3).
Previous studies indicate that REO affects total repetitions and thus the volume is greater when an exercise is placed at the beginning of an RT session (4). In untrained subjects, greater strength increases were reported for the first exercise of a given RE sequence (5-7). Results of the previous studies emphasize on placing important training exercises at the beginning of a training session sequence to meet individual needs (8-10). Strength and power during multiple joint exercises may be reduced when performed after several other exercises in an RE session (5). American College of Sports Medicine recommends performing large muscle group exercises first during a resistance-exercise session (11).
There is some evidence that glucoregulation homeostasis is altered with increasing levels of obesity and those alterations may mediate the cortisol response to exercise (10). It has been reported that obesity is accompanied by some endocrine disturbances during acute endurance and resistance exercises including; a blunted blood growth hormone release, and greater cortisol concentrations (8-12). It has been shown that obese subjects have lower bioactive GH and higher growth hormone binding protein concentrations compared to lean subjects (10).
Acute RE evokes metabolic, hormonal, neural and cardiovascular responses, the magnitude of which depends on the manipulated program variables (13). The effects of acute REO on metabolic hormones and whether obesity affects hormonal response to resistance exercise orders has not been widely studied.
2. Objectives
The present study was designed to assess the influence of REO on the number of repetitions, ratings of perceived exertion, metabolic hormone responses, and to see whether the hormonal response of obese young adult men to REO are different from age-matched normal-weight controls.
3. Materials and Methods
26 male volunteer students who did not have any regular physical activity during the past six months and had no history of taking any medicine, supplement or medical problems participated in this study. They were categorized into normal-weight (n = 15, mean age 21.73 ± 1.58 years, mean height 177 ± 5.89 cm, mean weight 68.21 ± 8.31 kg, mean body fat 16.1 ± 1.7%, and mean Body Mass Index [BMI] = 21.83 ± 2.88 kg/m2) and obese (n = 11, mean age 21.91 ± 1.58 years, mean height 173.82 ± 6.88 cm, mean weight 92.68 ± 10.73 kg, mean body fat 28.8 ± 3.6 %, and mean BMI = 30.39 ± 1.76 kg/m2) groups. The nature of the study and potential risks associated with it were explained to all subjects. Participants signed informed consent forms before enrolling in the research study. The study protocol was approved by the university research ethics committee.
As shown in Figure 1, one week before starting the first session of protocols, participants were familiarized with the equipment and exercise techniques. Ten repetition maximum (10 RM) of subjects was determined. The sequence of 10 RM for nine resistance exercises was chest press, leg press, lat pull-down machine, leg extension, overhead press, hamstring curl, biceps curl, calf raise, and triceps extension. To determine the reliability of the 10 RM testing, 40 percent of subjects in each group were selected randomly and 10 RM was measured again the following day. Listed in large to small muscles, the quantities of r for nine moves were 0.87, 0.82, 0.92, 0.92, 0.86, 0.91, 0.95, 0.89 and 0.90, respectively. BMI was calculated by dividing the weight (kg) of a person by his height (m2).
Research Plan
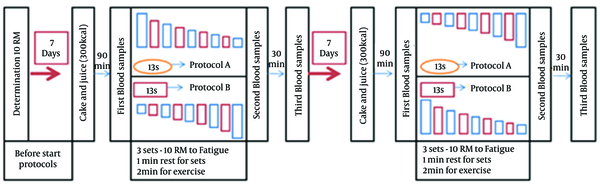
This double blind cross sectional study was conducted in two sessions, one week apart. A combination of upper and lower body muscle groups were used in two different protocols. In one protocol subjects began with large muscle group and progressed toward small muscle group exercises (A protocol). In another protocol subjects started with small muscle group and advanced to large muscle group exercises. In A protocol the exercise order was: (1) chest press, (2) leg press, (3) lat pull-down machine, (4) leg extension, (5) overhead press, (6) hamstring machine, (7) biceps curl, (8) calf raise, (9) triceps extension. In B protocol, they performed the same exercises in reverse order. In the second session, all steps were repeated exactly the same as the first one, but this time the subjects who did A protocol in the first session did B protocol and vice versa. Movements were performed in 3 sets of 10 RM to near fatigue. The subjects were verbally encouraged to produce their maximum effort. Rest intervals between repetitions of each set and between movements of resistance exercise were 1 and 2 minutes, respectively. We chose short rest intervals between sets to elicit a greater hormonal response as reported before (14).
There was no speed or time limit for doing exercise movements. At the end of the third set of each exercise, perceived exertion was determined based on the Borg Scale (15). The average run-time of each session was one hour. There was no significant difference between duration and volume of training protocols calculated based on volume load formula (16). Each repetition began with an eccentric phase followed immediately by a concentric phase with no pause between phases. Subjects were not allowed to eat anything or drink any beverages other than water during exercise sessions. They were not allowed to drink water during the last half hour of exercise session. Before each training session, subjects performed a 10 - 15 minute general and specific warm-up. General warm-up included stretching, and other general body movements. In specific warm up, subjects were asked to do, based on the protocol assigned to, the first activity of each order in 2 sets with 50% of 1 RM and 10 - 15 repetitions. Two days before starting the first session, participants received a form to record their daily diet and physical activity. All subjects were asked to adhere to the diet and physical activity they had before the start of the first session, and at least for two days before the starting of the second session.
Blood samples (6 mL) were drawn before, immediately after as well as 30 minutes post exercise from the antecubital vein under normal room temperature and were centrifuged for 15 minutes at 1500g and stored at -18° C until analyzed. To reduce the impact of hunger on blood variables and to prevent hypotension, half an hour before blood draws, a low calorie breakfast (juice, low-sugar cake about 300 kcal) was given to all subjects. The first blood samples were obtained at nine o’clock in the morning before starting of the exercise protocols. Second and third blood samples were taken at 0 and 30 minutes after the resistance exercise sessions. Subjects did not drink or eat in the interval between the second and third blood draw. Ambient temperature was between 20 - 25°C during both practice sessions. The second session was conducted at the same time one week later. Measurement of serum cortisol and testosterone levels was carried out using ELISA kit provided by Diametra Company, Italy (17). Sensitivities of the assays for cortisol and testosterone were 1.5 ng/mL and 0.07 ng/mL, respectively. The inter and the intra‐assay variation for cortisol and testosterone were ≤ 15%, ≤ % 8, and 10.5%, 5.8%, respectively. IGF-1 was measured by an Immunoenzymatic monoclonal assay (18). Sensitivity of the assay was 3.1 µg/l. The inter and the intra‐assay CV values for IGF-1 were 6.5% and 7.2%, respectively. Normality of the data was tested with the Kolmogorov-Smirnov test. Two-way ANOVA test with repeated measurements were used to evaluate changes within and between groups. Bonferroni post hoc analysis and independent t-test were used for within- session and across session significant changes, respectively. Data were analyzed with SPSS software (version 19) and P value of significance was set at 0.05.
4. Results
4.1. Number of Repetitions and Rate of Perceived Exertion (RPE)
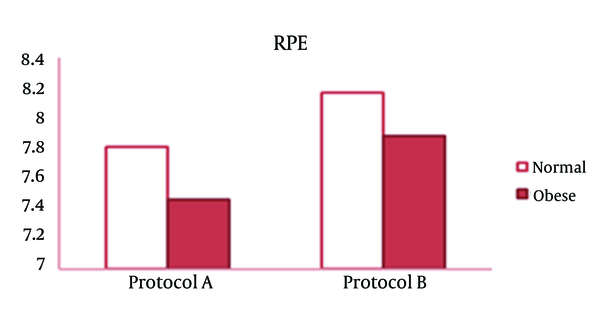
As it can be noted from Table 1, RE protocols did not affect the number of repetitions in none of the groups. Performing exercises at the end of an exercise sequence did not result in fewer repetitions in the 3 sets of an exercise, when an exercise was performed last in an exercise sequence. There were no differences between two protocols for the last exercise. For example, we observed a significant decrease in triceps extension exercise, the last exercise in A Protocol, from the 1st set to the 2nd set. The same thing happened from the 2nd set to the 3rd set in B protocol, in which triceps extension was the first exercise. In none of the exercises or sets there were significant differences between two protocols. Obesity did not affect the number of performed repetitions except for leg press in A protocol, in which obese subjects had higher average repetitions than normal-weight subjects. In obese subjects like normal subjects, the type of protocols did not affect the number of repetitions. In both groups the average exercise RPE was higher following B protocol but within and between group differences were not significant (Figure 2).
Number of Repetitions Performed in 3 Consecutive Sets for Two Different Resistant Exercise Orders in Normal-Weight and Obese Young Adult mena,b
| Exercise c | Set 1 | Set 2 | Set 3 |
|---|---|---|---|
| Chest Press | |||
| NA | 11.20 ± 3.70 | 9.20 ± 2.98 d | 8.13 ± 1.84 |
| OA | 11.55 ± 4.10 | 9.09 ± 2.11 | 8.91 ± 3.75 |
| NB | 10.87 ± 3.92 | 9.93 ± 3.61 | 9.20 ± 4.1 |
| OB | 10.87 ± 3.28 | 8.91 ± 5.08 | 7.00 ± 3.54 |
| Leg Press | |||
| NA | 13.73 ± 7.78 | 12.60 ± 3.18 | 10.67 ± 3.09 e |
| OA | 20.27 ± 5.14 | 20.73 ± 6.31 | 20.73 ± 6.75 |
| NB | 15.33 ± 5.55 | 14.20 ± 4.97 | 13.87 ± 5.40 |
| OB | 16.91 ± 7.74 | 17.55 ± 4.61 | 16.82 ± 7.76 |
| Lat | |||
| NA | 14.87 ± 5.83 | 11.73 ± 4.01 d | 10.47 ± 3.27 |
| OA | 14.18 ± 5.95 | 12.82 ± 3.71 | 11.18 ± 5.19 e |
| NB | 14.53 ± 4.64 | 13.07 ± 4.22 d | 11.07 ± 3.99 e |
| OB | 12.73 ± 6.18 | 12.55 ± 5.26 | 10.82 ± 4.33 |
| Leg Extension | |||
| NA | 12.53±3.78 | 12.07±4.96 | 10.87±2.17 |
| OA | 17.18 ± 6.42 | 15.64 ± 7.58 | 14.00 ± 5.08 |
| NB | 14.47 ± 3.04 | 14.80 ± 4.60 | 14.00 ± 5.57 |
| OB | 15.64 ± 5.97 | 13.82 ± 4.85 d | 14.00 ± 6.62 |
| Overhead Press | |||
| NA | 12.27 ± 5.69 | 9.53 ± 3.48 d | 7.47 ± 2.88 e |
| OA | 14.91 ± 7.73 | 12.91 ± 4.30 d | 9.55 ± 4.48 e |
| NB | 12.80 ± 5.05 | 11.00 ± 3.72 d | 9.60 ± 3.27 e |
| OB | 13.00 ± 3.71 | 9.55 ± 2.73 d | 7.82 ± 3.63 e |
| Hamstring Curl | |||
| NA | 14.07 ± 5.44 | 12.20 ± 3.61 d | 10.67 ± 3.81 |
| OA | 12.64 ± 4.15 | 13.00 ± 4.34 | 11.27 ± 5.04 e |
| NB | 15.40 ± 4.87 | 12.73 ± 3.10 d | 11.87 ± 3.31 |
| OB | 11.91 ± 3.62 | 11.00 ± 2.32 | 10.91 ± 4.09 |
| Biceps Curl | |||
| NA | 11.80 ± 5.20 | 9.53 ± 2.53 d | 8.13 ± 2.03 e |
| OA | 11.64 ± 4.76 | 8.91 ± 3.83 d | 7.36 ± 4.13 e |
| NB | 13.80 ± 5.45 | 13.40 ± 5.21 | 10.67 ± 4.62 e |
| OB | 12.27 ± 6.66 | 8.55 ± 2.42 d | 8.09 ± 2.17 |
| Calf Raise | |||
| NA | 30.33 ± 10.90 | 28.87 ± 9.09 | 28.00 ± 11.08 |
| OA | 35.73 ± 10.21 | 31.55 ± 7.15 | 27.82 ± 7.24 e |
| NB | 33.87 ± 11.38 | 33.13 ± 12.96 | 32.40 ± 12.31 |
| OB | 33.18 ± 11.09 | 30.00 ± 11.01 | 27.73 ± 10.79 |
| Triceps Extension | |||
| NA | 14.87 ± 5.14 | 12.13 ± 3.54 d | 11.93 ± 3.53 |
| OA | 15.55 ± 5.61 | 13.18 ± 4.53 | 10.64 ± 3.91 e |
| NB | 17.53 ± 6.63 | 15.13 ± 5.01 | 12.60 ± 4.22 e |
| OB | 16.64 ± 8.55 | 13.82 ± 4.17 | 11.73 ± 2.57 |
Effects of Two Different Resistant Exercise Orders on Rating of Perceived Exertion
4.2. Hormonal Responses to Various RE Order Protocols.
4.2.1. IGF-1
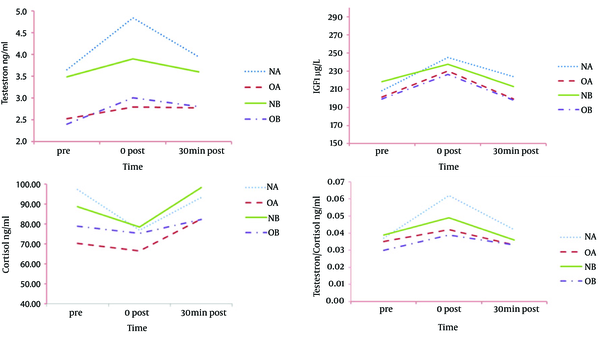
Serum levels of IGF-1 increased immediately after exercise in both groups, but returned to baseline levels after 30 minutes of recovery (Figure 3 A). Post exercise changes in serum IGF-1 levels were significant between protocols. In normal-weight group, the amount of increase in serum IGF-1, after the A protocol was almost double the amount of increase after the B protocol (~ 18% vs. ~9%). In obese group and for both protocols, baseline, 0 and 30 minutes post-exercise values of IGF-1 were lower than normal-weight group. The interaction of time-group was not significant for none of the protocols (P = 0.13, P = 0.08).
4.2.2. Testosterone
As shown in Figure 3 B, there was a significant increase in serum testosterone concentration immediately after the RE in comparison to baseline levels. In normal-weight subjects, immediately after performing the A protocol, testosterone levels increased to about 33%, while the amount of increase after performing the B protocol was about 12%. However, the response of testosterone to RE in obese group was reduced in comparison to normal-weight group. Between groups differences were not significant. Baseline testosterone levels in obese men were significantly lower than normal group (P = 0.02, vs. P = 0.03, respectively). The amount of increase in testosterone immediately after applying the A protocol was significantly higher in normal group compared with obese group (P = 0.02). The interaction of time-group was not significant for none of the protocols (P = 0.09 vs. P = 0.53). Obese subjects not only had lower baseline testosterone level but showed attenuated response to acute RE.
4.2.3. Cortisol
In normal subjects, serum cortisol levels decreased immediately after implementing both protocols. Cortisol level reduced about 22% after performing A protocol compared with 12% reduction in B protocol. Cortisol levels returned to baseline level (P < 0.05) after 30 minutes recovery (Figure 3 C) Cortisol levels did not change significantly in response to RE protocols in obese group (P > 0.05). Significant differences were not found between normal and obese groups (P > 0.05), neither immediately after exercise nor after 30 minutes recovery. Time-group interaction was not significant (P = 0.36 and P = 0.17, respectively).
Changes in the Serum Levels
5. Discussion
Results of the current study suggest that two different RE orders, moving from larger muscle groups to smaller ones and vice versa, do not affect the number of repetitions performed neither in normal-weight nor in age-matched obese men. Muscle fatigue and short rest periods between sets are possible explanations for the significant decrease in the number of repetitions performed in the third set in this study. Even though RPE was increased to a greater extent in the small to large REO, the difference between protocols was not significant. The effect of REO on RPE in this study is in agreement with what was reported before (2, 8). Previous studies have shown that rest interval length as a RE variable affects repetitions number and in training with greater loads, 3 - 5 minutes rest between sets allowes for greater repetitions over multiple sets (19). Performing large muscle group exercises first in RE training and progressing to small muscle group produced greater anabolic hormonal response relative to the reverse sequence in normal-weight young adult men. Obesity blunted anabolic hormonal response to REOs. We chose a short rest interval between sets as the results of previous studies indicated that short rest intervals produce greater anabolic hormonal response to RE, while longer rest intervals increase the total volume (1, 19).
5.1. Hormonal Response to REO
5.1.1. IGF-1
IGF-1 as a potent activator of the Akt/mTOR signalling pathway has profound effect in skeletal muscle adaptation to RE (20). We found significant increases in serum levels of IGF-1 immediately after REO protocols in both normal-weight and age-matched obese groups. In normal group, post exercise increase in IGF-1 after performing A protocol was almost doubled compared to the amount of increase in B protocol. In obese group, both baseline and post exercise levels of IGF-1 were lower than age matched normal weight group which is in agreement with what reported before (21, 22). Even though obesity has negative effect on circulating GH-IGF-1 axis, employing specific exercise programme variables can ameliorate attenuated GH response in obese people (23). Profound increase in circulating IGF-1 after A protocol suggests that exercising large muscles first in RE training programs can have more beneficial effects on skeletal muscle growth and adaptation to RE.
5.1.2. Testosterone
Testosterone has anabolic effects on muscle tissue and plays an important role in adaptations to RE in men. The response of testosterone to RE is dictated in large by RE variables (24). Previous study indicate that heavy RE is effective in increasing serum testosterone level in young men (25). It was reported that the protocol that included both the elbow flexor and resistance exercises of leg muscles significantly increased serum level of testosterone, GH and IGF-1 in young men (26). Even though serum testosterone increased immediately after exercise in both normal and obese groups in the present study, it was much more profound in case of A protocol in normal-weight group. RE is a strong stimulus for increasing plasma concentration of catecholamines, and obesity attenuates this response (21). We did not measure catecholamine levels to infer any conclusion in this regard. More profound increase in circulating testosterone in case of A protocol is in agreement with a previous report claiming involvement of multi-joint and big muscles incites better anabolic hormone responses (27).
5.1.3. Cortisol
Cortisol has catabolic effects on muscle tissue and can oppose the beneficial effects of anabolic hormones on protein synthesis and muscle mass. It was reported that cortisol response to acute endurance and resistance exercise was disturbed by obesity and obese people had greater cortisol release in response to RE training (8). We found severe reduction in serum level of cortisol in normal-weight subjects following implementing A Protocol, indicating that RE protocol starting with large muscle groups and progressing to small muscle groups can reduce cortisol more effectively than reverse protocol in RE programs. Baseline level of cortisol in obese subjects was lower than normal- weight counterparts but cortisol response was not affected by REO.
This study confirms previous findings that REO do not affect the number of repetitions performed and rating of perceiving exertion but, as a variable, using large muscles and multi-joints moves at the beginning of a RE protocol in normal-weight adult men produces better anabolic hormonal response and less catabolic cortisol release. These results indicate that in RE training, starting with large muscle group and progressing to small group brings about more beneficial muscular gains. Our data also reconfirmed previous findings that obesity blunts anabolic hormonal response to RE in young men. Given the lack of similar studies, further research on a bigger sample size is needed to generalize this result that in an RE training session more beneficial gain can be obtained by exercising large muscles first.
Acknowledgements
References
-
1.
Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674-88. [PubMed ID: 15064596].
-
2.
Simao R, de Salles BF, Figueiredo T, Dias I, Willardson JM. Exercise order in resistance training. Sports Med. 2012;42(3):251-65. [PubMed ID: 22292516]. https://doi.org/10.2165/11597240-000000000-00000.
-
3.
Farinatti PT, Simao R, Monteiro WD, Fleck SJ. Influence of exercise order on oxygen uptake during strength training in young women. J Strength Cond Res. 2009;23(3):1037-44. [PubMed ID: 19387369]. https://doi.org/10.1519/JSC.0b013e3181a2b3e4.
-
4.
Bellezza PA, Hall EE, Miller PC, Bixby WR. The influence of exercise order on blood lactate, perceptual, and affective responses. J Strength Cond Res. 2009;23(1):203-8. [PubMed ID: 19130645].
-
5.
Dias I, de Salles BF, Novaes J, Costa PB, Simao R. Influence of exercise order on maximum strength in untrained young men. J Sci Med Sport. 2010;13(1):65-9. [PubMed ID: 19243993]. https://doi.org/10.1016/j.jsams.2008.09.003.
-
6.
Gentil P, Oliveira E, de Araujo Rocha Junior V, do Carmo J, Bottaro M. Effects of exercise order on upper-body muscle activation and exercise performance. J Strength Cond Res. 2007;21(4):1082-6. [PubMed ID: 18076251]. https://doi.org/10.1519/R-21216.1.
-
7.
Simao R, Farinatti Pde T, Polito MD, Viveiros L, Fleck SJ. Influence of exercise order on the number of repetitions performed and perceived exertion during resistance exercise in women. J Strength Cond Res. 2007;21(1):23-8. [PubMed ID: 17313265]. https://doi.org/10.1519/R-18765.1.
-
8.
Hansen D, Meeusen R, Mullens A, Dendale P. Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity. Sports Med. 2012;42(5):415-31. [PubMed ID: 22455310]. https://doi.org/10.2165/11599590-000000000-00000.
-
9.
Simao R, Spineti J, de Salles BF, Oliveira LF, Matta T, Miranda F, et al. Influence of exercise order on maximum strength and muscle thickness in untrained men. J Sports Sci Med. 2010;9(1):1-7. [PubMed ID: 24149379].
-
10.
Thomas GA, Kraemer WJ, Kennett MJ, Comstock BA, Maresh CM, Denegar CR, et al. Immunoreactive and bioactive growth hormone responses to resistance exercise in men who are lean or obese. J Appl Physiol (1985). 2011;111(2):465-72. [PubMed ID: 21636569]. https://doi.org/10.1152/japplphysiol.00157.2011.
-
11.
American College of Sports M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687-708. [PubMed ID: 19204579]. https://doi.org/10.1249/MSS.0b013e3181915670.
-
12.
Thomas GA, Kraemer WJ, Comstock BA, Dunn-Lewis C, Volek JS, Denegar CR, et al. Effects of resistance exercise and obesity level on ghrelin and cortisol in men. Metabolism. 2012;61(6):860-8. [PubMed ID: 22146097]. https://doi.org/10.1016/j.metabol.2011.10.015.
-
13.
Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, et al. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol (1985). 1990;69(4):1442-50. [PubMed ID: 2262468].
-
14.
Rahimi R, Qaderi M, Faraji H, Boroujerdi SS. Effects of very short rest periods on hormonal responses to resistance exercise in men. J Strength Cond Res. 2010;24(7):1851-9. [PubMed ID: 20555276]. https://doi.org/10.1519/JSC.0b013e3181ddb265.
-
15.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-81. [PubMed ID: 7154893].
-
16.
Stone MH, Plisk SS, Stone ME, Schilling BK, O'Bryant HS, Pierce KC. Athletic Performance Development: Volume Load---1 Set vs. Multiple Sets, Training Velocity and Training Variation. STRENGTH COND J. 1998;20(6):22-31.
-
17.
Tietz NW. Clinical guide to laboratory tests. 3 ed. Philadelphia: WA Saunders Co; 1995.
-
18.
Burns C, Rigsby P, Moore M, Rafferty B. The First International Standard For Insulin-like Growth Factor-1 (IGF-1) for immunoassay: preparation and calibration in an international collaborative study. Growth Horm IGF Res. 2009;19(5):457-62. [PubMed ID: 19303800]. https://doi.org/10.1016/j.ghir.2009.02.002.
-
19.
de Salles BF, Simao R, Miranda F, Novaes Jda S, Lemos A, Willardson JM. Rest interval between sets in strength training. Sports Med. 2009;39(9):765-77. [PubMed ID: 19691365]. https://doi.org/10.2165/11315230-000000000-00000.
-
20.
Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol (1985). 2002;93(3):1159-67. [PubMed ID: 12183514]. https://doi.org/10.1152/japplphysiol.01264.2001.
-
21.
Eliakim A, Nemet D, Zaldivar F, McMurray RG, Culler FL, Galassetti P, et al. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol (1985). 2006;100(5):1630-7. [PubMed ID: 16373448]. https://doi.org/10.1152/japplphysiol.01072.2005.
-
22.
Kreitschmann-Andermahr I, Suarez P, Jennings R, Evers N, Brabant G. GH/IGF-I regulation in obesity--mechanisms and practical consequences in children and adults. Horm Res Paediatr. 2010;73(3):153-60. [PubMed ID: 20197666]. https://doi.org/10.1159/000284355.
-
23.
Thomas GA, Kraemer WJ, Comstock BA, Dunn-Lewis C, Maresh CM, Volek JS. Obesity, growth hormone and exercise. Sports Med. 2013;43(9):839-49. [PubMed ID: 23812873]. https://doi.org/10.1007/s40279-013-0064-7.
-
24.
Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40(12):1037-53. [PubMed ID: 21058750]. https://doi.org/10.2165/11536910-000000000-00000.
-
25.
West DW, Cotie LM, Mitchell CJ, Churchward-Venne TA, MacDonald MJ, Phillips SM. Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF-1 to skeletal muscle. Appl Physiol Nutr Metab. 2013;38(2):220-6. [PubMed ID: 23438236]. https://doi.org/10.1139/apnm-2012-0397.
-
26.
Hakkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol (1985). 1993;74(2):882-7. [PubMed ID: 8458810].
-
27.
Kraemer WJ, Noble BJ, Clark MJ, Culver BW. Physiologic responses to heavy-resistance exercise with very short rest periods. Int J Sports Med. 1987;8(4):247-52. [PubMed ID: 3667019]. https://doi.org/10.1055/s-2008-1025663.