Abstract
Background and Objectives:
Brucella is an intracellular gram-negative bacterium that can infect many kinds of mammals like humans, sheep, cattle, etc. Brucellosis is a contagious occupational disease caused by Brucella spp. that affects individuals who have close contact with infected animals. The clinical features of Brucellosis are not disease-specific and almost every organ can be affected. This zoonotic disease is a great health concern and economically important in many countries, such as Iran. The aim of this study was to detect Brucella spp. in pasteurized and non-pasteurized dairy products.Methods:
In this study, 208 samples, including goat, sheep, and cow raw and pasteurized milk as well as pasteurized and non-pasteurized cheese, were collected in Tehran province. The DNA was extracted, and then the real-time PCR was used for detection of the Brucella spp. gene.Results:
The prevalence of Brucella spp. contamination in the dairy products was: 45.5% in goat`s raw milk, 39.1% in non-pasteurized cheese, 27.3% in sheep`s raw milk, 26.3% in cow`s raw milk, 25% in pasteurized cheese, and 14.7% in pasteurized milk.Conclusions:
Rapid and exact detection of pathogens in dairy products is the most significant factor to prevent foodborne diseases. In addition, the real-time PCR assay is sensitive and specific enough to detect a low number of Brucella spp. in dairy products.Keywords
Real-Time PCR Brucellosis Pasteurization Dairy Products Brucella spp.
1. Background
Non-pasteurized dairy products are one of the most significant hosts of many foodborne pathogens including Enterobacteriaceae family, Brucella spp., and Campylobacter spp. (1). The genus of Brucella comprises facultative Gram-negative bacteria that are able to infect a vast variety of mammalians such as humans and chattel. Brucellosis is a contagious occupational disease caused by Brucella spp. that affects individuals who have close contact with infected animals. The most common way of Brucellosis transmission is ingestion of non-pasteurized milk and dairy products obtained from infected animals (2, 3).
Brucella spp. are accumulated in mammary glands and supra-mammary lymph nodes of infected animals and therefore the milk of these animals will be a source of pathogens (4). The clinical features of Brucellosis are not disease-specific; but almost every organ can be affected (5, 6). Infection with Brucella can cause a variety of problems in animals; for example, in females, it could be the cause of abortion and in males, usually orchitis and epididymitis are seen. Depending on conditions such as proper temperature, pH, and humidity, Brucella can remain in a contaminated environment for several months (7). Several epidemiological studies have reported a high frequency of brucellosis in endemic countries such as Saudi Arabia (19%), Iran (20%), Peru (8%), and Azerbaijan (10%) (8).
Four out of six major identified species of Brucella are human pathogens. Human brucellosis is mostly caused by B. melitensis while B. abortus is the second cause of human brucellosis, mostly infecting cattle, buffalos, elks, yaks, and camels; B. canis is the other cause of human brucellosis and B. suis that infects domestic pigs and rodents is the last one (9). Because Brucella can easily be transmitted as aerosols, it was used in the former U.S. biological weapons program (10).
Currently, diagnostic methods for detection of Brucella spp. rely on serological, microbiological, and molecular techniques. Serological techniques are standard for the epidemiological surveillance of brucellosis (11). The most common method for Brucella detection in milk and milk products is MRT (milk ring test) that has low sensitivity and accuracy (12). Molecular detection methods have been widely used for Brucella diagnosis in the last decades (13). Real-time PCR, which has less hazard and high sensitivity, has been developed for Brucella detection (14). Real-time PCR does not require extensive manipulation that minimizes the risk of contamination (15). Several nucleic acid sequences for Brucella spp. have been used to be amplified by PCR technique like16S rRNA, 16S-23S intragenic spacer region, omp2, and bcsp31 (16). A real-time Light-Cycler PCR (LC-PCR) assay that is based on the use of SYBR Green I DNA-binding fluorophore dye was developed by a clinical laboratory to simplify the molecular diagnosis of brucellosis (17).
Despite the decreased incidence of Brucellosis, in Iran and many endemic countries, it remains as an important public health.
Furthermore, a survey displayed that nearly 7.4% of cows in Iran were infected with Brucella spp. (18). Approximately, 500000 cases of human brucellosis globally are reported to the world health organization annually (19). Therefore, it seems that the contaminated dairy products are one of the most common causes of brucellosis. Therefore, the aim of this study was to detect Brucella spp. in different dairy products.
2. Methods
2.1. Preparation of Samples
In this case study, 208 different samples including 57 samples of cow raw milk, 34 samples of pasteurized milk (from different companies), 28 samples of pasteurized cheese (from different companies), 23 samples of unpasteurized cheese, 33 samples of goat raw milk, and 33 samples of sheep raw milk were collected in the province of Tehran. The samples were collected from 2014 to 2016 and stored at -20°C. For DNA extraction, 100 µL of each sample were obtained and DNA was extracted from samples with QIAamp DNA Mini Kit (Qiagen, USA), according to the protocols).
2.2. Real-Time PCR Technique
The real-time PCR assays were optimized and applied to all samples by the 7500 real-time PCR system (Applied Biosystems).
2.3. Real-Time PCR Reactions
The Taqman/ROX qPCR Master Mix (2X) (Applied Biosystems) was used. Each reaction mixture contained 100 ng of template DNA, 12.5 µL Master Mix (Applied Biosystems), 1 µL of each F/R (Forward/ Reverse) primers and probe (10 mM), and 9.5 µL nuclease-free water in a final volume of 25 µL per reaction. All reactions had a positive control that contained Brucella spp. DNA with exact concentration and a negative control that contained dilute water instead of DNA.
The mixture was subjected to the following PCR conditions: primary denaturation temperature at 95°C for 10 minutes to activate AmpliTaq Gold polymerase, followed by 40 cycles of denaturation at 95°C for 15 seconds, and annealing at 59°C for 1 minute. To minimize Ct values for experimental variability, the threshold cycle, in which the fluorescence signal raised significantly above background in the exponential phase of the amplification, was specified by the second derivative maximum method.
2.4. Template Preparation of the Standard Curve
The PCR amplification of the Brucella gene fragment was performed by using a universal primer. The reaction mixture contained Brucella DNA (1 - 20 ng), 1X PCR Buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 0.4 µM each F and R primers, and 2U Taq DNA polymerase (5 U/µL; Fermentas, USA) in a final volume of 25 µL. Amplification included a primary denaturation at 95°C for 3 minutes, followed by 25 cycles of denaturation at 94°C for 30 seconds, annealing at 54°C for 30 seconds, and extension at 72°C for 1 minutes. The PCR Amplicon (766 bp) was cloned into a pTZ57R vector and transformed in E. coli JM107 cells with T/A cloning Kit, (Fermentas, cat #: k1213) following the manufacturer’s instructions. DNA sequencing confirmed the cloning. The absorbance of the DNA solution was measured at 260 nm. The standard curve was drawn by the Fit Point’s analysis method that included the 7500 real-time PCR system (Applied Biosystems). The concentration log of a dilution series of the standard or reference template DNA (Brucella spp.) was plotted versus the cycle number in which the fluorescent signal increased up to the background or threshold (Ct value). The slope of the standard curve, which was provided for each detected approach, was put into the following equation to determine the reaction’s efficiency: efficiency = 10- (1/sl°pe) (20).
2.5. DNA Sequence Analysis and Design of the Primers and Probe
The target gene for the designed probe and primers set was Brucella spp. gene (Accession No. HE603359). The Primer Express Software provided by Applied Biosystems was used for designing primers. All primers were supplied by Bioneer (Korea). The sequences of each primer are listed in Table 1.
Primers Sequences Used for Detection of Brucella spp.
| Methods | Forward Primer (5’ - 3’) | Reverse Primer (5’ - 3’) | Fragment Size, bp |
|---|---|---|---|
| Real Time PCR | CATATCGTTGCGCGTAAGGA | GAAACGCGCTTGCCTTTC | 64 |
| Probe | FAM CAAACATCAAATCGGTCGCGGACC MGB | ||
| Universal primer | TGCCCGGTCTCGTAGCGACG | TCTGCGCCGGGATGCAGC | 766 |
2.6. Sensitivity and Specificity Determination of Real-Time PCR Assays
For sensitivity determination of real-time PCR, different dilutions of bacterial DNA from 1 million up to 10 particles were provided. The DNA of mice, humans, Salmonella, Shigella, Saccharomycescerevisiae, and Escherichiacoli were used to verify the PCR specificity.
3. Results
3.1. Testing of Samples for Brucella by Real-Time PCR
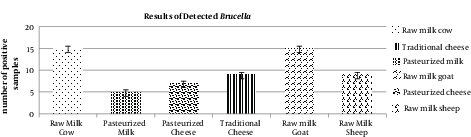
The results of real-time PCR show the following prevalence of contamination for Brucella spp.: 45.5% in goat raw milk, 39.1% in non-pasteurized cheese, 27.3% in sheep raw milk, 26.3% in cow raw milk, 25% in pasteurized cheese, and 14.7% in pasteurized milk. The highest and lowest prevalence rates of Brucella spp. were observed in goat raw milk and pasteurized milk, respectively. Most of the contaminated samples were collected from companies without any standard certification. In the milk and cheese produced in rural areas with no observation of sanitation organizations, a high number of Brucella spp. was detected.
The results of all samples are described in Table 2.
PCR Results of Brucella spp. Detection in Dairy Products
| Sample | No. | Real-time PCR | |
|---|---|---|---|
| Positive | % | ||
| Raw cow milk | 57 | 15 | 26.3 |
| Pasteurized milk | 34 | 5 | 14.7 |
| Pasteurized cheese | 28 | 7 | 25 |
| Traditional cheese | 23 | 9 | 39.1 |
| Raw goat milk | 33 | 15 | 45.5 |
| Raw sheep milk | 33 | 9 | 27.3 |
Real-Time PCR Results for Detection of Brucella spp.
3.2. Sensitivity and Specificity of Real-Time PCR
The real-time PCR after optimization had high specificity by showing no reactions to infectious agents except Brucella spp. The real-time sensitivity was 10 particles.
Sensitivity of Different Dilutions of Bacteria by the Standard Curve
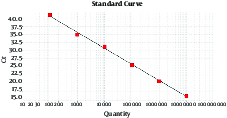
The threshold cycle values are plotted against the Brucella spp. input copy number.
4. Discussion
It seems that people who are living far from Brucella endemic regions are at low risk of infection; however, recently some reports indicated that brucellosis is increasing in non-endemic areas as in endemic regions. In addition, it seems that brucellosis could be easily transferred from rural to urban regions.
It is usually done by transferring raw milk and dairy products that are infected by Brucella spp. from far and near distances (2).
B. abortus can spread in food and water. Under conditions of high humidity, low temperatures, and no sunlight, these organisms can stay viable for a long time in the water, aborted fetuses, wool, feces, hay, clothes, and equipment. Brucella species can withstand drying, particularly when organic substances are present in the soil. In low temperature, survival is longer, especially when it is below freezing (21). Brucellosis affects many organs and tissues as a systemic infection (6). The highest significant incidence of brucellosis bacteremia occurs in spring and summer while the lowest occurrence is in winter. This is because of the consumption of unpasteurized milk products as a result of more travel to rural areas at these times (22). As mentioned by WHO, nowadays, brucellosis is the most common infection in the world with 500,000 infected cases each year (6, 23).
Endemic countries suffer from the lack of productivity and its adverse effects on human health (24). Several different factors like socioeconomic factors and some cultural habits cause various prevalence rates of brucellosis in distinct areas all over the world (25).
The prevalence of human brucellosis in different parts of Iran varied from 1.5 to 107.5 per 100,000 people in 2003. The highest levels of infection appeared in Hamadan with 107.5, Kurdistan with 83.5, Western Azarbaijan with 71.4, and Zanjan with 67.1 per 100,000 people (26).
Clearly, the prevalence of Brucella contamination varies according to the sensitivity of the used methods. Although isolation and phenotyping of Brucella are time-consuming and unsafe and need well-trained staff, they are still the gold standard for diagnosis of Brucella spp. (27). Today by using molecular detection techniques like PCR, the detection of brucellosis is significantly increasing (28, 29). Indeed, several studies have shown that agents such as lipids, enzymes, polysaccharides, proteins, and Ca2+ in high concentration that are present in dairy products can play the role of PCR inhibitor by interfering with nucleic acid degradation or with the amplification activity of polymerase (30). Unlike the circulating bacteria and DNA, antibodies against Brucella antigen remain in the blood for a long time, making sometimes PCR results negative while ELISA results are positive.
In the study performed by Lindahl-Rajala et al. in 2017, it was stated that 10.3% of non-pasteurized cow milk samples were infected with Brucella spp. in Tajikistan. They also declared that since the consumption of non-pasteurized milk is common in this area, this problem has caused anxiety and prevalence of the diseases (30). Similar to this study, they also used real-time PCR technique and stated that traditional detection methods are not sufficient for detection of this bacterium.
Probert et al. in 2004 established the multiplex PCR test for the detection of Brucella spp., B. abortus and B. melitensis, in a single test (31). In Iran, some limited studies have been performed in the field of detecting Brucella spp. by using real-time PCR on dairy products. In the study by Majid Yaran et al. in 2016, the prevalence of B. melitensis and B. abortus in raw milk and dairy products were evaluated by using real time PCR (3). In spite of the differences in the obtained results, in both studies, the necessity of using exact and sensitive molecular techniques for detection of Brucella spp. in dairy products was emphasized.
The assessment of real-time PCR technique for detection of B. melitensis in non-pasteurized milk was done by Wareth et al. in 2014. They notified that non-pasteurized dairy products are important sources for the prevalence of brucellosis and real-time PCR is qualified and efficient for detection of this pathogen (2).
As mentioned earlier, one of the most important ways for brucellosis infection transmission is consumption of infected dairy products. The results of this study showed that high percentages of non- pasteurized dairy products including milk and traditional cheese are infected with Brucella spp.
In spite of this fact, the consumption of non-pasteurized dairy products in many places still makes a great concern for the disease prevalence. In addition, since the results showed 14.7% of pasteurized milk and 25% of pasteurized cheese samples were infected with Brucella spp., it seems the pasteurization methods are not effective for destruction of this pathogen. As the dairy products are controlled before being distributed, it seems that commercial quality control of these products is not sufficient and exact. Therefore, reducing the possibility of being infected by this pathogen by using accurate molecular detection techniques like real-time PCR should be considered (3, 32).
In order to decrease the venture of Brucella infection due to the ingestion of contaminated dairy products, food safety management systems, which guarantee the sanitary quality of the products, have to control and improve the production of dairy products. Intransitive training of dairy makers and consumers should be provided and the consumers should be notified of serious health risks due to unpasteurized milk and dairy products (33).
Finally, for exact detection of bacteria and evaluation of the amount of pathogen, it is essential to use sensitive and specific methods such as real-time PCR to detect Brucella spp. in dairy products.
References
-
1.
Committee on Infectious D, Committee on N, American Academy of P. Consumption of raw or unpasteurized milk and milk products by pregnant women and children. Pediatrics. 2014;133(1):175-9. [PubMed ID: 24344105]. https://doi.org/10.1542/peds.2013-3502.
-
2.
Wareth G, Melzer F, Elschner MC, Neubauer H, Roesler U. Detection of Brucella melitensis in bovine milk and milk products from apparently healthy animals in Egypt by real-time PCR. J Infect Dev Ctries. 2014;8(10):1339-43. [PubMed ID: 25313613]. https://doi.org/10.3855/jidc.4847.
-
3.
Yaran M, Najafi S, Shoaei P, Ataei B, Fadaei R, Ramazanpour J, et al. Prevalence of Brucella melitensis and Brucella abortus in raw milk and dairy product by real time PCR technique. Ulutas Med J. 2016;2(1):7-11. https://doi.org/10.5455/umj.20151004054235.
-
4.
Ilhan Z, Solmaz H, Aksakal A, Gulhan T, Ekin IH, Boynukara B. Detection of Brucella melitensis DNA in the milk of sheep after abortion by PCR assay. Arch Med Vet. 2008;40(2):141-6. https://doi.org/10.4067/s0301-732x2008000200005.
-
5.
Tay BY, Ahmad N, Hashim R, Mohamed Zahidi J, Thong KL, Koh XP, et al. Multiple-locus variable-number tandem-repeat analysis (MLVA) genotyping of human Brucella isolates in Malaysia. BMC Infect Dis. 2015;15:220. [PubMed ID: 26033227]. https://doi.org/10.1186/s12879-015-0958-0.
-
6.
Celebi O, Celebi D, Eda Balkan C. Effects of boiling dairy products on human brucellosis. Eurasian J Med. 2013;45(2):73-6. [PubMed ID: 25610256]. https://doi.org/10.5152/eajm.2013.17.
-
7.
Wang Z, Bie P, Cheng J, Wu Q, Lu L. In vitro evaluation of six chemical agents on smooth Brucella melitensis strain. Ann Clin Microbiol Antimicrob. 2015;14:16. [PubMed ID: 25857255]. https://doi.org/10.1186/s12941-015-0077-1.
-
8.
Sanodze L, Bautista CT, Garuchava N, Chubinidze S, Tsertsvadze E, Broladze M, et al. Expansion of brucellosis detection in the country of Georgia by screening household members of cases and neighboring community members. BMC Public Health. 2015;15:459. [PubMed ID: 25934639]. https://doi.org/10.1186/s12889-015-1761-y.
-
9.
Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, et al. Strong Association Between Human and Animal Brucella Seropositivity in a Linked Study in Kenya, 2012-2013. Am J Trop Med Hyg. 2015;93(2):224-31. [PubMed ID: 26101275]. https://doi.org/10.4269/ajtmh.15-0113.
-
10.
Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278(5):399-411. [PubMed ID: 9244332]. https://doi.org/10.1001/jama.278.5.399.
-
11.
Colmenero JD, Reguera JM, Martos F, Sanchez-De-Mora D, Delgado M, Causse M, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore). 1996;75(4):195-211. [PubMed ID: 8699960]. https://doi.org/10.1097/00005792-199607000-00003.
-
12.
Izadi A, Moslemi E, Tabatabaei Panah AS, Kheiri Manjili H. Brucella Spp. detection in dairy products using Nested and Hemi Nested PCR techniques. Ann Biol Res. 2014;5(1):124-31.
-
13.
Baily GG, Krahn JB, Drasar BS, Stoker NG. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg. 1992;95(4):271-5. [PubMed ID: 1495123].
-
14.
Bricker BJ. PCR as a diagnostic tool for brucellosis. Vet Microbiol. 2002;90(1-4):435-46. [PubMed ID: 12414163]. https://doi.org/10.1016/S0378-1135(02)00228-6.
-
15.
Kami M, Fukui T, Ogawa S, Kazuyama Y, Machida U, Tanaka Y, et al. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin Infect Dis. 2001;33(9):1504-12. [PubMed ID: 11588697]. https://doi.org/10.1086/323337.
-
16.
Hamdy ME, Amin AS. Detection of Brucella species in the milk of infected cattle, sheep, goats and camels by PCR. Vet J. 2002;163(3):299-305. [PubMed ID: 12090772]. https://doi.org/10.1053/tvjl.2001.0681.
-
17.
Ohtsuki R, Kawamoto K, Kato Y, Shah MM, Ezaki T, Makino SI. Rapid detection of Brucella spp. by the loop-mediated isothermal amplification method. J Appl Microbiol. 2008;104(6):1815-23. [PubMed ID: 18248366]. https://doi.org/10.1111/j.1365-2672.2008.03732.x.
-
18.
Sanaei Dashti A, Karimi A, Javad V, Shiva F, Fallah F, Alaei MR, et al. ELISA Cut-off Point for the Diagnosis of Human Brucellosis; a Comparison with Serum Agglutination Test. Iran J Med Sci. 2012;37(1):9-14. [PubMed ID: 23115425].
-
19.
Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523-41. [PubMed ID: 21939378]. https://doi.org/10.1146/annurev-micro-090110-102905.
-
20.
Rasmussen R. Quantification on the LightCycler. In Rapid cycle real-time PCR. Springer Berlin Heidelberg; 2001. p. 21-34.
-
21.
Capparelli R, Parlato M, Iannaccone M, Roperto S, Marabelli R, Roperto F, et al. Heterogeneous shedding of Brucella abortus in milk and its effect on the control of animal brucellosis. J Appl Microbiol. 2009;106(6):2041-7. [PubMed ID: 19298512]. https://doi.org/10.1111/j.1365-2672.2009.04177.x.
-
22.
Memish Z, Mah MW, Al Mahmoud S, Al Shaalan M, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40(1):59-63. [PubMed ID: 10762113]. https://doi.org/10.1053/jinf.1999.0586.
-
23.
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-9. [PubMed ID: 16439329]. https://doi.org/10.1016/S1473-3099(06)70382-6.
-
24.
Cutler SJ, Whatmore AM, Commander NJ. Brucellosis--new aspects of an old disease. J Appl Microbiol. 2005;98(6):1270-81. [PubMed ID: 15916641]. https://doi.org/10.1111/j.1365-2672.2005.02622.x.
-
25.
Gwida M, Al Dahouk S, Melzer F, Rosler U, Neubauer H, Tomaso H. Brucellosis - regionally emerging zoonotic disease? Croat Med J. 2010;51(4):289-95. [PubMed ID: 20718081]. https://doi.org/10.3325/cmj.2010.51.289.
-
26.
Moradi G, Nasab NE, Ghaderi E, Sofi Majidpour M, Hamideh Salimzadeh. H. Brucellosis in Kurdistan Province from 1997 to 2003. Ann Alquds Med. 2006;2(1):32-7.
-
27.
Yu WL, Nielsen K. Review of detection of Brucella spp. by polymerase chain reaction. Croat Med J. 2010;51(4):306-13. [PubMed ID: 20718083].
-
28.
Garshasbi M, Ramazani A, Sorouri R, Javani S, Moradi S. Molecular detection of Brucella species in patients suspicious of Brucellosis from Zanjan, Iran. Braz J Microbiol. 2014;45(2):533-8. [PubMed ID: 25242938].
-
29.
Guarino A, Serpe L, Fusco G, Scaramuzzo A, Gallo P. Detection of Brucella species in buffalo whole blood by gene-specific PCR. Vet Rec. 2000;147(22):634-6. [PubMed ID: 11128080]. https://doi.org/10.1136/vr.147.22.634.
-
30.
Lindahl-Rajala E, Hoffman T, Fretin D, Godfroid J, Sattorov N, Boqvist S, et al. Detection and characterization of Brucella spp. in bovine milk in small-scale urban and peri-urban farming in Tajikistan. PLoS Negl Trop Dis. 2017;11(3). e0005367. [PubMed ID: 28296882]. https://doi.org/10.1371/journal.pntd.0005367.
-
31.
Probert WS, Schrader KN, Khuong NY, Bystrom SL, Graves MH. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J Clin Microbiol. 2004;42(3):1290-3. [PubMed ID: 15004098]. https://doi.org/10.1128/JCM.42.3.1290-1293.2004.
-
32.
Rodríguez-Lázaro D, López-Enríquez L, Ocampo-Sosa AA, Muñoz P, Blasco JM, Marín C, et al. Evaluation of eryC as a molecular marker for the quantitative detection of Brucella Spp. by real-time PCR in food samples. Food Anal Methods. 2017;10(5):1148-55. https://doi.org/10.1007/s12161-017-0822-5.
-
33.
Kaden R, Ferrari S, Alm E, Wahab T. A novel real-time PCR assay for specific detection of Brucella melitensis. BMC Infect Dis. 2017;17(1):230. [PubMed ID: 28340558]. https://doi.org/10.1186/s12879-017-2327-7.
