Abstract
Keywords
Intraoperative Hypotension Post-Induction Hypotension Anesthetic Fluid Deficits Carcinoid Crisis General Anesthesia Cancer Oncological Surgery
1. Context
Since the physiological correlation of blood pressure to cerebral and spinal perfusion pressure is well established, it is imperative to appreciate the relationship of normal physiological process and pathophysiological states where meticulous blood pressure control is warranted to ensure adequate perfusion of vital organs and tissue. Intraoperative hypotension is linked to significant morbidity and mortality (1-3). IOH is an absolute decrease of systolic blood pressure to less than 100 mmHg and/or a relative decrease of 30% from baseline, though there are several definitions in the literature (4). Studies show a consistent relationship between the magnitude and duration of IOH and postoperative renal, cardiac, and cerebral systems dysfunction in non-cardiac surgery (4).
The anesthesia team and preoperative/recovery room nurses are asked to monitor and manage each patient's physiology preoperatively (5-8). This management should maintain the patient’s physiological parameters within their normal range. Normal physiology of cerebral blood flow, including autoregulation, are directly affected by many factors, including PaCO2, mean arterial pressure, and oxygenation (Figure 1) (9).
Normal physiological relations between cerebral blood flow and mean arterial pressure autoregulation, PaCO2, and oxygenation
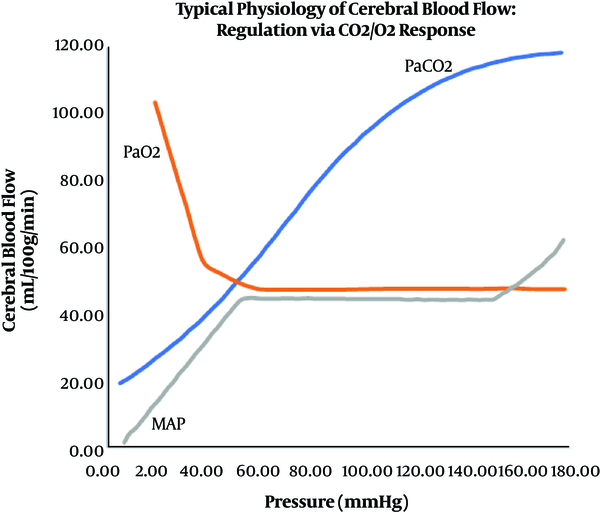
Various monitors, including an arterial line, can help determine physiological factors that may contribute to alterations in hemodynamics. When necessary, the physiology can be intentionally altered to reduce bleeding or avoid potential injuries related to the patient’s conditions or surgical considerations (e.g., deliberate hypotension or deliberate hypothermia) (10).
With regard to blood pressure management, in normal tissue, the cerebral and spinal cord blood flow is “auto regulated” and blood flow is maintained constant. Mean arterial pressure below the “lower limit of autoregulation” results in below-normal blood flow, which can compromise tissue. Previously, the lower limit for normal patients with normal blood pressures was 60 mmHg. Still, a reassessment of these studies suggests that it is more variable. A more accurate representative average value is 70 mmHg for healthy patients and 90-100 mmHg (or higher) with hypertensive patients (9-11).
Therefore, autoregulation can be utilized to support deliberately lowering the blood pressure (e.g., deliberate hypotension), for example, to reduce blood loss during selective spine or brain surgeries or to decrease the risk of catastrophic complications such as intracranial aneurysm rupture. In many patients, specifically young and healthy patients, lowering blood pressure is performed regularly with no significant risks to the brain or spinal cord. However, when the nervous system is compromised (e.g., ischemia), or if the patient auto regulates at higher pressures (e.g., hypertension), a higher pressure will be required to ensure adequate blood flow to the patient’s brain, spinal cord, heart, kidneys, and other vital organs (Figure 2).
The effect of autoregulation on cerebral blood flow and the “shift to the right phenomenon related to hypertensive patients
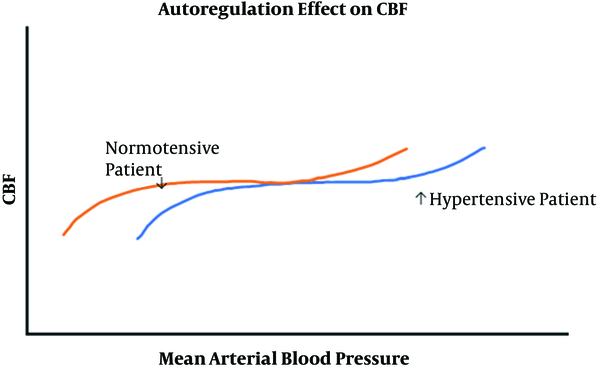
Therefore, excessive hypotension can result in ischemia of the brain, spinal cord, and critical organs such as the heart or kidneys, and this has been seen at blood pressures that are not usually associated with neural ischemia in healthy patients (e.g., systolic blood pressure above 90 mmHg systolic) (12). Other conditions that can result in ischemia include, regional hypo perfusion (e.g., obstructed artery to a limb), hypoxemia, excessive hyperventilation, severe anemia, and reduction in neural perfusion pressure (e.g., increased cerebrospinal fluid or intracranial pressure).
Time is also an important factor in the effects of blood pressure and ischemia on cortical and spinal cord neural tissue. As cerebral blood flow decreases below normal (50 cc/min/100 gm.), tissue blood flow falls at 18 - 20 cc/min/100 gm. This indicates a normal margin of safety in the brain (Figure 3). The electrical activity below this level becomes abnormal and absent at 12 - 15 cc/min/100 gm. corresponding with blood flow reduction is an increased potential risk of neural injury. Therefore, it may take up to 3 - 4 hours before a permanent neural deficit occurs at blood flows where the electrical activity is altered. The time to injury is shorter as the blood flow is further reduced (13).
Relationship of cerebral blood flow, ischemia, and time effects.
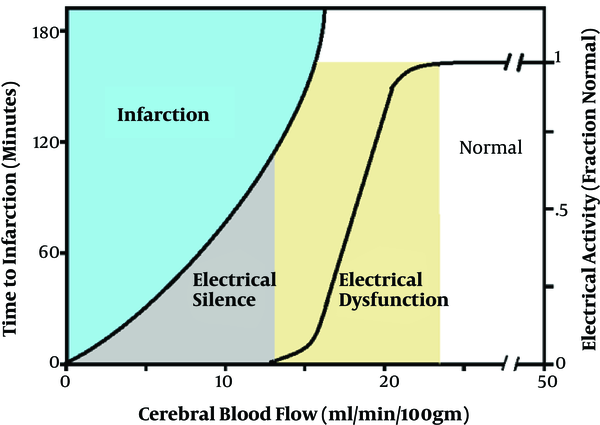
Positioning also plays a significant role in blood pressure and perfusion to critical organs and tissue. For example, in the beach chair position with the blood pressure cuff on the arm, there is an actual different blood pressure, which would be lower than recorded, since the center of the head is significantly higher than the arm. Therefore, the pressures noted are different than the true pressures (roughly for every 1 centimeter away from the head, the blood pressure is reduced by 1.3 mm Hg). General anesthesia with inhalational anesthetic agents such as sevoflurane, desflurane, and isoflurane impairs or attenuates autoregulation of cerebral blood flow (Figure 4).
Increasing doses of inhalational agent attenuate the autoregulation curve
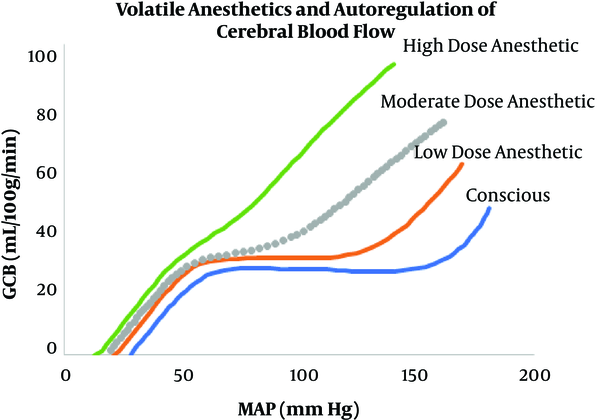
In this regard, a study from Daniel et al. demonstrated that a short duration of intraoperative mean arterial pressure of less than 55 mmHg is associated with myocardial injury and acute kidney injury (14). When examining the relationship between IOH in non-cardiac surgery and postoperative cognitive dysfunction, Luciano et al. found no relationship (15). However, a separate study with a larger sample size concluded that increased blood pressure fluctuation was a predictive factor of postoperative delirium (16).
In the present investigation, a literature search was evaluated, looking at the role of intraoperative hypotension on outcomes in patients undergoing general anesthesia with cancer.
2. Evidence Acquisition
Narrative Review. We performed a comprehensive search for English-language studies related to intraoperative hypotension in the oncological patient. We searched the following databases: PubMed, Medline, SciHub, Cochrane Database of Systematic Reviews, and Google Scholar. We used the following combinations of keywords: intraoperative hypotension, post-induction hypotension (PIH), anesthetic fluid deficits, carcinoid crisis, general anesthesia, cancer, oncological surgery. We tried to include as many recent manuscripts as possible (within the last three years) but also included papers that were older than three years if they were particularly relevant to our topic. We also attempted to search for, use, and cite primary manuscripts whenever possible.
3. Discussion
The present investigation reviewed a significant body of literature related to IOH in patients undergoing general anesthesia with cancer. Generally, intraoperative hypotension can be classified chronologically into 3 phases (17). The first phase is post-induction hypotension (PIH), which occurs during the first 20 minutes after anesthesia induction, the second phase is the early intraoperative hypotension, which occurs within 30 minutes of surgery, and lastly, the third phase is the late intraoperative hypotension, which is related to surgical factors (e.g., hemorrhage and secondary systemic inflammatory response).
With this framework in mind, we will focus on the contributing effects of cancer pathophysiology in the three phases of IOH.
3.1. Post-Induction Hypotension
Post-induction hypotension major predictors of PIH include patient age > 50 (e.g., the majority cancer patients), American Society of Anesthesiologists (ASA) physical status classification of III - IV, and choice of induction agent (18).
3.1.1. Cancer Therapy-Related Cardiac Dysfunction
The treatment modalities of cancer include cytotoxic chemotherapy, targeted molecular therapies, and tumor irradiation. One of the side effects of these modalities is cancer therapy-related cardiac dysfunction (CTRCD), which has been shown to increase the risk of IOH (19).
CTRCD is attributed to myocyte damage either related to apoptosis or loss of myofibrils, which leads to left ventricle dysfunction and heart failure. The CTRCD may be manifested within one year of initiating the chemotherapy (early-onset) or after years of treatment completion (late-onset) (20). Therefore, the anesthesiologist should be vigilant in preoperative surveillance for this chronic cardiotoxicity of late-onset. The American Society of Echocardiography and the European Association of Cardiovascular Imaging define CTRCD as “a decrease in left ventricular ejection fraction (LVEF) of more than 10% to below the lower limit of normal (LVEF = 53%) despite symptoms” (21). LVEF is the most commonly used investigative marker for preoperative cardiac assessment because of its ease of availability and relatively low cost. However, it should be noted that in the oncological population undergoing cancer therapy, myocardial deformation precedes LVEF changes. Therefore, a more sensitive modality is indicated to assess the cardiac function rather than a decrease in LVEF, which correlates with significant, late cardiac damage. The assessment of global longitudinal strain (GLS) by speckle tracking echocardiography is considered a good alternative where a decrease greater than 15% of the baseline GLS is considered a subclinical left ventricular dysfunction (22). Using GLS for contractility assessment is a newer, promising independent predictor of cardiac toxicity, which may help the anesthesiologist tailor the anesthetic plan to avoid PIH.
3.1.2. Fluid Deficit
Fluid deficit has been defined as loss of water with or without related electrolytes, especially sodium (23). The oncology patients could have a greater risk of development of fluid deficit due to multiple mechanisms caused by physiological and metabolic effects of either cancer itself or its treatment modalities. The postulated mechanisms include anorexia, cognitive dysfunction, diarrhea, and vomiting as side effects of chemotherapy, purposeful restriction of fluids for edema, and unsupervised diuretics use (24). Moreover, adrenal insufficiency (due to adrenal tumor or metastasis) and cerebral salt wasting (due to carcinomatous meningitis) may contribute to the hyponatremic fluid deficit (25). Alternatively, a hypernatremic fluid deficit may result from renal tubular defect (with multiple myeloma) and diabetes insipidus (due to pituitary tumor) (26)
Furthermore, the physiological compensatory response to fluid deficit is restricted in the oncology population, particularly elderly patients. This restriction is modulated or mediated by decreased thirst sensation, limited renin and aldosterone reserve, and relative renal resistance to antidiuretic hormone (27). Additionally, the fasting period before an elective oncology surgery could contribute to the development of hypovolemia. All these postulated mechanisms could lead to symptoms ranging from orthostatic hypotension up to fluid deficit shock when the loss is greater than 20% of intravascular volume. One study demonstrated that Induction of anesthesia decreased the stroke volume to 62% of baseline (28). This study further supports the hypothesis that PIH is more prevalent in the oncology population. The authors concluded that fluid volume optimization could ameliorate this decrease in stroke volume and that fluid responsiveness is greater in case of dehydration. Therefore, preoperative careful clinical assessment and laboratory tests (especially recent serum sodium) are key to diagnosing fluid deficit in oncology population and guide the preoperative fluid volume optimization. This may prevent the PIH.
3.1.3. Choice of Induction Agents
The choice of the induction agents and their dosage is one of the main determining factors of risk of development of PIH. The above-mentioned study compared the commonly used induction agents (propofol, thiopental, etomidate, and fentanyl) risk of PIH (18). The authors concluded that propofol (for induction of anesthesia) and fentanyl (increasing the induction dosage) are significantly related to the PIH. Moreover, they recommended smaller doses of fentanyl and advised to consider alternatives to propofol for anesthesia induction (e.g. etomidate) in patients with pre-induction mean arterial pressure < 70 mmHg. Another separate study found that propofol (2.5 mg/kg) can cause significantly more hypotension than thiopental (5 mg/kg) and etomidate (3 mg/kg) (29). Therefore, they reported that the risk of PIH may contraindicate the use of propofol in hypovolemic patients.
Another unique consideration in oncology patients who are presented with anterior mediastinal masses is the extrinsic compression of the heart, large vessels, especially pulmonary artery, and pericardial effusion (30). In such cases, induction of general anesthesia has been demonstrated to diminish the cardiac contractility and the cardiac output and to reduce the tone of the vascular structures, causing their compression. Therefore, reducing the doses of induction dosage is definitely recommended to decrease the incidence of PIH in such high-risk patients.
3.2. Early Intraoperative Hypotension
Early IOH occurs within 30 min of the start of surgery and has been related to multiple factors, including general and regional anesthesia, patient position, and early surgical intervention (11). Herein, we focused on general anesthesia for pelvic, abdominal, and intra-thoracic, non-cardiac surgery. In this context, the relationship between the tumor burden size and location and the development of early IOH may be heavily influenced by position, which may result in cardiac and respiratory compromise on supine position (31). Anesthesia in the supine and Trendelenburg positions could lead to a decrease in thoracic cavity dimensions and cephalic displacement of the diaphragm. Additionally, the institution of positive pressure ventilation and the gravitational effect of the tumor’s mass on the surrounding cardiac structures are important factors in this hemodynamic compromise, which could be severe and may lead to cardiac arrest (32).
3.3. Late Intraoperative Hypotension
Late intraoperative hypotension occurs after 30 minutes of induction of anesthesia and may be related to the surgical manipulation of the tumor. Such manipulation can result in intraoperative hypotension for a variety of reasons. In this discussion, we will focus on three factors found in high incidence in oncological surgery: hemorrhage, carcinoid crisis, and Venous thromboembolism.
3.3.1. Risk of Hemorrhage
The oncology patient could have an elevated risk of intraoperative bleeding because of neoplasm-specific pathophysiology and/or the anti-neoplastic therapy. The risk of bleeding may be higher in specific types of neoplasm and certain chemotherapy agents.
Myelosuppression is one of the detrimental complications of chemotherapy, especially thrombocytopenia (33). There are different mechanisms of chemotherapy-induced thrombocytopenia (34). The alkylating agents can affect stem cells, while the cyclophosphamide affects the megakaryocyte progenitors. Furthermore, bortezomib prevents platelet release from the megakaryocytes, and other agents may promote platelet apoptosis. One of the most challenging elements of cancer-related thrombocytopenia is transfusion refractoriness. Poor post-transfusion platelet response has been noticed in cancer patients, especially the critically ill subgroup with hyperproliferative thrombocytopenia (35). Transfusion refractoriness may be reduced by using single donor platelet transfusions and human leukocyte antigen matched platelets (36).
Neoplasm-specific alterations of hemostasis encompass a wide range of coagulation defects. Acute promyelocytic leukemia has been associated with hyper-fibrinolytic syndrome. Moreover, acquired hemophilia and acquired von Willebrand disease can also be triggered by a malignancy such as myeloproliferative and lymphoid neoplasms (37). Bleeding in such patients can quickly accelerate to disseminated intravascular coagulation, which could be attributed to the exposure of tissue factor to the intravascular space (38). Therefore, it is essential for the anesthesiologists to anticipate these defects preoperatively to guide transfusion therapy.
3.3.2. Carcinoid Crisis
Carcinoid tumors commonly arise from the neuroendocrine cells of the midgut (39). These neuroendocrine tumors secrete a variety of bioactive amines into the systemic circulation, which causes carcinoid syndrome. These bioactive amines include serotonin, bradykinins, tachykinins, prostaglandins, and histamine. Carcinoid syndrome development is associated with tumor grade, stage, and primary site (40). Moreover, the existence of hepatic metastasis may increase the incidence because of their direct drainage into the systemic circulation bypassing the portal circulation where all these amines could be deactivated by the liver. Carcinoid syndrome is characterized by deleterious cardiovascular and pulmonary complications such as hypotension, right‐sided heart failure, and bronchial constriction (41).
A sudden intraoperative release of the above-mentioned amines upon surgical palpation and manipulation of the tumor may result in carcinoid crisis. Carcinoid crisis is a serious and life-threatening complication of carcinoid syndrome and can lead to prolonged and profound hypotension and cardiac arrest (42). A prospective study reported that the pathophysiology of the crisis is consistent with distributive shock. The authors noticed that cardiac function was normal by using the transesophageal echocardiography while there was consistently intracardiac hypovolemia (43). On that account, volume expansion could be beneficial to restore the hemodynamic instability.
Carcinoid crisis has serious postoperative sequelae and increases the hospital length of stay. Recommendations for prophylaxis include anxiolysis and avoidance of exogenous catechol amines and histamine-release triggering agents. Furthermore, many clinical studies have recommended prophylactic octreotide either preoperatively or intraoperatively, which may decrease the incidence of the crisis (44-46). The role of prophylactic octreotide remains widely accepted to avoid profound hemodynamic instability in carcinoid patients (47).
3.3.3. Venous Thromboembolism
The hematological effects of the neoplasm and para-neoplastic processes include imbalances in the coagulation cascade, which can present as thrombosis, bleeding, and disseminated intravascular coagulation discussed above.
The oncologic population has up to a six-fold increase in the likelihood of developing Venous thromboembolism (VTE), including pulmonary embolism and deep vein thrombosis (48). The pathophysiology encompasses the release of procoagulants (including tissue factor and tumor mucin), which cause the formation of thrombin and fibrin. Moreover, an indirect mechanism includes activation of platelets, leukocytes, and endothelial cells with a release of cytokines, production of factor X‐activating cysteine proteases, and circulating tissue factor‐bearing micro-particles (49). Another hypothesis attributes thromboembolism to mechanical decompression of large stagnant veins upon resection of the large tumors. When such large tumors compress the pelvic vasculature, impacting pelvic, and lower limb venous tree and could increase the blood viscosity leading to VTE (50). Intraoperative VTE is a dreaded life-threatening emergent situation manifested by hemodynamic compromise leading up to cardiac arrest. The most common current recommendations advise the implementation of risk‐assessment tools and starting pharmacologic thromboprophylaxis preoperatively for high-risk patients (49). A prospective study examined the efficacy of implementing intermittent pneumatic compression at both intraoperative and postoperative and showed that it can decrease the incidence of the VTE in combination with pharmacological agents (51).
3.4. Final Thoughts
Hypotension predictive index (HPI) is a technology created by Edwards Lifesciences (Irvine, USA) to predict the onset of hypotension in 5, 10, 15 minutes before the upcoming event.
HPI has a unit-less number range from 1 to 100, with higher values indicating a higher likelihood of IOH (52). It necessitates the insertion of an arterial line, and further, no data is available regarding its ability to specifically predict the PIH and early intraoperative hypotension.
Such technology and algorithms can be modified to account for variables specific to the oncological population in defining a patient’s individual risk. While this would come at a high financial cost to collect the necessary data in prospective studies, it would allow anesthesiologists in the future to tailor anesthetic plans to achieve better IOH prevention in the cancer patient population.
3.5. Conclusions
Intraoperative hypotension reduces perfusion to critical organs and tissues and has adverse intraoperative and postoperative consequences. Certain populations have even greater consequences related to IOH. In the present investigation, we have highlighted the common causes of intraoperative hypotension and emphasized the particular vulnerability of the oncologic population to this life-threatening event. Stratification of risk based on tumor type, stage and therapy, and patient-specific factors and comorbidities can be complicated but is important to attempt successful prophylaxis.
References
-
1.
Vincent C. Patient Safety. 2 ed. BMJ Books; 2010.
-
2.
Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213-20. [PubMed ID: 17667564]. https://doi.org/10.1097/01.anes.0000270724.40897.8e.
-
3.
Hassani V, Movaseghi G, Safaeeyan R, Masghati S, Ghorbani Yekta B, Farahmand Rad R. Comparison of ephedrine vs. Norepinephrine in treating anesthesia-induced hypotension in hypertensive patients: Randomized double-blinded study. Anesth Pain Med. 2018;8(4). e79626. [PubMed ID: 30271750]. [PubMed Central ID: PMC6150924]. https://doi.org/10.5812/aapm.79626.
-
4.
Li D, Bohringer C, Liu H. What is "normal" intraoperative blood pressure and do deviations from it really affect postoperative outcome? J Biomed Res. 2017;31(2):79-81. [PubMed ID: 28808189]. [PubMed Central ID: PMC5445210]. https://doi.org/10.7555/JBR.31.20160167.
-
5.
Urits I, Jung JW, Amgalan A, Fortier L, Anya A, Wesp B, et al. Utilization of magnesium for the treatment of chronic pain. Anesth Pain Med. 2021;11(1). https://doi.org/10.5812/aapm.112348.
-
6.
Imani F, Zaman B, De Negri P. Postoperative pain management: Role of dexmedetomidine as an adjuvant. Anesth Pain Med. 2021;10(6). https://doi.org/10.5812/aapm.112176.
-
7.
Tully J, Jung JW, Patel A, Tukan A, Kandula S, Doan A, et al. Utilization of intravenous lidocaine infusion for the treatment of refractory chronic pain. Anesth Pain Med. 2020;10(6). https://doi.org/10.5812/aapm.112290.
-
8.
Hemati K, Zaman B, Hassani V, Imani F, Dariaie P. Efficacy of fentanyl transdermal patch in the treatment of chronic soft tissue cancer pain. Anesth Pain Med. 2015;5(1). e22900. [PubMed ID: 25789240]. [PubMed Central ID: PMC4350185]. https://doi.org/10.5812/aapm.22900.
-
9.
May DM, Jones SJ, Crockard HA. Somatosensory evoked potential monitoring in cervical surgery: identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85(4):566-73. [PubMed ID: 8814157]. https://doi.org/10.3171/jns.1996.85.4.0566.
-
10.
Drummond JC. The lower limit of autoregulation: Time to revise our thinking? Anesthesiology. 1997;86(6):1431-3. [PubMed ID: 9197320]. https://doi.org/10.1097/00000542-199706000-00034.
-
11.
Seyal M, Mull B. Mechanisms of signal change during intraoperative somatosensory evoked potential monitoring of the spinal cord. J Clin Neurophysiol. 2002;19(5):409-15. [PubMed ID: 12477986]. https://doi.org/10.1097/00004691-200210000-00004.
-
12.
Yousefshahi F, Tahmasebi M. Long-lasting orthostatic hypotension and constipation after celiac plexus block; a case report. Anesth Pain Med. 2018;8(1). e63221. [PubMed ID: 29868459]. [PubMed Central ID: PMC5970361]. https://doi.org/10.5812/aapm.63221.
-
13.
Sloan T, Kaye AD. Anesthesiology and Intraoperative Electrophysiological Monitoring. Essentials of Neurophysiology Monitoring Kaye and S Davis. Springer; 2014. p. 71-94.
-
14.
Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507-15. [PubMed ID: 23835589]. https://doi.org/10.1097/ALN.0b013e3182a10e26.
-
15.
Langer T, Santini A, Zadek F, Chiodi M, Pugni P, Cordolcini V, et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J Clin Anesth. 2019;52:111-8. [PubMed ID: 30243062]. https://doi.org/10.1016/j.jclinane.2018.09.021.
-
16.
Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115(3):418-26. [PubMed ID: 25616677]. [PubMed Central ID: PMC4533731]. https://doi.org/10.1093/bja/aeu458.
-
17.
Sudfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57-64. [PubMed ID: 28974066]. https://doi.org/10.1093/bja/aex127.
-
18.
Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622-8. [PubMed ID: 16115962]. https://doi.org/10.1213/01.ANE.0000175214.38450.91.
-
19.
Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ Heart Fail. 2016;9(1). e002661. [PubMed ID: 26747861]. [PubMed Central ID: PMC4709035]. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002661.
-
20.
Shaikh AY, Shih JA. Chemotherapy-induced cardiotoxicity. Curr Heart Fail Rep. 2012;9(2):117-27. [PubMed ID: 22382639]. https://doi.org/10.1007/s11897-012-0083-y.
-
21.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-92. [PubMed ID: 11248153]. https://doi.org/10.1056/NEJM200103153441101.
-
22.
Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin Med Insights Cardiol. 2019;13:1179546819866440. [PubMed ID: 31384135]. [PubMed Central ID: PMC6664629]. https://doi.org/10.1177/1179546819866445.
-
23.
Beers M, Berkow R. The merck manual of geriatrics. 2 ed. Merck & Co; 2000.
-
24.
Berk L, Rana S. Hypovolemia and dehydration in the oncology patient. J Support Oncol. 2006;4(9):447-54. discussion 455-7. [PubMed ID: 17080733].
-
25.
Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012;59(2):222-8. [PubMed ID: 22001181]. https://doi.org/10.1053/j.ajkd.2011.08.029.
-
26.
Sarhill N, Walsh D, Nelson K, Davis M. Evaluation and treatment of cancer-related fluid deficits: Volume depletion and dehydration. Support Care Cancer. 2001;9(6):408-19. [PubMed ID: 11585267]. https://doi.org/10.1007/s005200100251.
-
27.
Weinberg AD, Minaker KL. Dehydration. Evaluation and management in older adults. Council on scientific affairs. JAMA. 1995;274(19):1552-6. [PubMed ID: 7474224]. https://doi.org/10.1001/jama.274.19.1552.
-
28.
Li Y, He R, Ying X, Hahn RG. Dehydration, hemodynamics and fluid volume optimization after induction of general anesthesia. Clin Sao Paulo Braz. 2014;69(12):809-16. [PubMed ID: 25627992]. [PubMed Central ID: PMC4286668]. https://doi.org/10.6061/clinics/2014(12)04.
-
29.
McCollum JS, Dundee JW. Comparison of induction characteristics of four intravenous anaesthetic agents. Anaesthesia. 1986;41(10):995-1000. [PubMed ID: 3491551]. https://doi.org/10.1111/j.1365-2044.1986.tb12740.x.
-
30.
Dubey PK, Tripathi N. Anesthetic considerations in a patient with large anterior mediastinal mass. J Cardiothorac Vasc Anesth. 2019;33(4):1073-5. [PubMed ID: 29706571]. https://doi.org/10.1053/j.jvca.2018.03.023.
-
31.
Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth. 1989;36(6):681-8. [PubMed ID: 2490850]. https://doi.org/10.1007/BF03005421.
-
32.
Levin H, Bursztein S, Heifetz M. Cardiac arrest in a child with an anterior mediastinal mass. Anesth Analg. 1985;64(11):1129-30. [PubMed ID: 4051210].
-
33.
Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol. 2009;46(1 Suppl 2):S26-32. [PubMed ID: 19245931]. https://doi.org/10.1053/j.seminhematol.2008.12.007.
-
34.
Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncol Williston Park N. 2015;29(4):282-94. [PubMed ID: 25952492].
-
35.
Baron E, Charpentier J, Francois A, Ben Hadj Amor H, Habr B, Cariou A, et al. Post-transfusion platelet responses in critically ill cancer patients with hypoproliferative thrombocytopenia. Transfusion. 2020;60(2):275-84. [PubMed ID: 31724828]. https://doi.org/10.1111/trf.15596.
-
36.
Slichter SJ. Evidence-based platelet transfusion guidelines. Hematol Am Soc Hematol Educ Program. 2007:172-8. [PubMed ID: 18024626]. https://doi.org/10.1182/asheducation-2007.1.172.
-
37.
Mantha S. Bleeding disorders associated with cancer. Cancer Treat Res. 2019;179:191-203. [PubMed ID: 31317489]. https://doi.org/10.1007/978-3-030-20315-3_13.
-
38.
Dixit A, Chatterjee T, Mishra P, Kannan M, Choudhry DR, Mahapatra M, et al. Disseminated intravascular coagulation in acute leukemia at presentation and during induction therapy. Clin Appl Thromb Hemost. 2007;13(3):292-8. [PubMed ID: 17636191]. https://doi.org/10.1177/1076029607302435.
-
39.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934-59. [PubMed ID: 12569593]. https://doi.org/10.1002/cncr.11105.
-
40.
Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population-based study. Lancet Oncol. 2017;18(4):525-34. [PubMed ID: 28238592]. [PubMed Central ID: PMC6066284]. https://doi.org/10.1016/S1470-2045(17)30110-9.
-
41.
Dierdorf SF. Carcinoid tumor and carcinoid syndrome. Curr Opin Anaesthesiol. 2003;16(3):343-7. [PubMed ID: 17021482]. https://doi.org/10.1097/00001503-200306000-00017.
-
42.
Keskin O, Yalcin S. Carcinoid Crisis in the Intensive Care Unit. In: Nates JL, Price KJ, editors. Oncologic Critical Care. 33. Cham: Springer; 2020. p. 995-1002.
-
43.
Condron ME, Jameson NE, Limbach KE, Bingham AE, Sera VA, Anderson RB, et al. A prospective study of the pathophysiology of carcinoid crisis. Surgery. 2019;165(1):158-65. [PubMed ID: 30415870]. https://doi.org/10.1016/j.surg.2018.04.093.
-
44.
Borna RM, Jahr JS, Kmiecik S, Mancuso KF, Kaye AD. Pharmacology of octreotide: Clinical implications for anesthesiologists and associated risks. Anesthesiol Clin. 2017;35(2):327-39. [PubMed ID: 28526153]. https://doi.org/10.1016/j.anclin.2017.01.021.
-
45.
Woltering EA, Wright AE, Stevens MA, Wang YZ, Boudreaux JP, Mamikunian G, et al. Development of effective prophylaxis against intraoperative carcinoid crisis. J Clin Anesth. 2016;32:189-93. [PubMed ID: 27290972]. https://doi.org/10.1016/j.jclinane.2016.03.008.
-
46.
Mancuso K, Kaye AD, Boudreaux JP, Fox CJ, Lang P, Kalarickal PL, et al. Carcinoid syndrome and perioperative anesthetic considerations. J Clin Anesth. 2011;23(4):329-41. [PubMed ID: 21663822]. https://doi.org/10.1016/j.jclinane.2010.12.009.
-
47.
Massimino K, Harrskog O, Pommier S, Pommier R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J Surg Oncol. 2013;107(8):842-6. [PubMed ID: 23592524]. https://doi.org/10.1002/jso.23323.
-
48.
Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 133. Chest; 2008.
-
49.
Donnellan E, Kevane B, Bird BR, Ainle FN. Cancer and venous thromboembolic disease: from molecular mechanisms to clinical management. Curr Oncol. 2014;21(3):134-43. [PubMed ID: 24940094]. [PubMed Central ID: PMC4059798]. https://doi.org/10.3747/co.21.1864.
-
50.
Zhang W, Liu X, Cheng H, Yang Z, Zhang G. Risk factors and treatment of venous thromboembolism in perioperative patients with ovarian cancer in China. Medicine (Baltimore). 2018;97(31). e11754. [PubMed ID: 30075594]. [PubMed Central ID: PMC6081089]. https://doi.org/10.1097/MD.0000000000011754.
-
51.
Parry K, Sadeghi AH, van der Horst S, Westerink J, Ruurda JP, van Hillegersberg R. Intermittent pneumatic compression in combination with low-molecular weight heparin in the prevention of venous thromboembolic events in esophageal cancer surgery. J Surg Oncol. 2017;115(2):181-5. [PubMed ID: 28054341]. https://doi.org/10.1002/jso.24480.
-
52.
de Keijzer IN, Vos JJ, Scheeren TWL. Hypotension prediction index: From proof-of-concept to proof-of-feasibility. J Clin Monit Comput. 2020;34(6):1135-8. [PubMed ID: 31974829]. https://doi.org/10.1007/s10877-020-00465-3.
