-
PDF
- Split View
-
Views
-
Cite
Cite
Bruce Luber, Jason Steffener, Adrienne Tucker, Christian Habeck, Angel V. Peterchev, Zhi-De Deng, Robert C. Basner, Yaakov Stern, Sarah H. Lisanby, Extended Remediation of Sleep Deprived-Induced Working Memory Deficits Using fMRI-guided Transcranial Magnetic Stimulation, Sleep, Volume 36, Issue 6, 1 June 2013, Pages 857–871, https://doi.org/10.5665/sleep.2712
Close - Share Icon Share
Abstract
We attempted to prevent the development of working memory (WM) impairments caused by sleep deprivation using fMRI-guided repetitive transcranial magnetic stimulation (rTMS). Novel aspects of our fMRI-guided rTMS paradigm included the use of sophisticated covariance methods to identify functional networks in imaging data, and the use of fMRI-targeted rTMS concurrent with task performance to modulate plasticity effects over a longer term.
Between-groups mixed model.
TMS, MRI, and sleep laboratory study.
27 subjects (13 receiving Active rTMS, and 14 Sham) completed the sleep deprivation protocol, with another 21 (10 Active, 11 Sham) non-sleep deprived subjects run in a second experiment.
Our previous covariance analysis had identified a network, including occipital cortex, which demonstrated individual differences in resilience to the deleterious effects of sleep deprivation on WM performance. Five Hz rTMS was applied to left lateral occipital cortex while subjects performed a WM task during 4 sessions over the course of 2 days of total sleep deprivation.
At the end of the sleep deprivation period, Sham sleep deprived subjects exhibited degraded performance in the WM task. In contrast, those receiving Active rTMS did not show the slowing and lapsing typical in sleep deprivation, and instead performed similarly to non- sleep deprived subjects. Importantly, the Active sleep deprivation group showed rTMS-induced facilitation of WM performance a full 18 hours after the last rTMS session.
Over the course of sleep deprivation, these results indicate that rTMS applied concurrently with WM task performance affected neural circuitry involved in WM to prevent its full impact.
INTRODUCTION
Sleep deprivation has been estimated to affect 20% of the population1 and contributes to human error by pilots, truck drivers, shift workers, medical residents, and in other occupations that require long hours and sustained vigilance. The cognitive deficits caused by sleep deprivation are well described,2 and there has been much speculation as to their neural underpinnings.3–5
In our previous work we explored the effects of repetitive transcranial magnetic stimulation (rTMS) on a network involved in working memory and sensitive to the effects of sleep deprivation.6–8 We found that fMRI-guided rTMS could be used to remediate performance in sleep deprived individuals, benefiting subjects proportionally to the level of their deficit in expression of that network.8 In subjects who had experienced total sleep deprivation for two days, 5 Hz rTMS was applied during the retention phase of the working memory task. rTMS to left upper occipital cortex resulted in a reduction of the sleep-induced reaction time (RT) deficit without a corresponding decrease in accuracy, while stimulation at other sites did not. The degree of performance enhancement with upper occipital rTMS correlated with the degree to which each individual failed to sustain activation of the fMRI network as determined from pre- and post-sleep deprivation scans. A subset of participants performed the same rTMS procedure after recovering from sleep deprivation, and no effects of rTMS were found, suggesting that the benefits were specific to the sleep deprived state. These results demonstrated that rTMS applied to superior occipital cortex, part of a working memory network sensitive to sleep deprivation, specifically reduced the effects of sleep deprivation-induced working memory deficits, i.e., that rTMS had modulated a cortical network critical to the working memory task in a way that improved its resilience to sleep deprivation.
While such TMS-related cognitive enhancements suggest great promise in improving cognitive deficits, modulation of cortical activity involved with cognitive tasks by TMS has not been shown to be very long lasting. The duration of effects on performance measures has been on the order of 10 minutes9 to an hour,10 and the effects of a single TMS session on subsequent brain activity measured with EEG have also been estimated to last up to an hour or so.11 On the other hand, there has been some indication that increasing the duration of TMS stimulation increases the subsequent duration of cognitive effects.10 Repeated sessions of rTMS have already been shown to cause long-lasting changes in mood12 and in recovery of motor function from stroke.13 Repeated rTMS sessions may thus also prolong the duration of cognitive benefits as well.11 In addition, beneficial cognitive rTMS effects might be prolonged if rTMS is applied while subjects perform a cognitive task, as suggested by Thickbroom.14
In the present study, we implemented these two potential manipulations to prolong beneficial cognitive rTMS effects by applying rTMS while subjects performed the working memory task in multiple sessions over the course of two days of total sleep deprivation. We tested working memory performance at the end of the sleep deprivation period, 18 hours after the fourth TMS session, expecting that individuals receiving active rTMS would show less severe deficits in working memory performance due to sleep deprivation than a sham rTMS group, which would demonstrate a prolongation of benefit an order of magnitude longer than had been previously reported.
METHODS
Subjects
Fifty-five healthy volunteers participated in the study. Subjects were right handed (as determined by the modified Edinburgh Handedness Questionnaire), had normal or corrected-to-normal vision, and were native English speakers. Potential subjects were excluded if they had a history of current or past Axis I psychiatric disorder including substance abuse/dependence as determined by the Structured Clinical Interview for DSM-IV Axis I disorders (SCID - I/NP)15 or a history of neurological disease. All subjects were screened for contraindications for rTMS and for general health with physical and neurological examinations, blood and urine testing, urine drug screens, and pregnancy tests for women of childbearing capacity. The study was approved by the Columbia University and New York State Psychiatric Institute Investigational Review Board and was performed under an FDA-approved Investigational Device Exemption (IDE).
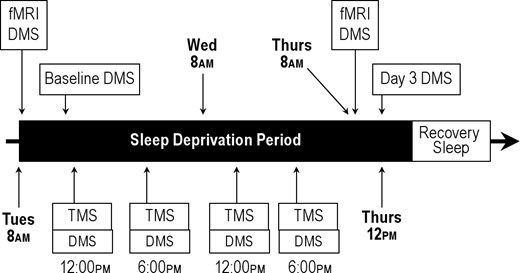
Thirty-three subjects took part in the full sleep deprivation protocol as described below. They were sleep deprived over a 3-day period, remaining awake from 07:00 Tuesday morning until about 03:00 Thursday, monitored in a sleep laboratory, which assured and maintained when necessary continued wakefulness, during which they participated in Baseline and Day 3 fMRI sessions, 4 rTMS sessions, and multiple daily sessions performing other cognitive tasks. These subjects were randomly assigned to 1 of 2 groups, one of which received active TMS (Active-sd (sleep deprived) group) and the other, sham rTMS (Sham-sd (sleep deprived) group). A total of 27 subjects completed the sleep deprived protocol, 13 in the Active-sd group and 14 in the Sham-sd group, with another 6 subjects assigned to the Sham-sd group dropping out after one night due to difficulty with sleep deprivation or, in one case, to illness. Gender was balanced between the Active-sd and Sham-sd groups, with 7 males in each. Age was also balanced, with a mean of 23.3 ± 0.6 years in sham group, and 23.3 ± 1.2 years in the active group. There was also no significant difference in years of education, with a mean of 15.9 ± 0.5 years of education in the sham group and 15.0 ± 0.6 years in the active group.
We could find no particular reason why all 6 dropouts were from the Sham-sd group and none from the Active-sd group. The dropouts did not differ demographically from those who completed the study. Gender was balanced (3 male, 3 female), as it was in the completers. Mean age of the dropouts (23.3 ± 1.9) was not significantly different from completers, nor was years of education (15.5 ± 0.8 years). While it is most likely that the dropouts all being from the Sham-sd group was a statistical aberration, it is also possible that the active rTMS could have had an additional reinforcing effect (besides the specific effect on the delayed match to sample [DMS] task performance we observed [see below]) in regard to sleep deprivation that might make it more tolerable, although it is difficult to see at this point what that might be.
A second group of 22 subjects were recruited for a second experiment in order to answer 2 questions that would aid in interpreting the results of the main sleep deprivation experiment: first, what is the effect of practice in the memory task due to a 4-session procedure, and second, what is the effect of 4 sessions of TMS alone (without sleep deprivation) on task performance? An answer to the first question was important because over the course of 4 TMS sessions, subjects performed 1024 trials of the DMS task. While subjects initially practiced the task for between 45 and 75 min in their screening session on a day prior to the 3-day sleep deprivation procedure, it was possible that performing over a thousand more trials would produce a practice effect (usually improved RT), and an accounting of the extent of performance enhancement due to such extensive practice (in contrast to any facilitation due to TMS) was needed. Answering the second question was needed because it was necessary to demonstrate that the TMS would not change the DMS performance of subjects not undergoing sleep deprivation. We had previously shown this to be the case for acute application of TMS: after 2 days of sleep deprivation, subjects showed remediated performance with TMS, while they showed no performance enhancements in the DMS task later, after they had recovered.8 However, the present experiment tested something different: the cumulative effects of 4 sessions of concurrent TMS and task performance. We did not know if subjects without sleep deprivation would show enhancement effects with cumulative sessions. If they did not, this provided evidence that the rTMS was specifically remediating a deficit caused by sleep deprivation.
The subjects in the second experiment were randomly assigned to 2 groups receiving either active (Active-non-sd group) or sham rTMS (Sham-non-sd group). These subjects had Baseline and Day 3 sessions using the working memory task, and had the same course of 4 rTMS sessions, but without sleep deprivation. However, the non-sleep deprived groups were not treated identically to the sleep deprived groups, in that they did not stay in the sleep lab and were instructed to sleep normally each night. They also did not participate in the 2 fMRI sessions, nor in the other cognitive task sessions that the sleep deprived subjects did.
Twenty-one subjects completed the study as controls (with one dropout from the Sham-non-sd group). There were 11 (6 female) in the Sham-non-sd group, and 10 (4 female) in the Active-non-sd group. These subjects were slightly older than the sleep deprived participants, with a mean age of 26.4 ± 4.5 years in the Active-non-sd group and 26.1 ± 4.4 years in the Sham-non-sd group. Active-non-sds had a mean of 16.5 ± 2.8 years of education, with a mean of 16.3 ± 2.6 years of education in the Sham-non-sds.
DMS Task
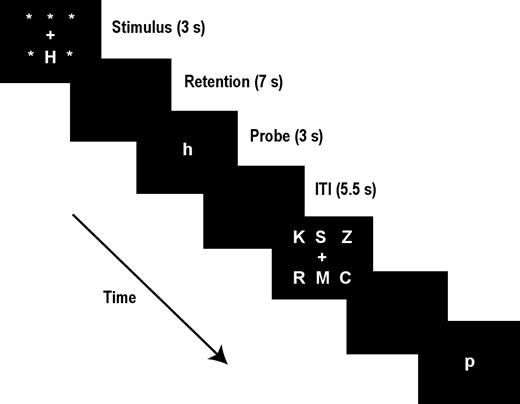
Participants were trained on a delayed-match-to-sample (DMS) working memory task described in our previous work.6–8 Each trial was 13 sec long according to the following sequence (see Figure 1): First, an array of 1 or 6 upper-case letters was presented on a computer screen for 3 s (stimulus phase). Each letter subtended 1.1 degrees of visual angle. Next, the screen was blank for 7 s (retention phase), during which time the subjects were asked to fixate on the center of the screen and keep the stimulus items in mind. Finally, a test stimulus, a single lower-case letter, appeared for 3 s at the center of the screen (probe period). At this time the subject was to indicate by a button press whether or not the probe letter matched a character in the stimulus array, using the right hand for matching probes and the left for non-matches. Subjects were instructed to respond as quickly and as accurately as possible. Following the probe phase was an inter-trial interval, which lasted 2 s, plus a randomized duration between 0 and 0.5 s, during which the computer screen was again blank. Choice of set size (1 or 6) and positive or negative probe for an individual trial was pseudo-randomized, with the restriction that there be 16 true positive and 16 true negative probes for each of the 2 set sizes over a block of 64 trials. Subjects were initially trained on the DMS task in a session prior to the beginning of testing. Practice in the task was continued until subjects produced stable accuracy and reaction time performance, generally after 192 to 320 trials. The DMS task was performed at noon of the first day (Baseline) and at noon on of the third day of the protocol (Day 3), as well as during the 4 rTMS sessions of the first and second days (see Figure 2, which provides a schematic of the full 3-day procedure).
Schematic diagram of the DMS paradigm. Two trials are shown, the first with a set size of one and requiring a “yes” response, and the second with a set size of 6 and requiring a “no” response. The trial phases and their durations are listed at the right (ITI = inter-trial interval).
Schematic diagram of the study procedure over the course of two days.
The DMS task was also performed at 08:30 during MRI sessions on the first and third days (Figure 2). For the MRI sessions there were 3 memory set sizes (1, 3, and 6) rather than the 2 (1 and 6) used in the rTMS procedures. The third set size was included so that fMRI responses to 3 levels of memory load could be assessed in the covariance analysis used to derive functional networks. In this case, each experimental block contained 10 trials for each of the 3 set sizes, with 5 true negative and 5 true positive probes per set size. Three experimental blocks were run in total, yielding 90 experimental trials per scanning session.
Other Tasks
Subjects performed 4 other tasks across the sleep deprivation period. Although deleterious effects of sleep deprivation on these tasks are already known, changes in performance in these tasks can be used to demonstrate that targeted rTMS specifically benefits only impairments in the DMS task, and not in a wide variety of other types of task.
The Psychomotor Vigilance Task (PVT) was modeled after that of Dinges16 and is considered to be one of the best measures of the effect of sleep deprivation on cognitive state.3,4 In this task, subjects responded as quickly as possible with a space-bar keyboard press to the appearance of a red “X” on a computer screen, which was followed by RT feedback. The Xs appeared randomly with an inter-trial interval between 2 to 10 sec over a block of trials lasting 10 minutes. The initial PVT session was administered before 09:30 of the first day after a normal night of sleep at home. This first session was considered a practice run, and the data were not included in analyses. Eight additional sessions were administered every 6 h beginning at noon of the first day and extending until 06:00 of the third and final day. The final session was after 48 h of sleep deprivation; this session was not used to avoid well-known end spurt effects. Thus, a total of 3 PVT test bouts from Day 1 were averaged together to make the “non-sleep deprived” data, and similarly, 3 test bouts from Day 2 were averaged to make the “sleep-deprived” data. Lapses were defined as RT > 500 ms; false starts as RT < 150 ms. RT was averaged over all other responses (those < 500 ms and > 150 ms).
The following 3 tasks were performed in the morning of the first day (non-sleep deprived) and at the same time after 48 h of sleep deprivation:
Tracking Task, performed during MRI acquisitions, consists of single- and dual-task conditions in which the primary task is visuomotor tracking. Participants use a joystick to perform compensatory tracking, moving a cursor back to a central cross after random perturbations occurring every 40 ms. In dual-task conditions, the secondary task requires a button press whenever circles diagonally across match in color. Details of the task can be found in Gazes et al.17
The Multi-Attribute Task Battery (MATB), a flight simulator for desktop computer developed by NASA, is a complex divided attention task that requires participants to continuously interact with 4 distinct subtasks.18 Participants were required to continuously track using a joystick while simultaneously monitoring and responding to a variety of secondary tasks, including warning lights, moving dials, a fuel resource management task, and auditory communications.
The Selective Reminding Test (SRT) was administered to participants to assess verbal learning and memory.19 Participants were presented with 6 trials to learn 12 semantically unrelated words aurally. After each attempt to recall the list, subjects were reminded only of the words that were not recalled and then asked to recall the entire list (“total recall”). Subjects were asked to recall as many words as possible from the list after a 15-min delay.
Sleep Deprivation
The sleep deprivation and MRI procedures are described in detail elsewhere.6,8 Briefly, the sleep deprivation procedure was carried out over the course of 56 h, beginning at 07:00 on day 1 and terminating by 15:00 on Day 3 (Figure 2). MRI sessions occurred within 3 h of study start and within 4 h of leaving. Participants were admitted to the sleep disorders center at 18:00 on the night before the sleep deprivation was to begin, to ensure each subject had a complete night's sleep before beginning. Experimental personnel accompanied and monitored the participants continuously during day and evening hours. Participants were not allowed to consume caffeine or other stimulants. While not participating in experimental protocols, participants had access to the Internet, music, and a TV with broadcast programming, movies, and video games. Sleep deprivation in the sleep lab was monitored continuously by sleep center staff and assured and maintained via EEG and video monitoring.
TMS Application
rTMS was applied using a vacuum-cooled figure-eight coil (5 cm diameter) powered by a Magstim Super-Rapid stimulator (Magstim Co., Whitland, South West Wales, UK). For sham rTMS, the coil was placed perpendicular to the subject's head (one-wing, 90° sham manipulation). Of note, the stimulation site was at a posterior location, so subjects could not see any differences in coil positioning. When asked at the end of the sleep deprivation period (after all testing) to make their best guess as to whether they had received real or sham rTMS, subjects were unable to do so above chance level (42% correct, χ2 = 1.3, P < 0.26). rTMS stimulus intensity was set at 100% of motor threshold (MT) of the left hemisphere, which was defined as the lowest intensity needed to evoke motor potentials ≥ 50 microvolts recorded from the first dorsal interosseus muscle in at least 5/10 stimulations.
The site chosen for rTMS application was guided by group fMRI results found using the DMS task in a previous study6 and replicated in a separate subject group in a follow-up fMRI/ rTMS study.8 Based on Luber et al.,8 the upper part of the left middle occipital gyrus in Brodmann Area 19, near the border with the temporal lobe, was chosen. This site was part of a network found with specialized covariance analyses (ordinal trends analysis) that was activated by the DMS task and sensitive to sleep deprivation, in that its activation decreased with sleep deprivation in individual subjects to a degree correlated with their drop in DMS performance.6 It was originally singled out of the network because it was also a region that had been sensitive to sleep deprivation manipulations during the performance of visual working memory tasks in a number of imaging studies.20–22 In Luber et al.,8 rTMS applied to this location but not others remediated DMS performance deficits caused by sleep deprivation, and the effects of rTMS were sleep-state sensitive, in that improved performance with rTMS only occurred in the sleep deprived state; no improvement on DMS performance with rTMS occurred during wakefulness in a non-sleep deprived state. Coil placement was guided by Brainsight, a computerized frameless stereotaxy system (Rogue Research, Montreal, Canada). This system used an infrared camera to monitor the positions of reflective markers attached to the participant's head. Head locations were correlated in real time with the participant's MRI data after the data were co-registered to a set of anatomical locations. Reflective markers were attached to the coil and the subject, so that relative positions of the coil to the head (and the MRI) could be tracked, allowing precise positioning of the coil with respect to annotated MRI locations.
Four blocks of 64 trials of the DMS task were run in each session. Five Hz active or sham rTMS was applied during the 7-s retention interval (35 pulses) of every other trial. Subjects were allowed breaks between each block, and their wakefulness was continuously monitored and maintained during task performance.
Over the course of the 2-day sleep deprivation period, rTMS was applied while subjects performed the memory test in four 1.5-h sessions (Figure 2). These 4 sessions were at 12:00 and 18:00, both on the first day, after subjects had a full night's sleep, and on the second day, after the first night of sleep deprivation. Performance level on the DMS task (with no concomitant rTMS) was measured with 2 blocks of trials at 12:00 at the beginning of the first session of the first day and at 12:00 on the third day, after the second night of sleep deprivation. A remediating effect of rTMS was thus assessed by comparing performance from these 2 end points.
Median reaction time (RT), lapses (trials without a subject response) and accuracy (% correct) were calculated for the baseline (Day 1) and the Day 3 Test for each subject. Results of the Active-sd and Sham-sd groups were compared using mixed-model ANOVAs with between-group factor of TMS group (Active-sd, Sham-sd), and repeated measures factors of Time (Baseline, Day 3), and Set Size (1, 6) were performed separately on median RT and accuracy data. For the second experiment, similar mixed-model ANOVAs were used. Based on the fact that the DMS task is designed to be sensitive to RT rather than accuracy, as well as our previous results using TMS in the DMS task,7,8 it was expected that RT rather than accuracy would show TMS effects. As the 2 most common cognitive effects of sleep deprivation are slowing and lapsing, TMS effects on RT and lapsing were anticipated here.23
fMRI Acquisition and Preprocessing
During the performance of each block of the DMS task, 207 BOLD images,24,25 were acquired with an Intera 1.5 T Phillips MR scanner equipped with a standard quadrature head coil, using a gradient echo echo-planar (GE-EPI) pulse sequence (TE / TR = 50 ms / 3,000 ms; flip angle = 90; 64 × 64 matrix, in-plane voxel size = 3.124 mm × 3.124 mm; slice thickness = 8 mm (no gap); 17 transaxial slices per volume). Four additional GE-EPI excitations were performed before the task began, at the beginning of each run, to allow transverse magnetization immediately after radio frequency excitation to approach its steady-state value; the images corresponding to these excitations were discarded. Data were spatially normalized using a T1-weighted spoiled gradient image (107 slices; 256 × 256 grid; FOV = 230 mm × 160.5 mm × 183.28 mm).
DMS task stimuli were back-projected onto a screen located at the foot of the MRI bed using an LCD projector, which participants viewed via a mirror system located in the head coil. All participants wore MR compatible glasses as needed to have vision at their best corrected acuity (manufactured by SafeVision, LLC. Webster Groves, MO). Responses were made on a LUMItouch response system (Photon Control Company) using the index fingers of either hand. Task administration and collection of response data were controlled using PsyScope 1.2.5 running on a Macintosh G3 iBook. Task onset was electronically synchronized with the MRI acquisition computer. A Carnegie Mellon Button Box (New Micros, Inc. Dallas, TX) provided digital input-output for the response system and synchronization with the MRI acquisition computer, as well as millisecond accurate timing of responses.
fMRI Time-Series (First-Level) Modeling
At the first-level GLM, the GE-EPI time-series were modeled with regressors that represented the expected BOLD fMRI response (relative to the blank intervals) to the 3 DMS trial components of memory set presentation, retention delay, and probe presentation, separately for set size (1, 3, and 6). DMS trials without motor responses from the subject during the probe period were modeled separately and were not included at the second-level GLM analysis. Rectangular functions were used for the trial components of memory set presentation and probe presentation lasting throughout that entire component (3000 ms), and a single rectangular function of 7000 ms duration was used for the retention delay. Contrasts were estimated for each load level and trial phase and were carried forward to the second-level group analyses. The second-level, voxel-wise GLM modeled the 9 repeated measures per subject per voxel, with a design matrix representing 2 repeated-measure factors (trial component and set size). Contrasts from this second-level group analysis were calculated and subjected to the multivariate sequential latent root testing. The covariance matrix of this repeated measures second-level analysis was estimated at each voxel and spatially averaged to approximate the known observation error covariance matrix used in the multivariate analyses.26
Second-Level Modeling: MLM Analysis
Group level analysis of BOLD image data used multivariate linear modeling (MLM)26 to identify significant load-dependent networks, or covariance patterns, comprising latent spatial variables within the BOLD image effects of interest, engaged by the active and sham groups. This analysis was designed to be sensitive to group differences in covarying network activity, and has already been successfully used for this purpose with the DMS task in a number of group analyses of BOLD data.27–30
A singular value decomposition (SVD) was performed on the spatially whitened effects of interest to identify covariance patterns. Sequential latent root testing was subsequently used to assess the number of significant latent spatial patterns (with α controlled at 0.05). The effects of interest for this study were the load-dependent working memory neural responses during the different phases of the experiment. These comprised 3 combinations of slopes of subject specific contrast maps with respect to set size (1, 3, 6) during the 3 trial components, each of which was computed separately for the Active-sd and Sham-sd groups. The effective number of trials per subject per set size was equal to 30. Significant latent spatial patterns are presented for descriptive purposes scaled by their singular values (analogous to SPM{t} images),26 thresholded for descriptive purposes at a t value corresponding to P < 0.001 uncorrected for multiple comparisons and a cluster size of 50 voxels. This threshold does not control map-wise statistical significance at α = 0.05, but does provide a condensed description of the significant latent spatial patterns. Once identified, the spatial patterns were multiplied voxel by voxel with the subject specific contrast maps that were entered into the MLM analysis, and then summed to calculate each subject network expressions. Possible outcomes for the MLM analyses are 0, 1, or 2 latent patterns. By design, the first latent pattern is indicative of common activation pattern between groups (Active-sd vs. Sham-sd in this case). In contrast, the second latent pattern, if identified, suggests group differences in brain activation. The signs of the voxel values in a latent spatial pattern and its corresponding expression across subjects (or groups) are only meaningful in their product (i.e., the signs of each in isolation may be thought of as completely arbitrary). One multiplies a particular latent spatial pattern by its predicted expression to yield the predicted contribution from that latent pattern to the effects of interest.26 Anatomic labels for cluster maxima were provided by Talairach Daemon.
Computational Estimate of Effective TMS
To estimate the depth of effective brain stimulation in the Active sleep deprived group, we modeled the electric field distribution induced by TMS using the finite element method simulation package MagNet (Infolytica Corp., Montreal, Canada). Three cancatenated spheres were fitted to the template MRI to model the human head (see Figure 8F). The posterior, middle, and anterior spheres have diameters of 8.5 cm, 8.25 cm, and 8 cm, respectively, and isotropic conductivity of 0.33 S/ m.31 The TMS coil (Magstim P/N 1640) was modeled based on manufacturer data and x-rays of the coil. The coil windings were assumed to be 1.5 cm from the surface of the cortex, to account for account for the thickness of the CSF, skull and scalp layers, and the coil insulation. The electric field was computed using the Time Harmonic solver of MagNet.32
RESULTS
The presentation of the results is ordered in the following sequence: First, the effects of sleep deprivation alone will be shown, in order to establish the performance deficits caused by sleep deprivation in our DMS task and in the other tasks. Second, rTMS modulation of these sleep deprivation effects is presented. Third, evidence that rTMS also modulated brain activity (via fMRI) is shown. Finally, results of the second experiment involving non-sleep deprivedgroups are presented. Statistical results involving sleep deprivation but not rTMS group will be presented in the first section, while those involving rTMS group comparisons will be presented in the second section. Thus the second section, which compares Baseline and Day 3 DMS performance (during sessions when rTMS is not applied), contains the critical tests of rTMS amelioration of sleep deprivation effects on the DMS task. The third section presents rTMS effects that occurred during the rTMS sessions. The fourth and fifth sections contain the results of the analysis of Baseline and Day 3 fMRI contrasts. The results of the second experiment examining practice and TMS effects in non- sleep deprivedgroups are presented in the final section.
Effects of Sleep Deprivation
As expected, 2 days of total sleep deprivation had profound effects on the sleep deprived subjects, demonstrated by scores in the Epworth Sleepiness Scale (ESS), as well as overall Day 3 – Baseline sleep deprivation performance effects in the DMS and the other cognitive tasks. For the ESS, a repeated measures ANOVA showed a strong main effect of Time (Baseline vs. Day 3: F1,21 = 28.6, P < 0.001), with a Baseline mean of 5.2 (± 3.2) and a Day 3 mean of 13.3 (± 5.7). The overall ESS mean exceeded the cutoff for clinically significant sleepiness (ESS > 10).
In the DMS task, sleep deprivation produced slowing of median reaction time (RT), decreased accuracy, and more performance lapses in Baseline and Day 3 testing for Sham-sd subjects. In the Baseline and Day 3 testing sessions, a repeated-measures ANOVA of RT in the Sham-sd group showed a main effect of set size (F1,13 = 67.5, P < 0.001), which was expected due to the robust effect of set size in the DMS task. There was also a main effect of time of testing (Baseline vs. Day 3; F1,13 = 5.8, P < 0.05). ANOVA for % Correct for the Sham-sd group across Time (Baseline vs. Day 3) and Set Size (1 or 6 items) showed almost significant main effects of Set Size (F1,13 = 4.6, P < 0.051) and Time (F1,13 = 4.3, P < 0.06), due to slightly decreased accuracy with increased set size and with sleep deprivation, and a Set Size × Time interaction (F1,13 = 5.3, P < 0.04), due to a greater drop in % Correct with sleep deprivation for set size of 6. Number of lapses is a central measure in sleep research. Sham-sd subjects averaged 6.4 (± 6.1) lapses (here, the absence of a response during a trial within the 4-sec probe window) at the Day 3 test, while in the Baseline test lapses were negligible, with 2 of the 14 Sham-sd subjects having had one lapse (in 128 trials), for a rate per session of 0.14.
As to DMS performance inside the MRI scanner, sleep deprived subjects again showed the median RT slowing, decreased accuracy, and increased lapsing typical of sleep deprivation. The RT and % Correct for Sham-sd (and also Active-sd) groups at Baseline and Day 3 for the DMS task performed during fMRI measurement are shown in Table 1 for the 3 memory set sizes used in the scanner. In ANOVAs on performance for all subjects, there were main effects of Time (Baseline vs. Day 3) for RT (F1,25 = 5.0, P < 0.035), % Correct (F1,25 = 40.1, P < 0.0001), and lapses (F1,25 = 34.5, P < 0.0001). In addition, there was the expected main effect of Set Size (1, 3, or 6 items) for RT (F2,24 = 30.1, P < 0.0001).
Group mRT ± SE and percentage correct ± SE in the DMS task in the MRI scanner during Baseline and Day 3 sessions for the sleep deprived participants
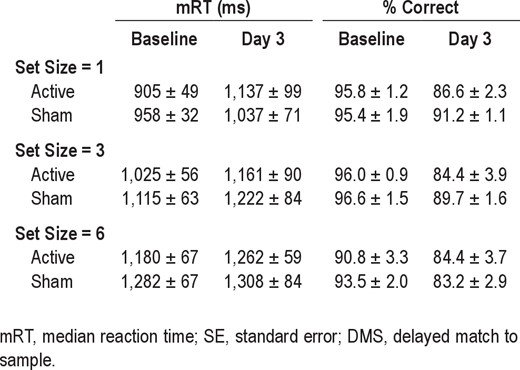
Group mRT ± SE and percentage correct ± SE in the DMS task in the MRI scanner during Baseline and Day 3 sessions for the sleep deprived participants

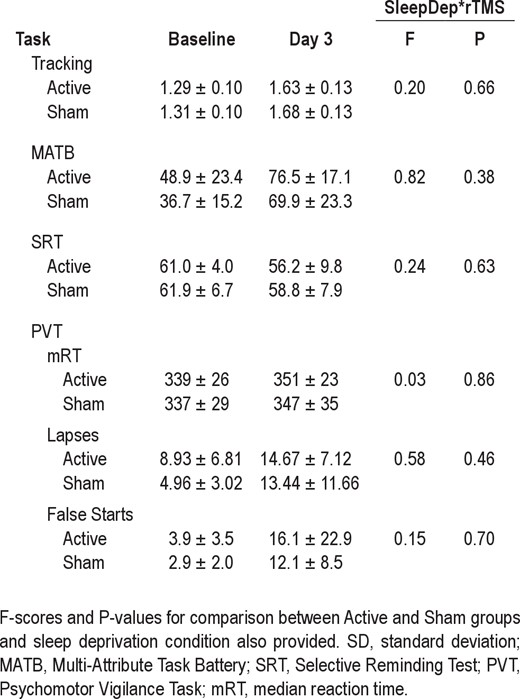
The deleterious effect of sleep deprivation could also be seen in the performance of the other cognitive tasks. The mean scores for both Active-sd and Sham-sd groups in the Tracking, MATB, SRT, and PVT tasks are shown in Table 2. Scores in the tracking task worsened (i.e., increased) from Baseline to Day 3 (F1,25 = 139.9, P < 0.001), as did those in the MAT-B multitasking (F1,25 = 99.5, P < 0.001). Scores also worsened (i.e., decreased) in the verbal SRT (F1,25 = 5.1, P < 0.04). PVT performance also worsened, with a significant increase in the number of lapses (F1,25 = 24.0, P < 0.001) and false alarms (F1,25 = 8.1, P < 0.01). RT did not increase with sleep deprivation, unusual for this task, but given the 4-fold rise in false alarms, subjects may have tried to maintain speed in the face of a deficit in processing caused by sleep deprivation at the expense of accuracy.
Effects of rTMS in Baseline vs. Day 3 comparisons
Active-sd and Sham-sd groups did not differ in measured subjective sleepiness, with Day 3 group ESS means of 12.4 (± 5.4) and 14.3 (± 6.0). There was no Group effect or Time × Group interaction in the ESS. The means of both groups exceeded the cutoff for clinically significant sleepiness (ESS > 10).
There were no significant differences between Active-sd and Sham-sd groups in the Baseline testing session (t25 = 0.7, P < 0.47 for RT and t25 = 1.5, P < 0.15 for % Correct). Beneficial effects of rTMS were seen in Baseline vs. Day 3 comparisons of RT performance and lapsing behavior of the Active-sd and Sham-sd groups during testing sessions out of the scanner (during which no rTMS was applied) and in comparisons with the non- sleep deprived active and sham groups. Beneficial effects of rTMS were also observed in DMS performance during rTMS sessions (see the next section).
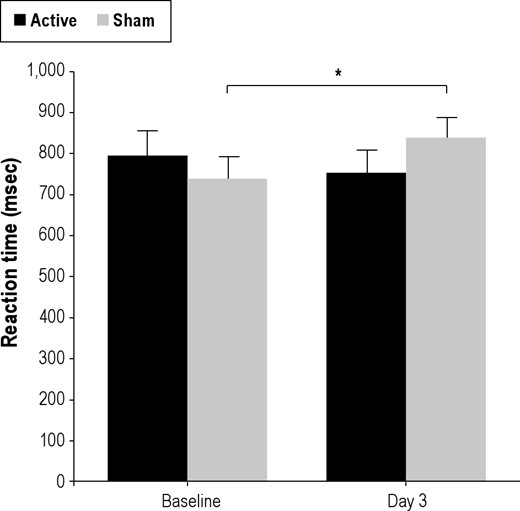
A mixed model ANOVA on median RT with factors of rTMS group (Active-sd, Sham-sd), Time (Baseline, Day 3), and Set Size (1, 6), yielded the expected main effect of Set Size (F1,25 = 118.6, P < 0.0001). There was a significant Time × rTMS Group interaction (F1,25 = 4.2, P < 0.05), a positive result in the critical test of rTMS amelioration of sleep deprivation effects on slowing in the DMS task. As can be seen in Figure 3, this interaction was due to a speeding of RT from Baseline to Day 3 in the Active-sd group and a slowing of RT in the Sham-sd group. In post hoc testing of the contributions of both groups to overall RT (since the interaction did not involve set size) at Baseline and Day 3, the mean RT for the active group did not change (t12 = 1.0, P < 0.19), while RT slowed on Day 3 compared to Baseline for the sham group (t13 = 2.3, P < 0.04, Bonferroni corrected). Thus while the sham group showed slowing in the DMS task typical in sleep deprivation, those who received active rTMS did not show a deficit.
Baseline and Day 3 RTs for Active-sd and Sham-sd groups. The Sham-sd group shows a significant increase in RT (*P < 0.04) after sleep deprivation, while the Active-sd group shows a drop (although nonsignificant) in RT. Bars show mean error.
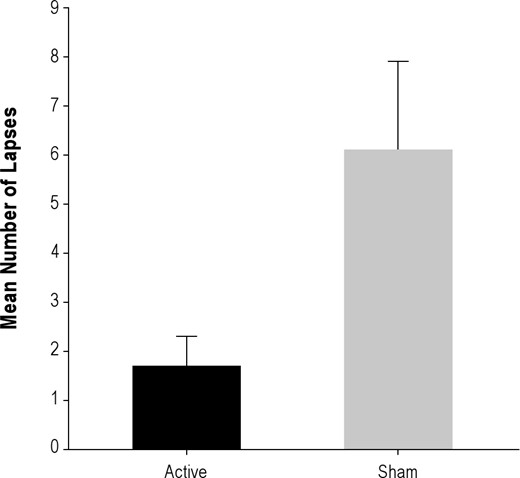
There was also a positive result in the critical test of rTMS amelioration of sleep deprivation effects on lapsing in the DMS task. Active-sd subjects had only a very small number of lapses (mean = 1.7) in the Day 3 session. In fact, 9 of the 13 subjects in the Active group had at most one lapse, with 5 having no lapses at all. This contrasted to the Sham-sd group, who, as reported in the previous section, averaged 6.4 lapses in the Day 3 session, with only 2 of 14 experiencing one or no lapses. The mean lapses are compared in Figure 4, and their difference was significant (t25 = 2.4, P < 0.025).
Mean number of lapses in active and sham groups in the DMS task performed in non-MRI testing sessions on Day 3, at the end of the sleep deprivation period. There were no lapses in either group in pre-sleep deprivation baseline performance.
The ANOVA on % correct responses had a main effect of Time (F1,25 = 7.2, P < 0.02), and no Time × rTMS Group or Time × Set Size interactions: subjects became slightly worse in accuracy in the DMS task due to sleep deprivation. Overall accuracy (percentage correct) decreased slightly from Baseline to Day 3, as indicated in the main effect of Time: 2.2% for the active group, and 3.0% for the sham group. Post hoc analysis showed that only the drop for the sham group was significant (t13 = 2.2, P < 0.05), although the active group did show a trend (t12 = 1.70, P < 0.12). In summary, the sham group demonstrated an overall performance deficit in the task, with both speed and accuracy worsening (and with significantly more lapses than the active group), while the active group maintained performance.
As in the Baseline testing session, there were no significant differences in DMS performance between the Active-sd and Sham-sd groups in the Tuesday scanning sessions (t25 = 0.9, P < 0.39 for RT and t25 = 0.2, P < 0.86 for % Correct). There were also no main effects or interactions of rTMS group for subject performance within the MRI scanner for any of the 3 performance measures. Also, there were no significant differences between Active-sd and Sham-sd groups over time on any of the 4 other tasks (Tracking, MATB, SRT, and PVT), as can be seen in the F- and P-values for the interaction between the TMS group and Time (Pre/Post sleep deprivation) included in Table 2.
TMS Effects in Sleep Deprivation Groups during rTMS Sessions
In order to see if an acute beneficial effect of rTMS in the alternating trials during which rTMS trains were applied might be present after a single night of sleep deprivation rather than after 2 nights, as we had previously demonstrated,8 mixed-model ANOVAs were run on overall RT (preliminary analyses showed no set size differences between active and sham groups), on accuracy, and on lapsing, with a between-group factor of TMS (Active-sd and Sham-sd groups) and repeated measures factor of Session (Sessions 1 through 4). There were no significant RT or % Correct baseline differences between the groups in the first TMS session. Overall RT had increased after one night of sleep deprivation by 83 ms for the Active-sd group, and by almost twice as much (156 ms) in the Sham-sd group, while accuracy did not differ between the groups. However, the RT difference between the groups was not significant. On the other hand, the ANOVA for lapses was significant for Time (F3,75 = 42.1, P < 0.0001) and the Time × TMS interaction (F3,75 = 4.6, P < 0.02). Lapsing grew more for the Sham-sd group compared to the Active-sd group. Between the first and third rTMS sessions, mean lapses grew from 2.8 to 17.6 with active rTMS, and 2.2 to 24.8 with sham, a 41% increase with sham over the active mean, although this difference was not significant. By the fourth session, the difference had become significant, as the mean for the Active-sd group dropped to 15.2, while it grew larger in the Sham-sd group to 31.5 (t25 = 2.7, P < 0.05 Bonferroni corrected).
Effects of rTMS were also sought for in the alternating trials in which no rTMS trains were applied in order to test for evidence of cumulative effects of the rTMS sessions. The same mixed-model ANOVAs used in the previous paragraph were run on overall RT, accuracy, and lapsing, this time for no-TMS trials. Again, there were no significant baseline RT or % Correct differences between the groups in the first session. Overall RT had increased more in the Sham-sd group than the Active-sd group, as it had in the rTMS trials (here, an increase of 45 ms with active rTMS and 112 ms for sham), while accuracy once again did not differ between the groups. However, while it was more than double, this RT difference was not signifi-cant. For lapsing, there again was a significant main effect of Time (F3,75 = 43.7, P < 0.0001) and a Time × TMS interaction (F3,75 = 5.7, P < 0.008). Lapsing increased more for the Sham-sd group than the Active-sd group. Between the first and third rTMS sessions, mean lapses grew from 4.7 to 20.4 with active rTMS, and 3.3 to 28.7 with sham, a 41% increase with sham over the active mean, although this difference was not significant. By the fourth session, the difference had grown significant, as the mean lapses for the Active-sd group dropped to 16.3, while it grew larger in the Sham-sd group to 35.5 (t25 = 2.8, P < 0.05 Bonferroni corrected).
Imaging Analysis Using the MLM Model: Effects of Sleep Deprivation
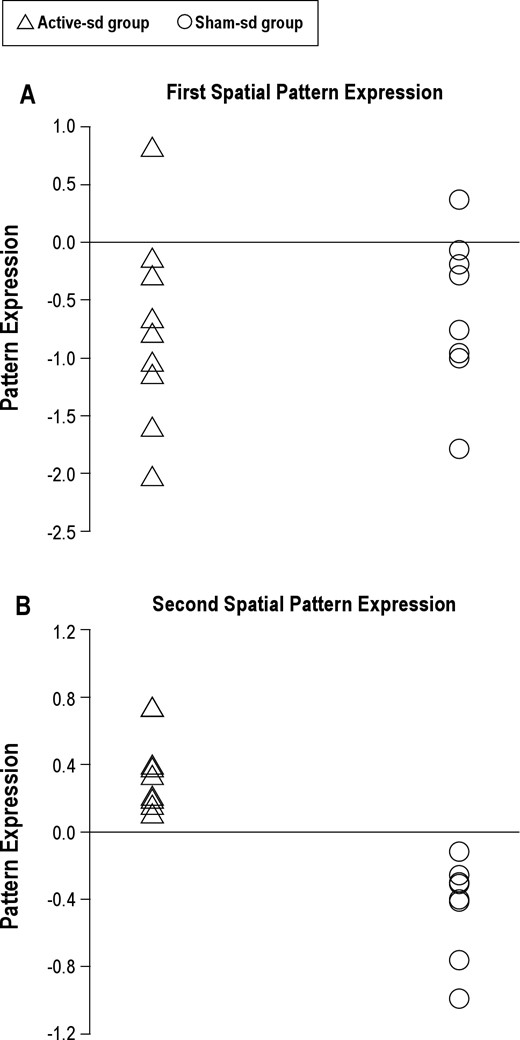
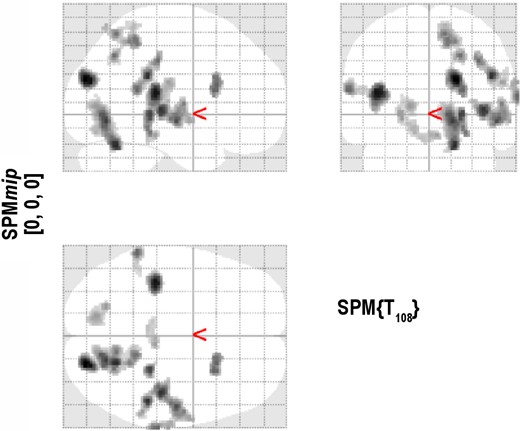
While no significant patterns contrasting Baseline and Day 3 were found during either the stimulus or the retention phase, there were 2 significant spatial patterns in the probe phase data. The first pattern represented the network activation shared by the Active-sd and Sham-sd groups attributable to the effects of sleep deprivation, while the second pattern represented the difference in activation between the two groups. Figure 5 shows the level of expression of the first pattern for the individuals in both the active and sham groups. As displayed in Figure 5A, the first pattern was expressed similarly for both active and sham participants (Kolmogorov-Smirnov Test: P = 0.96). The pattern of activation (see Figure 6 and Table 3) involved a wide range of brain regions including inferior parietal, superior temporal, hippocampal, basal ganglia, thalamus, and postcentral areas. These regions were more expressed during sleep deprivation during the probe phase for both active and sham participants.
(A) The expression of the first spatial fMRI pattern for each subject in the Active-sd group is plotted on the left while the expression of each Sham-sd subject is plotted on the right. (B) Same for subject expression of the second spatial pattern.
The activated regions in the first spatial fMRI pattern. These regions were similarly activated for both the Active-sd and the Sham-sd groups in the Day 3 – Baseline contrast.
Imaging Analysis Using the MLM Model: Effects of rTMS
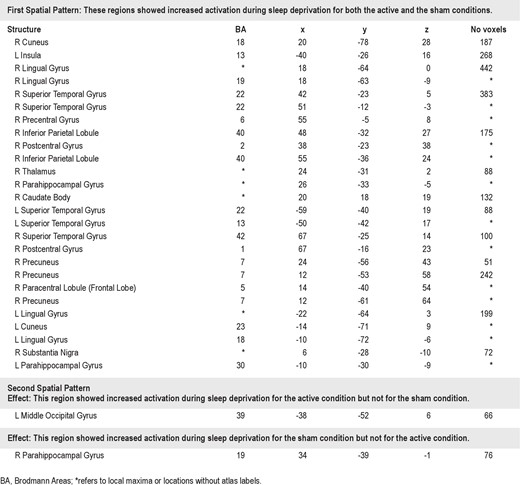
As displayed in Figure 5B, expression of the second pattern was neatly segregated between Active-sd and Shamsd participants (Kolmogorov-Smirnov Test: P = 0.0019). Only 2 areas defined the second pattern: a region in the left lateral occipital cortex near the temporal border which was more expressed after sleep deprivation by the Active-sd group, and a region in the right parahippocampal gyrus, which was more activated by the Sham-sd group (Table 3). As can be seen in Figure 7, the area activated for the Active-sd group was directly beneath the center of the rTMS coil. The targeted area for rTMS is shown as a blue circle, and was chosen from the network found in Ha-beck et al.,6 which resulted in facilitated subject performance in the DMS task when stimulated acutely at the end of 2 days of sleep deprivation in our previous study.8 The region activated was found to be in the same gyrus but located deeper than the targeted area.
Coronal (A), sagittal (C), and transverse (E) sections of a template MRI showing the region (in green) activated in the Active-sd group. The blue circle represents the area targeted by rTMS. B, D, F show the electric field strength distribution computed from three-sphere model (shown as inset in F) and superimposed on the coronal, sagittal, and transverse sections, respectively. L, left.
We used a computational model to estimate the region of direct neural stimulation by TMS relative to the region activated in the active group. Figure 7B, D, and F show the simulated electric field distribution superimposed on structural MRI slices indictaing the target (blue) and fMRI activation (green) regions. The maximum induced electric field in the target region at 100% resting motor threshold is 0.9 V/cm, which is consistent with other estimates of the electric field strength corresponding to TMS motor threshold.33–36 The induced electric field strength at the fMRI activation region is approximately 0.2–0.4 V/cm, which is considered subthreshold. However, subthreshold rTMS at stimulus intensity as low as 60% of active motor threshold has been shown to modulate corticosponal excitability.37 The active motor threshold is 20% lower than resting motor threshold on average.38 Therefore, stimulation at 60% of active motor threshold would correspond to a maximum induced electric field of about 0.4 V/cm in our model, suggesting that direct neuromodulatory effects of rTMS in the activation region are plausible. Furthermore, indirect (transynaptic) activation is also a potential mechanism linking the target region with the activation region.
Experiment 2: Effects of Practice and TMS in a Non-Sleep Deprived State
We performed the second experiment to provide evidence for 2 questions: first, what is the effect on DMS performance of practice alone after a course of 4 sessions without TMS or sleep deprivation, and second, what is the effect of TMS when sleep deprivation is not involved? To answer the first question, repeated measures ANOVAs with factors of Time (Baseline, Day 3), and Set Size (1, 6) were run on median RT and accuracy for the Sham-non-sd group. While there were the expected set size effects for RT (F1,10 = 59.6, P < 0.0001) and percent correct (F1,10 = 20.7, P < 0.002), no significant practice effect (i.e., in main effect for Time or Time × Set Size interaction) was found for either measure. For the second question, mixed model ANOVAs with between group factor of TMS group (Active, Sham) and repeated measures factors of Time (Baseline, Day 3), and Set Size (1, 6) were run on median RT and accuracy. Again, the expected main effects for Set Size were seen (F1,19 = 72.9, P < 0.0001 for RT and F1,19 = 40.8, P < 0.0001 for percent correct) but no main effects or interactions with TMS group were significant, indicating that while 4 sessions of TMS resulted in improvements in DMS performance in sleep deprivation subjects, it did not do so when subjects were not sleep deprived. In addition, as it was with just the Sham-non-sd group, there were no significant effects of practice with the Active-non-sd group included.
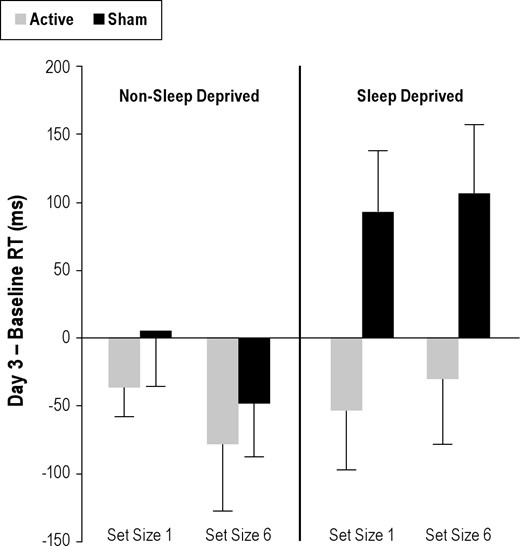
A summary comparison of the effects of TMS for the sleep deprived and non- sleep deprived groups can be seen in Figure 8, which shows their pre-post RT. The Active-sd group had an overall mean RT improvement on Day 3 at the end of sleep deprivation (compared to pre-sleep deprived baseline) of 41 ms. In contrast, the Sham-sd group had slowing of overall mean RT of 99 ms. In the Active-non-sd and Sham-non-sd groups, there were decreases in mean RT between Baseline and Day 3. Although these decreases were not significant, Active-non-sd and Sham-non-sd groups were speeded an average of 57 and 21 ms, respectively.
Difference in mRT (Day 3 – Baseline, non-MRI testing sessions) for non-sleep deprived (on the left) and sleep deprived (on the right) active and sham groups (gray and black, respectively) for set sizes 1 and 6. Positive values indicate relatively slower RTs on Day 3, while negative values indicate speeded responses. Active-sd subjects showed speeded responses similar to non-sleep deprived subjects, while Sham-sd subjects displayed RT slowing typical in sleep deprivation at both set sizes. Bars show mean error.
DISCUSSION
Consistent with our previous report,8 we again found that rTMS to left occipital cortex remediated DMS performance in sleep-deprived individuals. In this study, however, a beneficial cognitive effect of rTMS was demonstrated a full eighteen hours after the last of four sessions of concurrent rTMS/task performance given over the course of two days of sleep deprivation. Sleep deprived subjects receiving active 5 Hz rTMS performed the DMS task similarly to non-sleep deprived controls, while sleep deprived subjects receiving sham rTMS showed all the slowing of RT and lapses typical of sleep deprived individuals.23 The beneficial effect was quite specific to the DMS task, as there were no active/sham group differences in any of the other cognitive tasks. It was also specific to the sleep deprived state, as no active/sham difference was seen in the non-sleep deprived groups (as was also the case in Luber et al.8). Together, these results suggest that rTMS applied over the course of sleep deprivation, coupled with DMS task performance, affected neural circuitry involved in working memory to prevent the full impact of sleep deprivation from occurring.
Additionally, although the study was primarily designed to test for a cumulative rTMS benefit in performance of the DMS task after two days of sleep deprivation, evidence was also found concerning the development of that benefit. In the four rTMS sessions (which occurred on the first non-sleep deprived day and on the second day after one night of sleep deprivation), 5 Hz trains were applied every other DMS trial in order to provide a safe inter-train interval. For both DMS trials with rTMS and trials without rTMS, those in the Sham-sd group exhibited more lapsing than the Active-sd group by the third session, after one night of sleep deprivation, and this difference grew to statistical significance by the fourth session, with the Active-sd group actually showing decreases in lapsing from their previous rTMS session. The Sham-sd group also displayed more slowing in RT compared to the Active-sd group in both types of DMS trials, although these differences did not advance beyond statistical trends, perhaps because of a lack statistical power. It was previously shown (and replicated in the present study) that rTMS to the left occipital target provided no benefit when subjects were not sleep deprived.8 The growing difference between the Active-sd and Sham-sd groups in lapsing as well as the possible slowing after one night of sleep deprivation provide evidence of a growing remedial effect of rTMS over the course of sleep deprivation, although further research is clearly needed. Interestingly, the differences between Active-sd and Sham-sd groups for the non-rTMS trials were similar to those for rTMS trials, with evidence of a beneficial effect of rTMS on DMS performance. In our previous study, no effects of TMS were seen in the non-rTMS trials in a situation in which subjects had undergone two days of sleep deprivation.8 We concluded that the acute effects of rTMS in DMS performance in sleep deprived subjects, seen only in the alternating rTMS trials, were quite brief. Thus, acute TMS effects on non-TMS trials were not expected, and any effects seen in them should be due to some combination of the cumulative effects of rTMS sessions and the degree of sleep deprivation. Although it was not possible to separate the acute effects of rTMS (e.g., seen on Day 3 in Luber et al.8) from the cumulative effects of multiple rTMS sessions performed in the present study, the similarity of effects in nonrTMS and rTMS trials may indicate we were seeing primarily cumulative rTMS effects, and that the acute effects of rTMS had not yet developed after only one day of sleep deprivation.
It should be noted that there was no group difference in DMS behavioral performance in the scanner, and it might be questioned as to why, if Active rTMS benefitted DMS performance in one testing situation, it did not do so in both. In considering this, it is important to remember that (1) there was a signifi-cant difference between Active and Sham groups in the MRI scanner, with only the Active rTMS group showing an increase in activation post- sleep deprivation in a region directly beneath where the TMS coil had been placed; and (2) there were clear performance differences between Active-sd and Sham-sd groups in comparisons of Baseline to Day 3 testing done outside the scanner. There were also significant differences in comparisons with non-sleep deprived groups. The performance enhancements observed fit the context of the experiment, both in that there was RT facilitation in a task designed to be sensitive to RT rather than, say, accuracy, and in that the observed rTMS-related facilitation took the form of negating slowing and lapsing, the two strongest effects of sleep deprivation on cognitive performance. Consideration of the actual performance in and out of the scanner, especially at baseline before sleep deprivation, may explain the discrepancy. A comparison of in-scanner RT (see Table 1) with out-of-scanner performance (see Figure 3) at Baseline shows that, while performing at similar accuracy levels, subjects were about 300 ms (i.e., about 50%) slower at the DMS task during the MRI procedure for both set sizes 1 and 6 than when tested 3.5 hours later in the baseline session even when they were instructed on both occasions to respond as quickly as possible. Also at Baseline, the groups averaged 5.4 lapses, while there were only a total of four lapses in 27 subjects (i.e., an overall mean of 0.15 lapses) for the rTMS procedure. Lapsing should be a rare event when subjects are not sleep deprived, as it was in testing outside the scanner but not inside, and the degree of lapsing in the scanner is worrisome. On Day 3, RTs in the scanner were again about 300 ms slower than out of the scanner (also about twice the size of the active and sham group RT differences found in out-of-scanner sessions). The considerably slower RTs in the scanner at Baseline and Day 3 may have obscured any active and sham group differences. More importantly, both active and sham groups averaged many times the number of lapses in the Day 3 MRI procedure than they did when performing later that day outside the scanner, with the Active-sd group averaging 30.6 lapses and the Sham-sd group 29.7 in the scanner over 90 trials, compared to 1.7 and 6.4 lapses in Active and Sham group in 128 trials when tested outside the scanner 3.5 hours later. This discrepancy in lapses suggests a very different performance context inside the scanner. Moreover, RTs recorded inside the scanner provided a poorer estimate of performance. The high proportion of lapses in the scanner (i.e., averaging one-third of the trials) resulted in fewer trials (about 20/set size, compared with 64/set size out of the scanner), and most likely led to less sensitive and more variable estimates of subject RT compared with the out-of-scanner measures.
The much poorer DMS task performance in the same subjects in the MRI sessions compared to the out-of-scanner test sessions, even in the non-sleep deprived baseline, was probably due to a number of contextual effects. First, the fMRI measurements were done at 08:30, right after a nadir in circa-dian alertness, while the baseline and test DMS for the TMS procedure were done at 12:00, approaching a circadian peak in awareness.39 Also, in the MRI sessions, the task was performed lying down and in darkness, in contrast to being performed in a comfortable sitting position in bright ambient light in the sessions for the TMS procedures. Both lighting conditions40,41 and posture42 affect task performance, especially in the sleep deprived state. In addition, the banging noise of the MRI recording may have been more distracting, especially during sleep deprivation when subjects are already coping with the distraction of fatigue.43,44 Thus, a nadir in alertness due to time of day, lying down in a dark tube, and performing the DMS task simultaneously with distracting banging sounds, all may have contributed to worse performance at Baseline and at the Day 3 test. Moreover, these differences in context may have resulted in performance variability that obscured the facilitatory effects of rTMS found in the Active group when subjects performed the DMS task sitting comfortably in a brightly lit, quiet room at a time when circadian alertness was heightened. Conversely, it is also possible that Day 3 performance facilitation seen in the Active group in the noon testing session may have been in some way context-specific, such that it was only evident when testing in the same room where simultaneous TMS/DMS performance had previously been done. The results here indicate that the rTMS-caused performance enhancements, specific to the DMS task, can be overwhelmed by a different context in which other factors may dominate.
Induction of Extended rTMS Effects
Repetitive rTMS induces brain plasticity effects, where plasticity is defined as neural change lasting beyond the magnetic stimulation.45–47 For example, high-frequency rTMS, such as the 5 Hz stimulation used in the present study, demonstrably increases motor cortex excitability, as reflected in evoked motor potentials, with long term potentiation (LTP) or LTP-like mechanisms thought to underlie these effects.48–50 However, in all previous studies, changes caused by rTMS in healthy subjects have only lasted up to about an hour.45 This is a problem when considering the possible therapeutic use of rTMS, where more lasting changes are desired. The method used in rTMS therapy in depression is to perform repeated sessions of stimulation over a course of weeks, with the expectation that rTMS effects will accumulate over time. It has been found that daily sessions of high frequency rTMS for six weeks was effective in remediating depression.12 In the present study, we implemented this idea of accumulation of effect by having four-hour long rTMS sessions over the two-day course of sleep deprivation.
Another possible method to prolong cognitive effects of rTMS is to strategically use a behavioral task within different phases of rTMS application. According to Thickbroom,14 an rTMS session can be conceived as a four-part procedure: an immediate pre-rTMS period, an application period, an immediate post-application period during which plasticity mechanisms are up-regulated, and a later post-application period when neural excitability has returned to baseline. Having a subject practice a given task associated with a target cortical region prior to stimulation might prime the target network. Performing the task during rTMS application in a time-locked fashion might result in confluent Hebbian activation, potentiating the synapses central to processing the task. Task performance immediately after rTMS application, while the targeted cortical region remains modulated by the stimulation, could continue this neural network training. In the present study, we concentrated on having subjects perform the DMS task during rTMS application. We suggest that the combination of multiple rTMS sessions11 and concurrent rTMS/task performance14 worked together to generate sufficient neuroplasticity and subsequent neural changes in the distributed circuitry involved in processing the DMS task to enable superior cognitive working memory performance in subjects receiving active rTMS a day after the last rTMS session, presumably long after the acute action of rTMS at the local site of stimulation wore off.
This argument is also relevant to the claim made here that the rTMS effects were task-specific. While only the DMS task, and not the others tested at Baseline and Day 3, was used in the four TMS sessions, the fact that we only affected DMS performance, and not that of other cognitive tasks (particularly the PVT, which is generally profoundly affected by sleep deprivation) is an important observation. A TMS pulse affects not only cortex at the site of stimulation, but a network of brain locations, and processing at these other locations may be important for other tasks. Conversely, a single cortical location, such as the one being stimulated, may play a role in a number of different cognitive processes. As such, it cannot be assumed that multiple sessions of TMS applied to a given cortical region would affect only a single task; indeed, it might affect an array of them. This is the first study we are aware of in which the effect of TMS on a cognitive task has persisted into at least the next day, and thus effects on other tasks have not been previously tested. It was assumed that that application of TMS during DMS was causal in preserving DMS performance, but it was not given a priori that one must perform a task while TMS is applied to create a long-lasting TMS effect on that task. It was hoped that applying TMS while subjects performed a specific task that the target region was known to be sensitive to when in a sleep deprived state might affect performance only in that task, due to a proposed Hebbian-like mechanism. The finding that the TMS affect was limited to the DMS task and not others provides new data concerning the possible specificity of longer-lasting TMS effects in a multiple session paradigm and also supports the proposed Hebbian-like mechanism of action.
The Left Lateral Occipital Target Region in Working Memory and Sleep Deprivation
Only the DMS task was facilitated by rTMS, and not any others that were also tested. This provided evidence that visual working memory function, which was not a large component of the other tasks, was being affected rather than some other process or processes (e.g., visual, attentive, motor) that the DMS task shared with other tasks. In our previous work with the DMS task,7,8 we were able to demonstrate the specificity of rTMS effects in relation to rTMS frequency, task phase, and cortical target, as well as to the sleep-deprived state (rTMS to left lateral occipital cortex did not enhance performance in non-sleep deprived subjects). The fact that the targeted rTMS affected only sleep deprived subjects in their DMS performance and not in the other tasks narrows the scope of possible underlying neural processes affected. However, a clear conclusion cannot be drawn from the present study that the rTMS acted directly on general working memory function. This is most likely true if one's conception of working memory only revolves around the executive processes that actively manipulate the contents to be remembered. However, even in Baddeley and Hitch's original model of working memory,51 the function was composed of three elements: a phonological store, a visuo-spatial scratchpad, and a central executive. Modern theories of working memory include both the processes that manipulate contents, most likely centered in prefrontal cortex, and the “stores” where the actual contents are represented, most likely in perceptual and association areas in posterior cortex.52,53
The cortical region targeted in this study was in left lateral occipital cortex near the border with the temporal lobe. This region has been activated in a number of imaging studies of working memory, particularly when verbal stimuli were used (e.g., see the meta-analysis by Wager and Smith).22 It was part of an fMRI network identified using Ordinal Trends covariance analysis which was involved with resilience to sleep deprivation in the DMS task.6 Five Hz rTMS applied to this region caused sleep deprivation-specific performance facilitation both in our previous report8 and in the present study. Other imaging studies using working memory tasks have also reported decreases in lateral occipital activity associated with sleep deprivation.20,21,54,55 It has been suggested that sleep deprivation results in degraded perceptual processing, resulting in a percept generated in the encoding phase that is harder to maintain for comparison in the probe phase of a working memory task.56 Sleep improves the formation of procedural, skill-based memory,57,58 the kind that may have been responsible for the improvement seen in the DMS task in the non-sleep deprived subjects over the course of three days practice. Such procedural memory formation may have involved visual processing in the lateral occipital cortex: perhaps comparison of representations of memory items with the probe letter. Sleep deprivation may have interfered with the consolidation of this particular skill-based learning, while active TMS applied while practicing the task may have had an opposing effect. This scenario may explain why active rTMS subjects did not display the slowing and lapsing expected with sleep deprivation.23
With varying degrees of emphasis, theories of the cognitive effects of sleep deprivation assert that it mainly affects prefrontal and parietal control processes, such that effects are most evident in monotonous or less engaging tasks which require more attention and vigilance than in other tasks in which there is more bottom-up control and less need for continuous monitoring.59 Here, with a procedural memory specific to the DMS task in place (strengthened by rTMS), responses might be subject primarily to bottom-up processing, and thus less subject to lapses and slowing associated with controlled, directed attention.55 This would also explain why the subjects receiving active rTMS differed from those receiving sham only in the DMS task, as the procedural memory would be specific to that task. The above scenario may also be reflected in the relative fMRI activation of the lateral occipital region while performing the DMS task in a sleep deprived state that occurred only in the subjects who had received active stimulation of that region while they practiced the task over the course of the sleep deprivation period. By contrast, the subjects who had received sham stimulation had relatively increased activation in the right parahippocampal gyrus. Intriguingly, the right parahippocampal gyrus has been linked to neural compensation on this type of working memory task in the context of aging.6,20 While controversial, it has been hypothesized that utilization of the parahippocampal gyrus may reflect less reliance on recollection and instead a greater reliance on compensating with a less efficient familiarity-based memory strategy.20,21 In the sleep deprived sham subjects, diminished visual processing, reflected in the reduction of visual cortex activation,55 may have led to a need for more top-down processing, in which case slowing and lapses would be expected.23
This interpretation of the fMRI results supports the idea that differences in sleep deprivation-caused performance deficits in cognitive tasks may result from differences in cognitive reserve,5 in which some individuals are less vulnerable due to greater preexisting neural resources.3 Previous fMRI studies have shown that greater network activation at resting baseline led to less diminishment in both network activation and DMS performance after sleep deprivation61 and that greater network activation before sleep deprivation was correlated with better DMS performance after sleep deprivation.62 This led to suggestions of a cognitive reserve explanation for why some individuals are less cognitively vulnerable to sleep deprivation.3,62 Our own work has been consistent with the cognitive reserve explanation, as we were able to show that individuals who did not sustain activation of a working memory network after two days of sleep deprivation did not sustain DMS performance,6 and in fact rTMS applied to that network remediated their performance deficits in direct relation to their sleep deprivation-related lessening of the network's activation.8 The results of the present study were also consistent with a reserve hypothesis, as it was found that those that received active rTMS had greater activation in a cortical region beneath where the coil had been placed, suggesting that the rTMS had acted on the targeted working memory network to make it more resilient to sleep deprivation—that is, to add to the cognitive reserve of the network. However, there have been two neural mechanisms suggested to be responsible for cognitive reserve.5 One is that in some individuals the network used to perform a cognitive task has greater resilience to insult (e.g., aging or sleep deprivation), and evidence for this mechanism has been found in the studies just mentioned. Another suggested mechanism is that as the brain structures originally responsible for task performance deteriorate, network reorganization can occur as the brain attempts to use other brain regions to support task performance.5 This compensatory mechanism may explain the activation seen in right parahippocampal gyrus in those receiving sham rTMS and represents a possible extension of the cognitive reserve hypothesis in the sleep deprivation domain.
CONCLUSION
This report is the fourth in a series of studies which together illustrate the integrated fMRI-rTMS paradigm and its use both to causally identify functional brain networks underlying brain-behavior relationships and to develop potential brain modulation therapies. Novel aspects of this paradigm include the use of sophisticated covariance methods such as Ordinal Trends Modeling60 and MLM to identify functional networks in imaging data, and the use of fMRI-targeted concurrent rTMS/task performance to modulate plasticity effects over a longer term. One interesting extension of this paradigm centers on the exploration of working memory function in the elderly, a literature that shares similar concepts of cognitive reserve and compensatory processing with that of sleep deprivation.3,5
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Yunglin Gazes for her help with behavioral analysis. This research was supported by a grant from the Defense Advanced Research Projects Agency (DARPA; approved for public release, distribution unlimited), National Institute of Aging (NIA) grant T32 AG00261, and from the National Center for Research Resources (NCRR; grant # UL1 RR024156). Work was performed at Columbia University, New York, NY.





Comments