Published online Nov 6, 2017. doi: 10.5527/wjn.v6.i6.243
Peer-review started: June 28, 2017
First decision: September 4, 2017
Revised: September 12, 2017
Accepted: November 1, 2017
Article in press: November 1, 2017
Published online: November 6, 2017
Atypical hemolytic-uremic syndrome (aHUS) is a rare disease of complement dysregulation leading to thrombotic microangiopathy (TMA). Renal involvement and progression to end-stage renal disease are common in untreated patients. We report a 52-year-old female patient who presented with severe acute kidney injury, microangiopathic hemolytic anemia, and thrombocytopenia. She was managed with steroid, plasma exchange, and dialysis. Kidney biopsy shows TMA and renal cortical necrosis. Genetic analysis reveals heterozygous complement factor I (CFI) mutation. Eculizumab was initiated after 3 mo of presentation, continued for 9 mo, and stopped because of sustained hematologic remission, steady renal function, and cost issues. Despite this, the patient continued to be in hematologic remission and showed signs of renal recovery, and peritoneal dialysis was stopped 32 mo after initiation. We report a case of aHUS due to CFI mutation, which, to the best of our knowledge, has not been reported before in Saudi Arabia. Our case illustrates the challenges related to the diagnosis and management of this condition, in which a high index of suspicion and prompt treatment are usually necessary.
Core tip: Atypical hemolytic-uremic syndrome (aHUS) is a rare disorder. In some cases, complement mutation can be identified. The most common cases involved mutations of genes for complement factor H and membrane co-factor protein, whereas rare cases were linked to complement factor I (CFI) mutation. We here describe the first case of aHUS due to CFI mutation in Saudi Arabia. Our case presented with interesting characteristics including late recovery of kidney failure from dialysis upon initiation of eculizumab and persistent hematologic and renal remissions despite discontinuation of eculizumab.
- Citation: Almalki AH, Sadagah LF, Qureshi M, Maghrabi H, Algain A, Alsaeed A. Atypical hemolytic-uremic syndrome due to complement factor I mutation. World J Nephrol 2017; 6(6): 243-250
- URL: https://www.wjgnet.com/2220-6124/full/v6/i6/243.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i6.243
Thrombotic microangiopathy (TMA) is a life-threatening syndrome characterized by systemic microvascular occlusions, thrombocytopenia, microangiopathic hemolytic anemia, and renal or other end-organ damage[1,2]. TMA may complicate several conditions, such as systemic infections, cancer, pregnancy complications, autoimmune diseases, organ transplantation, and severe hypertension[1].
Atypical hemolytic-uremic syndrome (aHUS) is a rare, progressive, life-threatening form of TMA caused by a disorder in complement regulation, predominantly dysregulation of the complement alternative pathway[3,4]. The undelaying pathogenesis is complement gene mutations in the majority of patients and antibodies to complement factor H (CFH) in a smaller subset of patients (approximately 6%)[5-7]. Several cases of aHUS had been reported at different age groups in association with mutations of genes for complement factor H (CFH)[8-10], membrane co-factor protein (MCP)[8,11], and complement factor I (CFI)[8,12,13]. The most frequent mutation detected involves CFH (approximately 30%), then MCP (approximately 10%), and lastly CFI (approximately 5%)[12]. Although approximately 80% of patients present acutely with thrombocytopenia, microangiopathic hemolytic anemia, and renal impairment[14,15], the onset may be more gradual in other patients[16]. Because of systemic vascular involvement[17], extrarenal manifestations occur in up to 48% of patients, with frequent neurologic and cardiovascular involvement[6,18-20].
Untreated aHUS has been linked to high morbidity and mortality, with a 33%-40% risk of progression to end-stage renal disease (ESRD) or death during the first clinical manifestation[6]. Patients treated with plasma exchange remains to have a poor pro-gnosis, with up to 65% experiencing permanent renal damage, ESRD progression, or death within 1 year of diagnosis[8].
The understanding of the disease pathogenesis, which involves activation of alternative complement pathway, resulting in the formation of membrane attack complex (MAC) C5b-9[3], had contributed to the advances in treatment, which is eculizumab, a monoclonal IgG antibody that binds to C5 and prevents subsequent formation of terminal complement[21]. Eculizumab has significantly decreased the risk of ESRD in adult patients with aHUS from 60%-70%[6,14] to approximately 15%-20%[22-24].
Globally, CFI mutation had been rarely reported in association with aHUS, and to our knowledge, this is the first case reported in Saudi Arabia. Our case highlights important challenges related to the diagnosis and treatment of this rare disorder.
A 52-year-old Saudi female, with hypothyroidism on
| Laboratory parameter | Before admission (Nov 25, 2012) | Day of admission (Nov 28, 2012) |
| Hematologic tests | ||
| Complete blood count | ||
| WBCs (normal, 4-10 × 109/L) | 14.4 | 14.1 |
| Hemoglobin (normal, 12-15 g/dL) | 11.8 | 8.9 |
| Platelets (normal, 150-400 × 109/L) | 399 | 103 |
| Coagulation profile (s) | ||
| PT (normal, 11-14) | 12 | 10 |
| INR (normal, 0.9-1.2) | 0.9 | 0.9 |
| PTT (normal, 20-40) | 24 | 29 |
| Schistocytes on peripheral blood film | Not done | Yes |
| Serum biochemical tests | ||
| Renal function tests (mmol/L) | ||
| Sodium (normal, 135-145) | 142 | 129 |
| Potassium (normal, 3.5-5.5) | 3.9 | 4.1 |
| Bicarbonate (normal, 22-28) | 15 | 11 |
| Chloride (98-106) | 110 | 99 |
| Urea (normal, 2.1-7.1) | 3.2 | 19 |
| Creatinine (normal, 62-106) | 54 | 773 |
| Liver function tests | ||
| Total bilirubin (normal, 5-20 mmol/L) | 17.1 | 25.8 |
| AST (normal, 0-40 U/L) | 17 | 142 |
| ALT (normal, 0-30 U/L) | 18 | 240 |
| ALP (normal, 40-129 U/L) | 104 | 90 |
| GGT (normal, 8-65 U/L) | 21 | 18 |
| Albumin (normal, 35-50 g/L) | 42 | 29 |
| LDH (normal, 140-280 U/L) | 273 | 3377 |
Three days later (November 28, 2012), she returned to the emergency department with similar complaints, along with nausea, vomiting, shortness of breath, and reduced urine output for 2 d. She had no fever, joint pain, hematuria, or diarrhea. A systemic review was otherwise unremarkable. She is a housewife and mother of five healthy kids. Family history was negative for any hematologic, rheumatologic, malignant, or familial disorders. There was no significant history of renal disease in the family. On examination, she was conscious and oriented but pale. She had no signs of respiratory distress. She was afebrile, with blood pressure of 149/70 mmHg and normal heart rate. She had bilateral lower limb edema and bruises over her abdomen and lower limbs. Abdominal palpation was tender on the left flank and lumbar areas. Examination of other systems was unremarkable. Laboratory investigations showed persistent leukocytosis, a newly developing anemia, thrombocytopenia, and acute derangement in renal and liver function tests (Table 1) (serum creatinine: 773 µmol/L; normal: 62-106 µmol/L); C-reactive protein: 239 mg/L; normal: 0.3-5.0 mg/L); erythrocyte sedimentation rate, 64 mm/h); lactic acid: 0.9 mmol/L, normal: 0.7-2.1 mmol/L). As shown in Table 1, the presence of anemia, thrombocytopenia with elevated total bilirubin, and lactate dehydrogenase (LDH) alerted the treatment team to observe for hemolysis. Peripheral blood smear revealed significant schistocytes, whereas the result of the direct antiglobulin test was negative. Urinalysis was positive for protein, red blood cells, and leukoestrase and was negative for nitrite. Complement (C3 and C4) levels, autoantibody panel, antiphospholipid antibody panel, hepatitis serology, and tumor markers were all negative.
Because of the presence of microangiopathic features and acute kidney injury, hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura was considered, and she was started on hemodialysis along with daily plasma exchange and pulse steroid. Methylprednisolone was given for 3 d, and then oral prednisolone was started and then gradually tapered. She was treated for possible urinary tract infection with intravenous piperacillin-tazobactam 2.25 g every 8 h, and her vaginal bleeding was treated with medroxyprogestrone injections. Her platelet count improved with plasma exchange and steroids but started to decrease, and thus, diagnostic kidney biopsy was performed. The biopsy was complicated with left renal subcapsular hematoma and retroperitoneal bleeding. The bleeding was successfully controlled with embolization; how-ever, the procedure also revealed bilateral renal vein thrombosis. During this bleeding episode, plasma exchange was intermittently stopped because of rapidly decreasing platelet count and increasing LDH, which necessitated reinstitution of plasma exchange with fresh frozen plasma infusion. The dose of plasma exchange was increased from 1 to 1.5 plasma volumes daily.
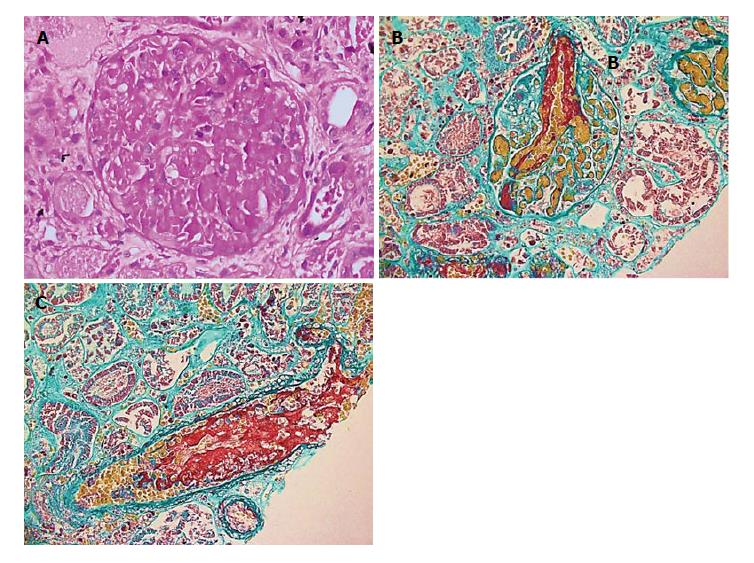
Renal biopsy showed findings of renal cortical necrosis, TMA, and acute tubular necrosis (15 glomeruli, none with global sclerosis or significant tubular atrophy/interstitial fibrosis, arteriosclerosis, or hyaline arteriolosclerosis). Immunofluorescence was negative, and electron microscopy was negative for immune deposits, but it showed significant subendothelial widening with flocculent material and areas of organized structures resembling fibrin. There was foot process effacement involving short segments 10%-15% (Figure 1).
The patient also underwent endometrial bio-psy, which only showed necrotic decidua with no fetal elements. Bone marrow aspirate showed normocellular active bone marrow with trilineage hematopoiesis. Mammogram and bone scan results were unremarkable. For the hemodialysis and plasma exchange, a right internal jugular tunneled cuffed catheter was inserted; however, shortly after, she developed exit-site infection due to Pseudomonas, but it was successfully treated with ciprofloxacin. Despite plasma exchange, steroids, and antibiotics, the patient remained anuric, dependent on dialysis, and with fluctuating platelet count. Because of logistical reasons and the patient’s preferences, she was shifted from hemodialysis to peritoneal dialysis (PD). After control of bleeding and improvement of her platelet count, she was started on systemic anticoagulation using warfarin.
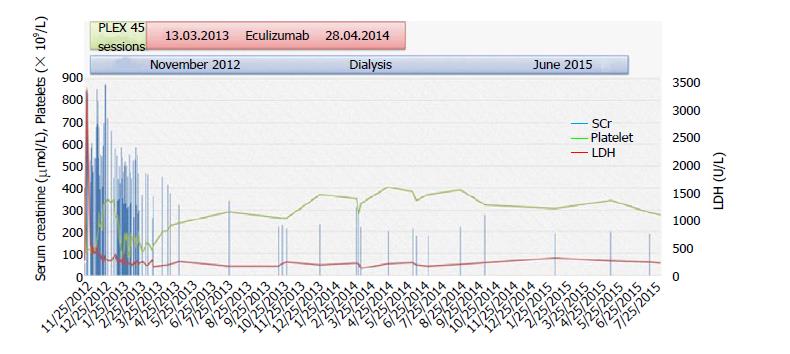
The diagnosis of aHUS was considered because the patient had a refractory picture of TMA with renal failure. Treatment with eculizumab was decided, as it is the only FDA-approved drug for this disorder. Molecular genetic analysis of known genes for aHUS and related disorders by next-generation sequencing was performed. The result of the molecular testing showed that the patient carries a heterozygous CFI variant, c.944G > A (p.Arg315Lys), which was the possible cause of her disorder. During admission, because of the unavailability and high cost of eculizumab, she received 46 sessions of plasma exchange (once a day initially, then reduced to three times weekly, twice weekly, once weekly, and then every 2 wk). She received meningococcal vaccine. Eculizumab was started on March 1, 2013, with induction dose of 900 mg weekly for 4 wk, 1200 mg on week 5, and then maintenance dose of 1200 mg every 2 wk. She tolerated the treatment fairly well and her condition improved, and she was discharged on April 9, 2013. Eculizumab was continued in the outpatient setting until November 2013 and was stopped because of sustained hematologic remission, steady renal function, and cost issues.
In March 2014, she was admitted with PD-related peritonitis due to coagulase-negative Staphylococcus aureus, which responded to intraperitoneal van-comycin. Eculizumab was restarted. In April 2014, she remained anuric and dependent on dialysis, but she was in hematologic remission and eculizumab was discontinued due to cost issue. Systemic anticoagulation was also stopped, as she had completed more than 6 mo of treatment.
In June 2015, during routine follow-up in the PD clinic, she reported improving urine output, as she was oligoanuric until then, with normal platelet count and stable renal function [white blood cells (WBC): 4.6 × 109/L; hemoglobin: 11.6 g/dL; platelets: 248 × 109/L; urea: 3.6 mmol/L; creatinine: 174 µmol/L; 24-h urine collection creatinine clearance: 24 mL/min]. PD was stopped, and her kidney function remained stable with no uremic symptoms, and thus her PD catheter was removed. She was last seen in the clinic on February 2017 with no symptoms, and her laboratory investigations are as follows: WBC: 6.1 × 109/L; hemoglobin: 11.8 g/dL; platelets: 204 × 109/L; urea: 9.3 mmol/L; creatinine: 195 µmol/L; sodium: 141 mmol/L; potassium: 4.4 mmol/L; bicarbonate: 18 mmol/L; LDH 206 U/L. She continues to be healthy and in hematologic remission with stable kidney function without the need for dialysis, eculizumab, or anticoagulation. The course of events is summarized in Figure 2.
We reported a case of a middle-aged woman with aHUS due to rare CFI mutation presenting with severe acute kidney injury. She required dialysis and remained dialysis-dependent for 32 mo. Eculizumab treatment was associated with complete hematologic remission. Interestingly, she is now off dialysis and continues to be in remission despite not taking eculizumab for more than 3 years. There are limitations and difficulties related to the management of this case. Most importantly, the time needed to confirm the diagnosis of complement factor mutation was significant due to lack of a laboratory facility for this test. In addition, eculizumab cost and availability was a limitation against prompt and continued use. Accordingly, these might have delayed the institution of appropriate treatment and impacted the clinical response and renal recovery.
The human complement cascade can be activated through three different pathways: The classical pathway, the lectin pathway, and the alternative pathway. CFH and MCP are regulatory proteins of the alternative pathway[25]. Human CFI is a serine proteinase that cleaves the α chain of C3b and plays a key role in the inhibition of the alternative pathway amplification loop, which generates C3 convertase from C3b[26]. Several familial and sporadic cases of aHUS were linked to mutations in these regulatory proteins of alternative pathway. Initially, the two complement alternative pathway proteins, CFH and MCP (CD46), have been identified to foster the development of aHUS[10,27-30]. More recently, genetic analysis in patients with aHUS with no factor H mutation demonstrated presence of CFI mutations[31]. In their novel description, Fremeaux-Bacchi et al[31] identified two heterozygous CFI mutations leading to a stop codon that are associated with functional deficiency in CFI. Later, more cases had been reported, and currently, CFI mutations account for 4%-10% of all cases of aHUS[32]. The clinical outcomes are characterized by high rate of renal failure, end-stage renal disease (ESRD), post–kidney transplant recurrence, and graft loss.
In a report by Waters and Licht[32,33] in 2010 and a previous cases series by Bell et al[33] in 1991, 33 cases of aHUS with complete or functional deficiency of CFI were described. The age ranges from the time of birth to 57 years, with a mean age of 19 years. Using the varying follow-up data available and before the introduction of eculizumab, the following outcomes were noted among the 33 cases: Two deaths, 74% rate of ESRD, 48% of whom underwent kidney transplantation with a recurrence rate of 82% and very high rate of graft loss after recurrence (up to 100% after first graft). Re-transplantation is associated with recurrence. The response to immunotherapy including FFP infusion with or without plasma exchange was not consistent in cases where it was reported.
Similar to patients described in literature, our patient presented with severe renal failure requiring dialysis and had remained dependent on dialysis for 32 mo. With eculizumab introduction, she developed sustained hematologic remission and later recovered some kidney function and is currently off dialysis for 18 mo, with a serum creatinine level of approximately 200 µmol/L. Because of the unavailability and high cost of eculizumab, it was not provided for the past 3 years, but despite this, she remained in hematologic remission and off dialysis.
Cases with CFI mutation manifest increased susceptibility to infection with encapsulated or-ganisms. Such susceptibility is linked to functional C3 deficiency as a result of uncontrolled activation of the alternative com-plement pathway[34]. Our patient received meningococcal vaccine upon confirmation of the diagnosis of CFI mutation. She developed one episode of hemodialysis catheter–related exit-site Pseudomonas infection and one episode of PD-related staphylococcal peritonitis; however, apart from this, no exceptionally increased rate of infections with encapsulated organisms was noted. The susceptibility to infections in CFI mutations might be more prominent with complete CFI deficiency than with heterozygous mutation. Additionally, the late-age presentation and continued remission despite being off eculizumab in our patient may suggest a less severe genetic defect.
Similar to our patient, several cases of eculizumab discontinuation had been described. The largest case series reported the development of TMA in 5 (31%) of 16 patients following discontinuation[35,36]. Those patients had received eculizumab for a median of 4.3 (range, 0.5-14.4) mo. In those 5 patients, relapse was identified, using regular home urine dipstick testing, within 6 mo of the last eculizumab dose. Eculizumab therapy was restarted, and it resulted in rapid improvement in serum creatinine levels and proteinuria.
In an attempt to address the possibility and safety of eculizumab discontinuation, Macia et al[37] studied the demographics, disease characteristics, and outcomes of aHUS cases in whom eculizumab was discontinued. The post-discontinuation follow-up ranged from few days to 17 mo. According to the data collected from cases reports, TMA developed in 16 (31%) of 52 cases who discontinued the drug after multiple doses and in 4 (80%) of 5 cases who discontinued the drug after a single dose. TMA development was found to be higher with a single dose of eculizumab, and hence, current evidence does not support single-dose eculizumab.
According to the data collected from eculizumab clinical trials, TMA developed in 12 (20%) of 61 patients during a median post-discontinuation follow-up of 24 wk[37]. The median time to TMA development was 13 (range, 4-127) wk. Three patients progressed to ESRD. The authors compared patients who experienced relapse (n = 12) to those who did not (n = 49) post-dis-continuation across several demographic and disease characteristics. No particular trend to predict TMA development was noted with regard to sex, identified complement mutation or antibody, time from diagnosis to start of eculizumab, duration on eculizumab treatment, kidney function and dialysis (at the time of initial treatment and discontinuation), and kidney status (native versus transplant)[37]. However, compared with patients who experienced no relapse, there was a trend to a lower median age (19.5 vs 27 years) and a higher proportion of CFH mutation (42% vs 18%) among those who experienced relapse. It had been noted that the use of functional tests of complement activity and measurement of complement proteins to predict development of TMA is of limited value[37]. The authors concluded that the development of TMA following eculizumab discontinuation is unpredictable.
To our knowledge, this is the first case of aHUS due to heterozygous CFI variant c.944G > A (p.Arg315Lys) reported in Saudi Arabia. Several lessons can be learned from our case and similar cases. Although rarely reported, it is important to keep a high index of suspicion for such severe disease when acute kidney injury presents with unexplained microangiopathic anemia. In such presentations, the severity of renal disease, lack of significant neurologic symptoms, and absence of diarrhea make aHUS the likely clinical diagnosis. In addition, a renal benefit with discontinuation of dialysis was possible in our case despite relatively late initiation of eculizumab and prolonged period of dialysis-dependent kidney disease. Lastly, although eculizumab discontinuation was possible in our patient and seems to be safe in some other reported cases during the reported follow-up periods, predicting the risk of TMA development remains difficult. Therefore, informed decision-making requires a more comprehensive collection of data on eculizumab discontinuation and finding a more sensitive tool to monitor complement activation and disease activity.
A middle age woman who presented with acute kidney injury (AKI) and features of thrombotic microangiopathy (TMA).
TMA, most likely atypical hemolytic uremic syndrome.
Causes of TMA with AKI: hemolytic uremic syndrome, thrombotic thrombocytopenic purpura (primary and secondary causes).
Thrombocytopenia, elevated lactate dehydrogenase, schistocytes on peripheral blood film, acute kidney injury with normal coagulation profile and complement factor I mutation on genetic testing.
Computer tomography to exclude underlying malignancy as secondary causes.
Renal biopsy showing features of TMA with renal cortical necrosis, and acute tubular necrosis.
Plasma exchange, dialysis and eculizumab.
Previous cases of aHUS showing remission with initiation of eculizumab and maintenance of remission despite its discontinuation.
AKI: Acute kidney injury; aHUS: Atypical hemolytic uremic syndrome; CFH: Complement factor H; CFI: Complement factor I; MCP: Membrane co-factor protein; PLEX: Plasma exchange; TMA: Thrombotic microangiopathy; TTP: Thrombotic thrombocytopenic purpura.
aHUS is a serious diagnosis that requires a high index of suspicion in cases presenting with unexplained AKI associated with microangiopathy. Renal benefit of eculizumab may be seen even with late initiation of the drug.
The authors acknowledge and thank the staff of the Pathology Laboratory for their contribution in obtaining the renal biopsy slide pictures and the staff of the Laboratory Department for their contribution in tracking the full genetic analysis report, which was done outside the country.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and Nephrology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang CC, Watanabe T S-Editor: Cui LJ L-Editor: A E-Editor: Zhao LM
| 1. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 698] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 2. | Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 877] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 3. | Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 906] [Cited by in F6Publishing: 846] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 4. | Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 420] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Kavanagh D, Goodship TH. Update on evaluating complement in hemolytic uremic syndrome. Curr Opin Nephrol Hypertens. 2007;16:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 679] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 7. | Dragon-Durey M-A, Loirat C, Cloarec S, Macher M-A, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V. Anti–factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Pérez-Caballero D, González-Rubio C, Gallardo ME, Vera M, Lopez-Trascasa M, de Córdoba SR, Sánchez-Corral P. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet. 2001;68:478-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, Porrati F, Gamba S, Remuzzi G; International Registry of Recurrent and Familial HUS/TTP. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362:1542-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Kavanagh D, Goodship TH, Richards A. Atypical haemolytic uraemic syndrome. Br Med Bull. 2006;77:5-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Kavanagh D, Kemp EJ, Mayland E, Winney RJ, Duffield JS, Warwick G, Richards A, Ward R, Goodship JA, Goodship TH. Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:2150-2155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey M-A, Ngo S, Moulin B, Servais A, Provot F, Rostaing L. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8: 554-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 15. | Noris M, Remuzzi G. Hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:1035-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Campistol JM, Arias M, Ariceta G, Blasco M, Espinosa L, Espinosa M, Grinyó JM, Praga M, Torra R, Vilalta R. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrología (Engl Ed). 2015;35:421-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 40] [Reference Citation Analysis (0)] |
| 17. | Nayer A, Asif A. Atypical hemolytic-uremic syndrome: a clinical review. Am J Ther. 2016;23:e151-e158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Geerdink LM, Westra D, van Wijk JA, Dorresteijn EM, Lilien MR, Davin J-C, Kömhoff M, Van Hoeck K, van der Vlugt A, van den Heuvel LP. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27:1283-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Neuhaus TJ, Calonder S, Leumann EP. Heterogeneity of atypical haemolytic uraemic syndromes. Arch Dis Childhood. 1997;76:518-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Sellier-Leclerc A-L, Fremeaux-Bacchi V, Dragon-Durey M-A, Macher M-A, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18:2392-2400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389-1401. [PubMed] [Cited in This Article: ] |
| 22. | Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, Menne J, Minetti EE, Provôt F, Rondeau E. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 23. | Legendre CM, Licht C, Muus P, Greenbaum L, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169-2181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1034] [Cited by in F6Publishing: 1015] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 24. | Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 25. | Walport MJ. Complement. N Engl J Med. 2001;344:1058-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2152] [Cited by in F6Publishing: 2093] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 26. | DiScipio R. Ultrastructures and interactions of complement factors H and I. J Immunol. 1992;149:2592-2599. [PubMed] [Cited in This Article: ] |
| 27. | Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Müslümanogğlu MH, Kavukcu S, Filler G, Pirson Y. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003;100:12966-12971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Rougier N, Kazatchkine MD, Rougier J-P, Fremeaux-Bacchi V, Blouin J, Deschenes G, Soto B, Baudouin V, Pautard B, Proesmans W. Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9:2318-2326. [PubMed] [Cited in This Article: ] |
| 29. | Thompson R, Winterborn M. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981;46:110. [PubMed] [Cited in This Article: ] |
| 30. | Ying L, Katz Y, Schlesinger M, Carmi R, Shalev H, Haider N, Beck G, Sheffield VC, Landau D. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 1999;65:1538-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Fremeaux-Bacchi V, Dragon-Durey M, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet. 2004;41:e84. [PubMed] [Cited in This Article: ] |
| 32. | Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011;26:41-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: clinical experience in 108 patients. N Engl J Med. 1991;325:398-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 539] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Vyse TJ, Morley BJ, Bartók I, Theodoridis EL, Davies KA, Webster A, Walport MJ. The molecular basis of hereditary complement factor I deficiency. J Clin Invest. 1996;97:925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis. 2015;66:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Ardissino G, Testa S, Possenti I, Tel F, Paglialonga F, Salardi S, Tedeschi S, Belingheri M, Cugno M. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64:633-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | Macia M, de Alvaro Moreno F, Dutt T, Fehrman I, Hadaya K, Gasteyger C, Heyne N. Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J. 2017;10:310-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |