Revised: May 15, 2014
Accepted: June 27, 2014
Published online: August 6, 2014
The slit diaphragm bridging the neighboring foot processes functions as a final barrier of glomerular capillary wall for preventing the leak of plasma proteins into primary urine. It is now accepted that the dysfunction of the sit diaphragm contributes to the development of proteinuria in several glomerular diseases. Nephrin, a gene product of NPHS1, a gene for a congenital nephrotic syndrome of Finnish type, constitutes an extracellular domain of the slit diaphragm. Podocin was identified as a gene product of NPHS2, a gene for a familial steroid-resistant nephrotic syndrome of French. Podocin binds the cytoplasmic domain of nephrin. After then, CD2 associated protein, NEPH1 and transient receptor potential-6 were also found as crucial molecules of the slit diaphragm. In order to explore other novel molecules contributing to the development of proteinuria, we performed a subtraction hybridization assay with a normal rat glomerular RNA and a glomerular RNA of rats with a puromycin aminonucleoside nephropathy, a mimic of a human minimal change type nephrotic syndrome. Then we have found that synaptic vesicle protein 2B, ephrin-B1 and neurexin were already downregulated at the early stage of puromycin aminonucleoside nephropathy, and that these molecules were localized close to nephrin. It is conceivable that these molecules are the slit diaphragm associated molecules, which participate in the regulation of the barrier function. These molecules could be targets to establish a novel therapy for nephrotic syndrome.
Core tip: The slit diaphragm located between neighboring foot processes of a glomerular podocyte functions as a final barrier to retain plasma proteins. Recently several molecules such as nephrin and podocin were identified as functional molecules of the slit diaphragm. However, the precise molecular compositions of the slit diaphragm are still unclear and the mechanism regulating its barrier function is not fully understood yet. Recently we have reported that synaptic vesicle protein 2B, ephrin-B1 and neurexin are expressed in podocyte and the decreased function of these molecules participates in the initiation of proteinuria. These molecules could be targets for a novel therapy for proteinuria.
- Citation: Fukusumi Y, Miyauchi N, Hashimoto T, Saito A, Kawachi H. Therapeutic target for nephrotic syndrome: Identification of novel slit diaphragm associated molecules. World J Nephrol 2014; 3(3): 77-84
- URL: https://www.wjgnet.com/2220-6124/full/v3/i3/77.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i3.77
A glomerular capillary wall preventing the leak of plasma proteins is consisted of three layers: an endothelial cell, a glomerular basement membrane, and a glomerular epithelial cell (podocyte). Podocyte is characterized as its highly sophisticated shape with the primary processes and the interdigitating secondary processes. The secondary process is called as a foot process[1]. The interdigitating foot processes are bridged by a structure called slit diaphragm. In 1988, Orikasa et al[2] of our group reported that the murine monoclonal antibody recognizing the extra-cellular site of the slit diaphragm caused massive proteinuria if injected into rats. The finding clearly indicated that the slit diaphragm is one of the essential structures of the barrier of the glomerular capillary wall[2]. In 1998, Kestilä et al[3] found the responsible gene for the Finnish type congenital nephrotic syndrome, and reported that its gene product, they called nephrin, was an extracellular component of the slit diaphragm[3,4]. Podocin was identified by Bout et al[5] as a protein coded by the responsible gene for familial steroid-resistant nephrotic syndrome in 2000. Following nephrin and podocin, CD2 associated protein (CD2AP), NEPH1 and canonical transient receptor potential-6 (TRPC6) were identified as crucial molecules of the slit diaphragm[6-10]. Several studies showed that the functional loss of these molecules participated in the initiation of proteinuria in acquired glomerular diseases[11-18]. It is now accepted that the slit diaphragm is a final barrier of glomerular capillary wall preventing proteinuria[19-23]. To explore the targets for the novel therapy of proteinuria, the subtraction hybridization assay was done with a normal rat glomerular cDNA and cDNA of rats showing proteinuria. We identified some molecules downregulated at proteinuric states. In this article, first we review the characteristics of the critical slit diaphragm molecules previously reported, and then we introduce the novel slit diaphragm-associated molecules, synaptic vesicle protein 2 (SV2) B, ephrin-B1 and neurexin.
Zonula occludens-1 (ZO-1) was originally identified as a molecule of the tight junction[24]. ZO-1 belongs to the membrane-associated guanylate kinase homologue (MAGUKs)[25]. It is reported that ZO-1 was expressed at the slit diaphragm in podocyte[26]. ZO-1 is the first protein reported to constitute the slit diaphragm. Splicing variants, ZO-1 α+ and ZO-1 α- which lacks motif α have been reported[27]. Both are expressed in tight junctions of the tubular epithelial cells, but only ZO-1α- is expressed at the slit diaphragm[28].
Nephrin is a product of gene mutated in Finish type congenital nephrotic syndrome[3]. Nephrin is now accepted as the most important component of the slit diaphragm. Nephrin is a transmembrane protein of 1241 amino acid residues of the immunoglobulin super family. Nephrin contains eight Ig-like modules and a single fibronectin type III module. The nephrin homologues of mouse[29] and rat[12,30] were cloned. Rat nephrin has 82.2% homology to human nephrin. We have shown the anti-slit diaphragm antibody previously reported, which cause proteinuria if injected into rat, binds the extracellular site of rat nephrin[12,31,32], indicating that nephrin is an essential slit diaphragm molecule.
Podocin was found as a protein coded by NPHS2, the responsible gene of autosomal recessive steroid-resistant nephrotic syndrome. Podocin is reported to bind nephrin and is accepted to be a slit diaphragm molecule[5]. Podocin is a 42 kDa protein with a single transmembrane domain. Because immunoelectron microscopic study demonstrated that both N- and C- termini were in cytoplasm, podocin is considered to have a hairpin-like structure[33]. Podocin homologues of rat and mouse were cloned. Identity between rat and mouse, mouse and human, rat and human, are 92.7%, 86%, 84.3%, respectively[14]. It is demonstrated that podocin interacts with nephrin and CD2AP[34]. It is reported that podocin is a raft-associated component of the slit diaphragm and to serve a scaffolding function.
CD2AP is understood to be one of critical molecules of the slit diaphragm[6]. CD2AP was originally reported to be an adaptor protein binding the cytoplasmic domain of CD2, a membrane protein on natural killer cell and T cell[35]. CD2AP is an 80 kDa protein containing an actin-binding site at the N terminus. CD2AP bound nephrin and anchored nephrin to the cytoskeleton[6]. It is reported that mice lacking CD2AP exhibit loss of foot process, a nephrotic range proteinuria and advance renal failure. It is reported that a mutation of the gene for CD2AP were detected in two human patients with focal segmental glomerulosclerosis (FSGS)[36].
NEPH1 is a nephrin associated protein identified by a gene trap method[7]. NEPH1 has five extracellular immunoglobulin-like domains[7]. NEPH1 interacts with C-terminal domain of podocin[8], ZO-1 and nephrin[37]. The foot processes effacement and proteinuria were detected in NEPH1 knockout mice. All NEPH1 knockout mice died before 8 week of age[7], indicating that NEPH1 is an essential molecule in podocyte.
In 2005, it was reported that the mutation in TRPC6 channel can cause familial FSGS[9,10]. TRPC6 belongs to the transient receptor potential superfamily of non-selective cation channels. TRPC6 is understood to be a receptor-operated channel leading to the influx of calcium in response to phospholipase C-mediated signals[38]. Immunoelectron microscopy study showed TRPC6 localized at major processes, foot processes and at the slit diaphragm[9,10,18,39]. TRPC6 is reported to be colocalized with nephrin, podocin, and CD2AP. In addition, nephrin and podocin are co-immunoprecipitated with TRPC6 in cultured podocyte[10]. Winn et al[9] reported that the mutation of TRPC6, proline-to-glutamine substitution at position 112 (P112Q) which is detected in patients of familiar FSGS leads to both increased amplitude and duration of calcium influx in the over expression system. Reiser et al[10] showed that R895C and E897K, other TRPC mutants detected in the patients, displayed increased current amplitude in the system with HEK293 cells. It is understood that the FSGS-associated mutations could lead to be a gain-of-function alteration in activity and thus increased calcium influx. Recently, Eckel et al[40] showed that albuminuria caused by continuous injection of angiotensin II was significantly less in TRPC-deficient mice than in wild type mice and discussed that TRPC6 promotes albuminuria by promoting angiotensin II-dependent increase in calcium. It is now accepted that TRPC6 channel activity at the slit diaphragm is essential for proper regulation of podocyte structure and function.
It was reported that the expression of nephrin decreased in patients of minimal change nephrotic syndrome (MCNS)[13,41]. Our group has investigated the expression of nephrin in rat puromycin aminonucleoside (PAN)-induced nephropathy[12]. PAN nephropathy is widely used as the model of MCNS. Our group showed mRNA expression for nephrin declined already at 1h after PAN injection into rats. We also observed that the immunofluorescence staining of nephrin changed to a discontinuous pattern from a continuous pattern along glomerular capillary wall on day 10 of PAN nephropathy when proteinuria peaked. The downregulation of nephrin in PAN nephropathy was reported by another group[11]. We reported that podocin is colocalized with nephrin in normal rats, whereas the podocin staining is apart from nephrin in rats with PAN nephropathy[14]. These observation showed that the molecular structure of the slit diaphragm was rearranged in PAN nephropathy, and that these molecular rearrangement led proteinuria in this model. It is conceivable that these alterations of the slit diaphragm participate in the development of MCNS. It is reported that nephrin staining changed to a discontinuous pattern in patients with membranous nephropathy[15]. It is reported that such an alteration in the staining of nephrin was detected in rats of passive Heymann nephritis model, mimic of human membranous nephropathy[42]. We observed that CD2AP and podocin were already apart from nephrin at the early phase of passive Heymann nephritis before the onset of proteinuria[43]. These reports showed that the altered localization of the slit diaphragm molecules is involved in the development of proteinuria also in membranous nephropathy. We reported that the expressions of nephrin, podocin and NEPH1 altered in rats of adriamycin (ADR)-induced nephropathy, which is accepted as a mimic of FSGS[44]. The rats with ADR nephropathy showed severe and continuous proteinuria. The dissociation of NEPH1 from nephrin was observed at the early phase of ADR nephropathy, when the dissociation of podocin from nephrin is not observed. It is postulated that the NEPH1- nephrin dissociation initiates proteinuria in this disease. In contrast to other slit diaphragm molecules, it was discussed that TRPC6 expression in podocytes is up-regulated in several diseases[18].
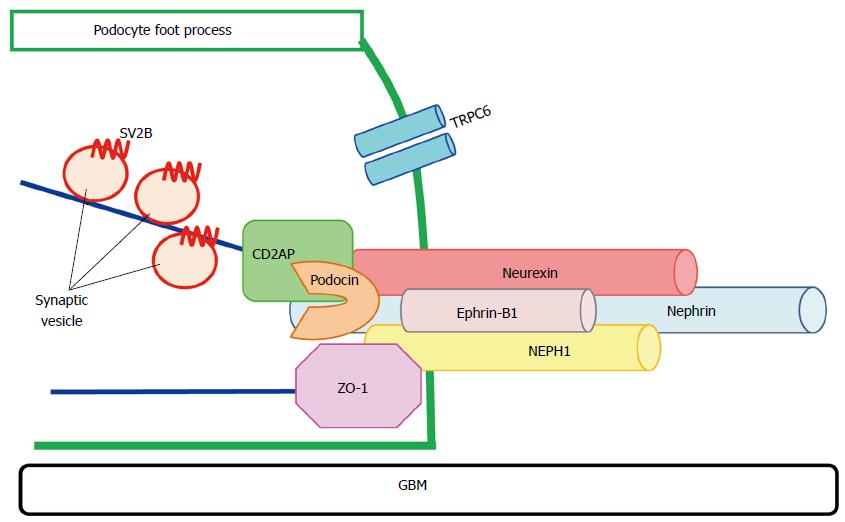
Although some critical molecules maintaining the barrier function of the slit diaphragm were identified, the precise molecular compositions of the slit diaphragm and the mechanism regulating its barrier function were not fully understood yet. To further analyze the molecular compositions of the slit diaphragm and to identify the target molecule for the therapy for nephrotic syndrome, we tried to purify the novel molecules and a subtraction assay with cDNA from normal rat glomeruli and nephrotic syndrome rat glomeruli was done[45]. It is plausible that, nephrin, podocin, CD2AP and NEPH1 were downregulated at the proteinuric states. Therefore, it is conceivable that the molecule whose expression decreased at the proteinuric state might be a molecule relating the development of proteinuria. We investigated the localization and the role of the molecules identified by the subtraction assay in podocyte. In addition, those of their associated molecules were also investigated. We focused on synaptic vesicle protein 2B[45], ephrin-B1[46] and neurexin[47] in these molecules. It is considered that these molecules are essential molecules of the slit diaphragm (Figure 1), and that they are the candidates for the target of novel therapy for nephrotic syndrome. The nature of these molecules is described below.
SV2 has three isoforms, SV2A, SV2B and SV2C. Our group found SV2B was downregulated in PAN nephropathy. The expression of mRNA of SV2B was already reduced when abnormal proteinuria was not detected yet. It is considered that the observation showed the decrease of the SV2B expression is not a mere consequence of proteinuria but has an etiological significance causing proteinuria. The decrease of the expression of SV2B was also observed from the early phase in anti-nephrin antibody-induced nephropathy. The observation indicated that SV2B is the slit diaphragm-associated molecule.
SV2 is known to regulate calcium-mediated synaptic transmission. It is understood that SV2 plays an essential role in vesicle trafficking[48-50]. SV2 was understood to be exclusively expressed in the neuronal tissue. However, some reports have shown that SV2s are expressed in several other tissues[51,52]. SV2B is detected in the microvesicles of pinealocytes. Both pinealocyte and podocyte are characterized by their specialized processes[52]. These properties also suggested that SV2B plays a role in the trafficking to the terminal of processes.
To analyze the role of SV2B in podocyte, RNA silencing analysis was performed using cultured podocytes, and the expression of CD2AP, one of critical molecules of the slit diaphragm[45] was analyzed. CD2AP was detected at the tip of the process in control cells, whereas the CD2AP staining was detected mainly at the cytoplasm in the cells treated with siRNA for SV2B. From these observations, we concluded that SV2B contributed to the maintaining of the normal molecular structure of the slit diaphragm. It is postulated that the dysfunction of SV2B is involved in the development of proteinuria via the redistribution of proteins of the slit diaphragm such as CD2AP in PAN nephropathy and in other proteinuric states. Not only SV2B but also Rab3A, another synaptic vesicle molecule and rabphilin-3a, an effecter of Rab3A were expressed in the podocyte, and played an important role in maintaining the podocyte function[53]. More elucidation of the role of the synaptic vesicle like vesicle expressing SV2B and Rab3A in podocyte is awaited.
The molecules belonging to the Eph-ephrin family were identified by the subtraction assay. Both Ephs and ephrins are transmembrane proteins and they function as ligand-receptor pairs[54-56]. Eph-ephrin family have many biological functions such as the cell migration and axon guidance[57-59]. It is also reported that the Eph-ephrin-B family play a role in the regulation of the permeability between epithelial cells[57]. These characteristics of Eph-ephrin-B prompted us to analyze a role of Eph-ephrin-B in podocyte. Ephrin-B1, ephrin-B2, EphB1 and EphB2 mRNA expressions were detected in normal rat glomeruli and in murine cultured podocytes. Our group observed that the mRNA expression and immunofluorescence findings of ephrin-B1 were found to be decreased at 24 h of the nephropathy caused by the anti-nephrin antibody injection, whereas EphB1 or ephrin-B2 was not altered. Ephrin-B1, an original name Lerk-2, is a membrane-anchored protein[54,55]. Ephrin-B1 contains a cytoplasmic tail, a single transmembrane domain and an extracellular domain[55,56]. An immunoelectron microscopic study showed that ephrin-B1 was detected at the slit diaphragm[46]. Interaction of ephrin-B1 with nephrin was observed by the immuno-precipitation assay with glomerular lysate. It is conceivable that these observations showed ephrin-B1 is a slit-diaphragm-associated protein. The expression of ephrin-B1 was decreased already at the early phase of the anti-nephrin antibody-induced nephropathy when the alteration of nephrin staining is not remarkable yet. The podocyte injury in this model is caused by the binding of antibody to nephrin. The observation that the expression of ephrin-B1 altered more rapidly than nephrin in this model is very interesting, and we believe that the findings suggested that ephrin-B1 is highly associated with nephrin.
To investigate the function of ephrin-B1 in podocyte, a knockdown system with siRNA was done in cultured podocyte. CD2AP was detected at the tip end of the processes in control cells, whereas the staining of CD2AP was detected in cytoplasmic area around the nuclei. The finding suggested that ephrin-B1 plays a role for the trafficking of CD2AP to the tip of process. All of these results suggest that ephrin-B1 is also a component of the slit diaphragm complex. Recently, Wnuk et al[60] reported that Eph-B4 and ephrin-Bs were expressed in podocyte and that the expressions were altered in glomeruronephritis model. These findings suggested that members of the Eph-ephrin-B family could be targets for a novel therapy for proteinuria.
After the discovery of SV2B as a critical molecule of the slit diaphragm, we analyzed the expression of several synaptic vesicle associated molecules in podocyte, and found that neurexin is expressed in rat and human podocyte[47]. Neurexin was originally identified as a cell surface receptor for α-latrotoxin, a component of black widow spider venom, and was considered to play a critical role in cell-cell interaction in across the synapse[61-65]. Neurexin has an interaction with synaptotagmin, a synaptic vesicle associated molecule, and is postulated to play a role in synaptic vesicle docking[66,67]. It is also reported that neurexin binds calcium/calmodulin-dependent serine protein kinase (CASK), a member of the MAGUK family[68]. It is reported that CASK interacts with nephrin[69]. CASK is also accepted to be an essential molecule of the slit diaphragm. Our group demonstrated that neurexin was restrictedly expressed in the glomeruli of the kidney[47]. Dual-labeling immunofluorescence studies showed that neurexin located close to CD2AP. It was also detected that some portions of the neurexin staining are coincident with the staining of Rab3A, a synaptic vesicle molecule. We observed that the staining intensity of neurexin in the glomeruli was clearly reduced, and their staining pattern shifted to a discontinuous patchy pattern in PAN nephropathy and in anti-nephrin antibody induced nephropathy. The alteration in the staining of neurexin in these models was detected more clearly and rapidly than that in the nephrin staining. These observations suggest that neurexin is one of the essential molecules regulating the slit diaphragm function. It is postulated that neurexin is a candidate of the targets for a novel therapy for nephrotic syndrome.
It is now widely understood that the dysfunction of the slit diaphragm participates in the initiation of proteinuria in several kinds of glomerular diseases. We summarized the nature of the major slit diaphragm molecules in Table 1. We reviewed the novel slit diaphragm associated molecules SV2B, ephrin-B1 and neurexin. Because proteinuria is an independent risk factor for the vascular episode in brain, heart and other organs[70], the novel more effective therapy for proteinuria should be established. It is reported that dysregulation of the synaptic vesicle function is involved in several neuronal diseases, and the drug targeting synaptic vesicle is used for the treatment for epilepsy. SV2B and other synaptic vesicle associated proteins could be novel therapeutic targets for nephrotic syndrome.
| Ref. | Molecules | Predicted molecule weight | Functions in the slit diaphragm |
| Schnabe et al[26] | ZO-1 | 225 kDa | Interact with NEPH1 |
| Kestilä et al[3] | Nephrin | 180 kDa | Maintaining the barrier function |
| Schwarz et al[34] | Podocin | 42 kDa | A raft-associated component and interact with nephrin and CD2AP |
| Shih et al[6] | CD2AP | 80 kDa | Interact with nephrin and anchor nephrin to the cytoskeleton |
| Liu et al[37] | NEPH1 | 110 kDa | Interact with ZO-1 and nephrin |
| Winn et al[9], Reiser et al[10] | TRPC6 | About 110 kDa | Interact with nephrin and podocin |
| Miyauchi et al[45] | SV2B | 80 kDa | The proper arrangement of CD2AP |
| Hashimoto et al[46] | Ephrin-B1 | 50 kDa | Maintaining the slit diaphragm structure |
| Saito et al[47] | Neurexin | 150 kDa | Regulating the slit diaphragm function |
P- Reviewer: Kong WY, Nihalani D, Quiroga B, Tanaka H, Yorioka N S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970;23:489-496. [PubMed] [Cited in This Article: ] |
| 2. | Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988;141:807-814. [PubMed] [Cited in This Article: ] |
| 3. | Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1425] [Cited by in F6Publishing: 1308] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 4. | Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962-7967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 548] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 5. | Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1085] [Cited by in F6Publishing: 1003] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 6. | Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 7. | Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829-4836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 2003;17:115-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 797] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 10. | Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 624] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Ahola H, Wang SX, Luimula P, Solin ML, Holzman LB, Holthöfer H. Cloning and expression of the rat nephrin homolog. Am J Pathol. 1999;155:907-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int. 2000;57:1949-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Furness PN, Hall LL, Shaw JA, Pringle JH. Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant. 1999;14:1234-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol. 2003;14:46-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Horinouchi I, Nakazato H, Kawano T, Iyama K, Furuse A, Arizono K, Machida J, Sakamoto T, Endo F, Hattori S. In situ evaluation of podocin in normal and glomerular diseases. Kidney Int. 2003;64:2092-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179:1719-1732. [PubMed] [Cited in This Article: ] |
| 18. | Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol. 2010;299:F689-F701. [PubMed] [Cited in This Article: ] |
| 19. | Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1067] [Cited by in F6Publishing: 1078] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 20. | Kawachi H, Suzuki K, Miyauchi N, Hashimoto T, Otaki Y, Shimizu F. Slit diaphragm dysfunction in proteinuric states: identification of novel therapeutic targets for nephrotic syndrome. Clin Exp Nephrol. 2009;13:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 22. | Menzel S, Moeller MJ. Role of the podocyte in proteinuria. Pediatr Nephrol. 2011;26:1775-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Schlöndorff JS, Pollak MR. TRPC6 in glomerular health and disease: what we know and what we believe. Semin Cell Dev Biol. 2006;17:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141-1149. [PubMed] [Cited in This Article: ] |
| 25. | Fanning AS, Lapierre LA, Brecher AR, Van Itallie CM, Anderson JM. Protein interactions in the tight junction: the role of MAGUK proteins in regulating tight junction organization and function. Curr Topics Membranes. 1996;43:211-235. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255-1263. [PubMed] [Cited in This Article: ] |
| 27. | Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992;262:C1119-C1124. [PubMed] [Cited in This Article: ] |
| 28. | Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1992;89:7075-7079. [PubMed] [Cited in This Article: ] |
| 29. | Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Holthöfer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D. Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol. 1999;155:1681-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest. 1999;104:1559-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Kawachi H, Koike H, Shimizu F. Molecular structure and function of the slit diaphragm: expression of nephrin in proteinuric states and in developing glomeruli. Nephrol Dial Transplant. 2002;17 Suppl 9:20-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attié T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 254] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 574] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 36. | Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 392] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 37. | Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol. 2006;572:359-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Goel M, Sinkins WG, Zuo CD, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1241-F1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Wernerson A, Dunér F, Pettersson E, Widholm SM, Berg U, Ruotsalainen V, Tryggvason K, Hultenby K, Söderberg M. Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol Dial Transplant. 2003;18:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol. 2002;13:946-956. [PubMed] [Cited in This Article: ] |
| 43. | Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int. 2005;67:2239-2253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Otaki Y, Miyauchi N, Higa M, Takada A, Kuroda T, Gejyo F, Shimizu F, Kawachi H. Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis. Am J Physiol Renal Physiol. 2008;295:F1376-F1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Miyauchi N, Saito A, Karasawa T, Harita Y, Suzuki K, Koike H, Han GD, Shimizu F, Kawachi H. Synaptic vesicle protein 2B is expressed in podocyte, and its expression is altered in proteinuric glomeruli. J Am Soc Nephrol. 2006;17:2748-2759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, Shimizu F, Kawachi H. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 2007;72:954-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Saito A, Miyauchi N, Hashimoto T, Karasawa T, Han GD, Kayaba M, Sumi T, Tomita M, Ikezumi Y, Suzuki K. Neurexin-1, a presynaptic adhesion molecule, localizes at the slit diaphragm of the glomerular podocytes in kidneys. Am J Physiol Regul Integr Comp Physiol. 2011;300:R340-R348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 49. | Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem. 1996;271:27770-27775. [PubMed] [Cited in This Article: ] |
| 50. | Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem. 2004;279:52124-52131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Wang MM, Janz R, Belizaire R, Frishman LJ, Sherry DM. Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. J Comp Neurol. 2003;460:106-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Hayashi M, Yamamoto A, Yatsushiro S, Yamada H, Futai M, Yamaguchi A, Moriyama Y. Synaptic vesicle protein SV2B, but not SV2A, is predominantly expressed and associated with microvesicles in rat pinealocytes. J Neurochem. 1998;71:356-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Rastaldi MP, Armelloni S, Berra S, Li M, Pesaresi M, Poczewski H, Langer B, Kerjaschki D, Henger A, Blattner SM. Glomerular podocytes possess the synaptic vesicle molecule Rab3A and its specific effector rabphilin-3a. Am J Pathol. 2003;163:889-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 376] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 55. | Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 878] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 56. | Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 801] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 57. | Martínez A, Soriano E. Functions of ephrin/Eph interactions in the development of the nervous system: emphasis on the hippocampal system. Brain Res Brain Res Rev. 2005;49:211-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Cheng N, Brantley DM, Chen J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002;13:75-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 60. | Wnuk M, Hlushchuk R, Janot M, Tuffin G, Martiny-Baron G, Holzer P, Imbach-Weese P, Djonov V, Huynh-Do U. Podocyte EphB4 signaling helps recovery from glomerular injury. Kidney Int. 2012;81:1212-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 62. | Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 63. | Missler M, Fernandez-Chacon R, Südhof TC. The making of neurexins. J Neurochem. 1998;71:1339-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50-56. [PubMed] [Cited in This Article: ] |
| 65. | Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, Südhof TC. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269:11987-11992. [PubMed] [Cited in This Article: ] |
| 66. | Hata Y, Davletov B, Petrenko AG, Jahn R, Südhof TC. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993;10:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Perin MS. The COOH terminus of synaptotagmin mediates interaction with the neurexins. J Biol Chem. 1994;269:8576-8581. [PubMed] [Cited in This Article: ] |
| 68. | Hata Y, Butz S, Südhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488-2494. [PubMed] [Cited in This Article: ] |
| 69. | Lehtonen S, Lehtonen E, Kudlicka K, Holthöfer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Gutiérrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, Warnock DG, Safford MM. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |