Published online Dec 6, 2012. doi: 10.5527/wjn.v1.i6.155
Revised: November 13, 2012
Accepted: November 25, 2012
Published online: December 6, 2012
Circulating toxins namely: free radicals, cytokines and metabolic products induce glomerular endothelial dysfunction, hemodynamic maladjustment and chronic ischemic state;this leads to tubulointerstitial fibrosis in chronic kidney disease (CKD). Altered vascular homeostasis observed in late stage CKD revealed defective angiogenesis and impaired nitric oxide production explaining therapeutic resistance to vasodilator treatment in late stage CKD. Under current practice, CKD patients are diagnosed and treated at a rather late stage due to the lack of sensitivity of the diagnostic markers available. This suggests the need for an alternative therapeutic strategy implementing the therapeutic approach at an early stage. This view is supported by the normal or mildly impaired vascular homeostasis observed in early stage CKD. Treatment at this early stage can potentially enhance renal perfusion, correct the renal ischemic state and restore renal function. Thus, this alternative therapeutic approach would effectively prevent end-stage renal disease.
- Citation: Futrakul N, Futrakul P. Urgent call for reconsideration of chronic kidney disease. World J Nephrol 2012; 1(6): 155-159
- URL: https://www.wjgnet.com/2220-6124/full/v1/i6/155.htm
- DOI: https://dx.doi.org/10.5527/wjn.v1.i6.155
Homeostasis of the vital organs in the body depends on the balance between nutrient supply through vascular perfusion and the integrity of structure and function of the organs. In this regard, kidney integrity depends mainly on the renal vascular supply. Philosophically, normal homeostasis of the kidney follows the so-called Natural Wisdom “The Middle Tract is the Balance of Nature”, which implies that the normal integrity of the kidney depends on normal vascular perfusion. Any deviation of blood perfusion, either too much or too little would be harmful to the kidney[1]. Such wisdom can be illustrated by the correlation between blood perfusion and its organ’s structure and function. Under normal circumstances, the intact tubulointerstitium has been shown to be surrounded by an adequate supply of peritubular capillary plexus. In contrast, under pathological conditions, such as in chronic kidney disease (CKD), the normal tubulointerstitial structure is replaced by tubulointerstitial fibrosis, along with the disappearance of peritubular capillary plexus which is replaced by renal microvascular disease[2,3]. The spatial relationship between renal perfusion and kidney integrity will be the context of the following issues: (1) Renal microvascular disease and tubulointerstitial fibrosis; (2) Why does the present therapeutic strategy fail to restore renal function in CKD (a) CKD is recognized and treated at a rather late stage; and (b) Altered vascular homeostasis in late CKD; and (3) An innovative therapeutic strategy to implement the treatment at early stage CKD.
Accumulating evidence supports the suggestion that there are toxins such as free radicals, cytokines and metabolic products circulating through the renal microcirculation in a variety of CKDs. Abnormally elevated oxidant and antioxidant deficiencies have been repeatedly documented in both mild as well as severe forms of CKD[4-11].
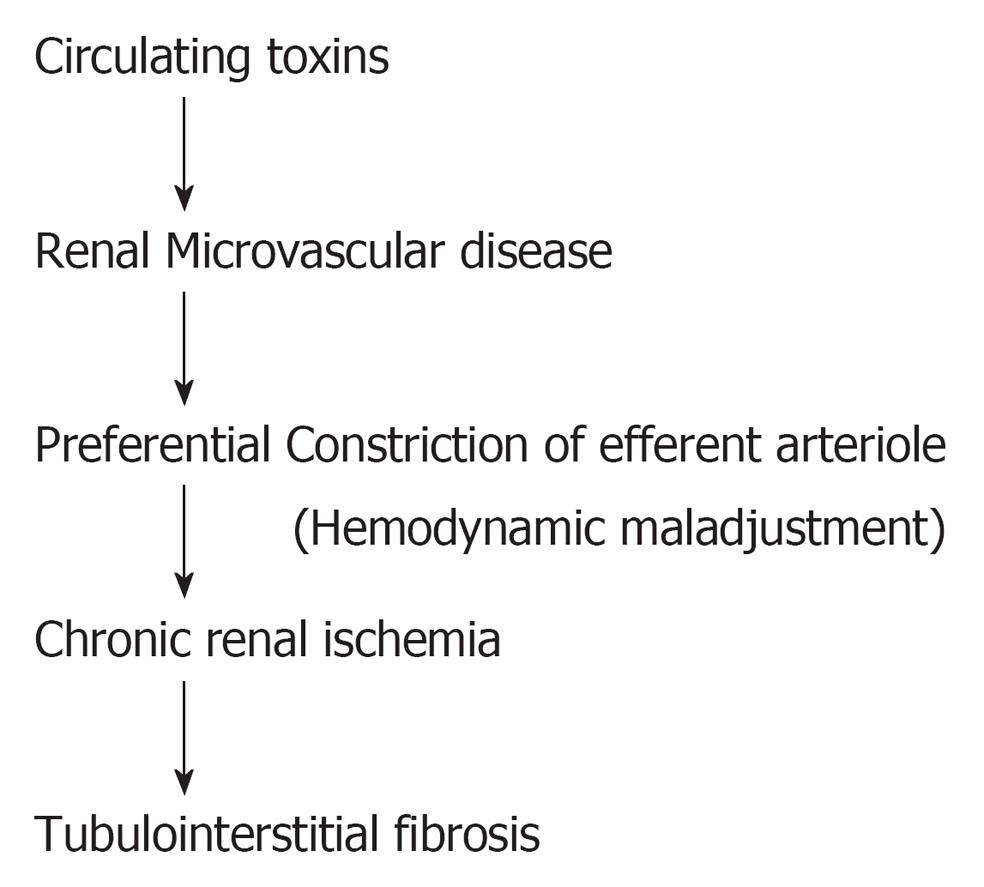
The circulating toxins can induce injury to renal microvasculature. Such vascular injury detaches the endothelial cell from the vascular wall and this is reflected in an increase in the number of circulating endothelial cells in a variety of CKD patients[12,13]. In addition to the increased number of circulating endothelial cells, the remaining endothelial cells also become dysfunctional. Glomerular endothelial dysfunction is characterized by upregulation of vasoconstrictors such as angiotensin II, endothelin, thromboxane A2, adhesion molecules, procoagulant activity and reactive oxygen species. Enhanced expression of vasoconstrictors induces hemodynamic maladjustment characterized by a preferential constriction at the efferent arteriole and thus a corresponding reduction in peritubular capillary flow supplying the tubulointerstitial structure (Figure 1). This phenomenon is well documented in CKD[14-17]. It is interesting to observe that an intact tubulointerstitial structure is usually associated with normal level of peritubular capillary flow. Reduced peritubular capillary flow has been observed in all CKD patients. A mild reduction in peritubular capillary flow has been noted to precede the development of tubulointerstitial fibrosis. An increased reduction in peritubular capillary flow leads to the appearance of tubulointerstitial fibrosis. A further reduction in peritubular capillary flow is associated with a higher degree of tubulointerstitial fibrosis (Table 1)[18-23]. This suggests that the reduction in peritubular capillary flow determines the development of tubulointerstitial fibrosis. In addition, the dysfunctioning endothelial cell expresses procoagulant activity which is reflected in blood hypercoagulalilty, blood hyperviscosity, a shortened platelet half life and a shortened fibrinogen half life, indicating an increased consumption of local intravascular coagulation, plausibly in the renal microcirculation. Thus correcting both the altered blood coagulability with anticoagulant and antiplatelet agents[22-24], as well as correcting the chronic ischemic state with vasodilators[13,14,17] is an appropriate therapeutic target.
| Clinical setting | Peritubular capillary flow mL/min per 1.73 m2 | Tubulointerstitial fibrosis |
| Normal | 480 | Negative |
| Early CKD | 250-400 | + |
| Late CKD | < 250 | + + → + + + + |
Under the current definition of CKD, which includes only patients associated with creatinine clearance under 60 mL/min per 1.73 m2, or serum creatinine greater than 1 mg/dL, recognition of CKD is practically limited to late CKD (stages 3-5) since serum creatinine does not change until the creatinine clearance drops to the 50% level[25-27]. This implies that treatment of CKD is usually initiated at a rather late stage. Early stage CKD patients have generally been left untreated, and the disease allowed to progress without any appropriate therapeutic intervention. Treatment of these CKD patients with vasodilators shows therapeutic unresponsiveness and fails to correct the chronic ischemic state[28-33]. This issue leads us to propose that vascular homeostasis explains such therapeutic failure.
It has been recently demonstrated that altered vascular homeostasis and impaired nitric oxide (NO) production are responsible for therapeutic resistance to vasodilators in late CKD.
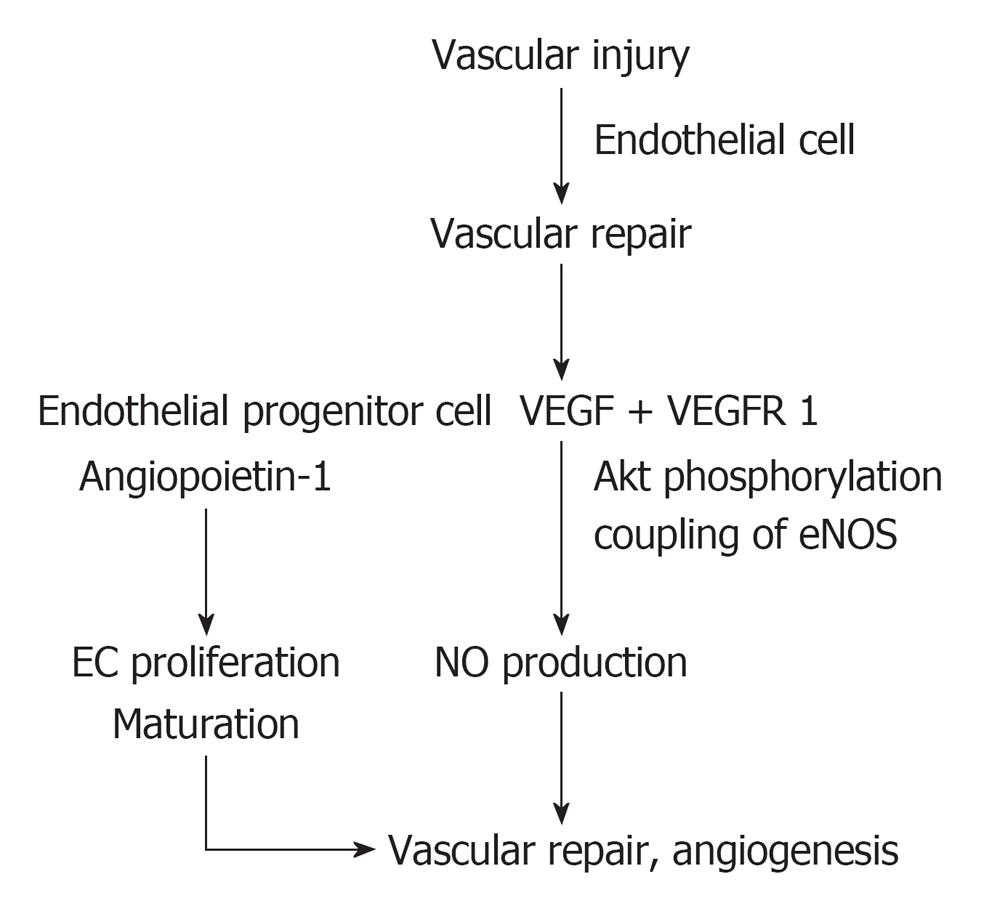
With respect to vascular homeostasis, a normal vascular homeostasis is the balance between vascular injury and vascular repair (Figure 2). Under normal circumstances, vascular injury results in an increased number of endothelial cells detaching from the diseased vascular wall into the circulation, so-called circulating endothelial cells which express receptor-bound vascular endothelial growth factor (VEGF) as suggested by Hohenstein et al[34]. Such vascular injury would trigger vascular repair by recruiting angiogenic factors such as VEGF which would activate through VEGF receptor 1 (VEGFR 1) inducing Akt phosphorylation, coupled with endothelial nitric oxide synthase (eNOS), and enhanced NO production[35-37]. Enhanced NO production, in conjunction with endothelial progenitor cells and angiopoietin 1, stimulate endothelial cell proliferation, maturation and reendothelialization. Collectively, they would integrate in a normal vascular repair and angiogenesis[38,39].
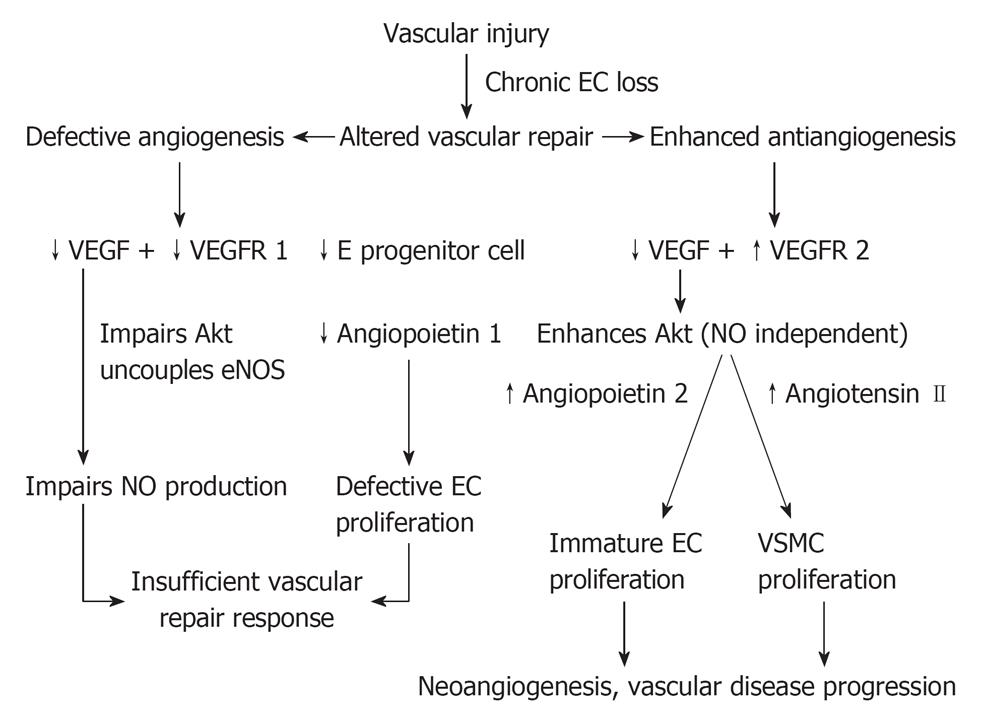
In late stage CKD, we as well as others[40-44] have recently demonstrated that there is an altered vascular homeostasis characterized by defective angiogenic factors namely VEGF, VEGFR 1, endothelial progenitor cells and angiopoietin 1, in conjunction with abnormally elevated antiangiogenic factors namely: VEGFR 2 and angiopoietin 2. Such altered vascular homeostasis observed in late stage CKD canbe summarized as follows.
With respect to defective angiogenic factors, as indicated on the left hand side of Figure 3, the defective VEGF and VEGFR 1 would impair the Akt phosphorylation, uncouple eNOS, and impair NO production. An impaired NO production in conjunction with defective endothelial progenitor cells and defective angiopoietin 1 would impair the physiological stimulation of endothelial cell proliferation and maturation. They would integrate together in an insufficient vasculogenesis and vascular repair. With respect to the abnormally elevated antiangiogenic factors, VEGF activates through VEGFR 2 inducing abnormal Akt phosphorylation through an NO-independent pathway, resulting in a proliferation of abnormally immature endothelial cells. The presence of defective angiopoietin 1, is responsible for the immature endothelial cell proliferation. In addition, in the presence of abnormally elevated angiopoietin 2, endothelial cells would be further destabilized and endothelial apoptosis induced. Collectively, they would integrate in the formation of abnormally immature endothelial cells, which would be consistent with endothelial myofibroblast transition cell indicated by Li[45]. With respect to the vascular smooth muscle cell (VSMC) proliferation, this is triggered by the upregulation of angiotensin II secondary to endothelial cell dysfunction, activating NADPH oxidase, oxidative stress, NFKB and p 38, JAK STAT and eventually stimulating VSMC proliferation[46]. The VSMC proliferation would induce a thickening of the vascular wall, a narrowing of vascular lumen, and eventually a reduction in vascular perfusion, which eventually leading to the development of neoangiogenesis and progressive vascular disease. The altered vascular homeostasis observed in late stage CKD indicates both an insufficient vasculogenesis associated with an impaired NO production, which explains the therapeutic resistance to vasodilators, as well as the clinical progression of renal microvascular disease of increasing severity. Also, the progression of renal microvascular disease correlates with the altered renal hemodynamics characterized by a progressive reduction in peritubular capillary flow along with a progressive decline in renal function[1]. Such therapeutic failure of current practice with vasodilators in late stage CKD requires an alternative strategy to focus the treatment at the new target group of CKD patients at the early stage of renal function impairment (Figure 3).
The alternative therapeutic strategy would focus on early stage CKD patients, who mostly have been untreated or received inappropriate treatment. Our recent study on vascular homeostasis in early stage CKD supports this concept[47,48]. In type 2 diabetic nephropathy, the vascular homeostasis observed in the normoalbuminuric stage indicated that both angiogenic factors namely: VEGF, angiopoietin 1 and VEGF receptor 1; as well as antiangiogenic factors namely: angiopoietin 2 and VEGF receptor 2, were within normal limits. In non-diabetic early stage CKD patients, the vascular homeostasis indicated that angiopoietin 1 was the only angiogenic factor showing a mild decrease, and that angiopoietin 2 was the only antiangiogenic factor showing a mild elevation. Thus these findings render support the theory that vascular homeostasis would likely be adequately functional in early stage CKD. With the adequate vasculogenesis observed in early stage CKD, vasodilator treatment would relax the efferent arteriole by enhancing the peritubular capillary flow. Increased peritubular capillary flow would inhibit the process of tubulointerstitial fibrosis indicated by the decline in Fe-Mg value following vasodilator treatment - an index indicating renal regeneration. Vasodilator treatment would also relax the afferent arteriole and thereby increase the glomerular filtration rate - an index indicating renal function improvement. In fact, therapeutic implementation with appropriate vasodilators at this early stage in CKD has indeed been able to enhance peritubular capillary flow, as well restore renal function[49,50].
In conclusion, therapeutic implementation of vasodilator treatment in early stage CKD, in an environment favorable to renal angiogenesis and regeneration, can effectively prevent end-stage renal disease.
Peer reviewers: Robert G Fassett, Professor, Department of Renal Medicine, Royal Brisbane and Women’s Hospital and the University of Queensland, Brisbane 4029, Australia; Gianni Bellomo, MD, Department of Nephrology, San Giovanni Battista Hospital Via Arcamone, 1 06034 Foligno, Italy
S- Editor Song XX L- Editor Hughes D E- Editor Zheng XM
| 1. | Futrakul P, Sitprija V, Yenrudi S, Poshyachinda M, Sensirivatana R, Watana D, Singklwa V, Jungthirapanich J, Futrakul N. Glomerular endothelial dysfunction determines disease progression: a hypothesis. Am J Nephrol. 1997;17:533-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Yenrudi S, Laohapaibul A, Kittidiwit W, Suteparuk S, Futrakul N. A correlation between renal morphology and renal circulation in pediatric nephrotic syndrome. Ren Fail. 2001;23:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Futrakul N, Kittikowit W, Yenrudi S. Reduced endothelial factor VIII staining in renal microcirculation correlates with hemodynamic alteration in nephrosis. Ren Fail. 2003;25:759-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005-L1028. [PubMed] [Cited in This Article: ] |
| 5. | Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220-9225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1109] [Cited by in F6Publishing: 1036] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 6. | Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524-2531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Calhan C, Santra A. Oxidative stress in the metabolic syndrome. Oxidative stress, inflammation and angiogenesis in the metabolic syndrome. New York: Springer-Verlag 2009; 33-63. [Cited in This Article: ] |
| 8. | Shah SV. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989;35:1093-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 198] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Futrakul N, Tosukhowong P, Valyapongpichit Y, Tipprukmas N, Futrakul P, Patumraj S. Oxidative stress and hemodynamic maladjustment in chronic renal disease: a therapeutic implication. Ren Fail. 2002;24:433-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chan JCM. Oxidative injury in focal segmental glomerulosclerosis. Asian Biomed. 2008;2:16-26. [Cited in This Article: ] |
| 11. | Futrakul N, Panichakul T, Butthep P, Futrakul P, Jetanalin P, Patumraj S, Siriviriyakul P. Ganoderma lucidum suppresses endothelial cell cytotoxicity and proteinuria in persistent proteinuric focal segmental glomerulosclerosis (FSGS) nephrosis. Clin Hemorheol Microcirc. 2004;31:267-272. [PubMed] [Cited in This Article: ] |
| 12. | Futrakul N, Butthep P, Patumraj S, Siriviriyakul P, Futrakul P. Microvascular disease and endothelial dysfunction in chronic kidney diseases: therapeutic implication. Clin Hemorheol Microcirc. 2006;34:265-271. [PubMed] [Cited in This Article: ] |
| 13. | Futrakul N, Butthep P, Vongthavarawat V, Futrakul P, Sirisalipoch S, Chaivatanarat T, Suwanwalaikorn S. Early detection of endothelial injury and dysfunction in conjunction with correction of hemodynamic maladjustment can effectively restore renal function in type 2 diabetic nephropathy. Clin Hemorheol Microcirc. 2006;34:373-381. [PubMed] [Cited in This Article: ] |
| 14. | Futrakul P, Pochanugool C, Poshyachinda M, Thamaree S, Yenrudi S, Buranasiri K, Saleekul P, Watana D, Sensirivatana R, Kingwatanakul P. Intrarenal hemodynamic abnormality in severe form of glomerulonephritis: therapeutic benefit with vasodilators. J Med Assoc Thai. 1992;75:375-385. [PubMed] [Cited in This Article: ] |
| 15. | Futrakul N, Butthep P, Futrakul P, Sittipreja V. Glomerular endothelial dysfunction in type 2 diabetes mellitus. Ren Fail. 2006;28:523-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Futrakul N, Siriviriyakul P, Futrakul P. Hemodynamic correction and early detection of tubulointerstitial fibrosis prevent disease progression in chronic kidney disease. Ren Fail. 2004;26:199-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 17. | Futrakul N, Vongthavarawat V, Sirisalipotch S, Chairatanarat T, Futrakul P, Suwanwalaikorn S. Tubular dysfunction and hemodynamic alteration in normoalbuminuric type 2 diabetes. Clin Hemorheol Microcirc. 2005;32:59-65. [PubMed] [Cited in This Article: ] |
| 18. | Futrakul N, Yenrudi S, Sensirivatana R, Watana D, Laohapaibul A, Watanapenphaibul K, Kingwatanakul P, Futrakul P, Futrakul S. Peritubular capillary flow determines tubulointerstitial disease in idiopathic nephrotic syndrome. Ren Fail. 2000;22:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806-816. [PubMed] [Cited in This Article: ] |
| 21. | Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, Johnson RJ. Tubulointerstitial disease: role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens. 2003;12:233-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Futrakul P, Poshyachinda M, Mitrakul C. Focal sclerosing glomerulonephritis: a kinetic evaluation of hemostasis and the effect of anticoagulant therapy: a controlled study. Clin Nephrol. 1978;10:180-186. [PubMed] [Cited in This Article: ] |
| 23. | Futrakul P, Poshyachinda M, Mitrakul C. Hypercoagulability in the nephrotic syndrome: Use of anticoagulation. Advances in basic and clinical nephrology. Basel: Karger 1981; 297-301. [Cited in This Article: ] |
| 24. | Futrakul P. Coagulation in glomerulonephritis and nephrotic syndrome: Its therapeutic intervention. Asian Mannual of Nephrology. Tokyo: Southeast Asian Medical Information Center 1981; 89-96. [Cited in This Article: ] |
| 25. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Futrakul N, Sila-asna M, Futrakul P. Therapeutic strategy towards renal restoration in chronic kidney disease. Asian Biomed. 2007;1:33-44. [Cited in This Article: ] |
| 27. | Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1-12. [PubMed] [Cited in This Article: ] |
| 28. | Ruggenenti P, Perna A, Benini R, Bertani T, Zoccali C, Maggiore Q, Salvadori M, Remuzzi G. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. J Am Soc Nephrol. 1999;10:997-1006. [PubMed] [Cited in This Article: ] |
| 29. | Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1439] [Cited by in F6Publishing: 1365] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 30. | Hilgers KF, Mann JF. ACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal disease. J Am Soc Nephrol. 2002;13:1100-1108. [PubMed] [Cited in This Article: ] |
| 31. | Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1207] [Cited by in F6Publishing: 1114] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 32. | Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Deferrari G, Ravera M, Berruti V, Leoncini G, Deferrari L. Optimizing therapy in the diabetic patient with renal disease: antihypertensive treatment. J Am Soc Nephrol. 2004;15 Suppl 1:S6-S11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69:1654-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Liao JK. Fine-tuning the angiogenic response to vascular endothelial growth factor. Circ Res. 2008;103:229-230. [PubMed] [Cited in This Article: ] |
| 38. | Kolatsi-Joannou M, Li XZ, Suda T, Yuan HT, Woolf AS. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev Dyn. 2001;222:120-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc. 2008;38:201-207. [PubMed] [Cited in This Article: ] |
| 41. | Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in type 2 diabetic nephropathy. Ren Fail. 2009;31:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Bortoloso E, Del Prete D, Dalla Vestra M, Gambaro G, Saller A, Antonucci F, Baggio B, Anglani F, Fioretto P. Quantitave and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol. 2004;150:799-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Woolf AS, Gnudi L, Long DA. Roles of angiopoietins in kidney development and disease. J Am Soc Nephrol. 2009;20:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Le-Thi-Thu H, Casañola-Martín GM, Marrero-Ponce Y, Rescigno A, Saso L, Parmar VS, Torrens F, Abad C. Novel coumarin-based tyrosinase inhibitors discovered by OECD principles-validated QSAR approach from an enlarged, balanced database. Mol Divers. 2011;15:507-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282:31038-31045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Futrakul N, Futrakul P. A mildly altered vascular homeostasis in early stage of CKD. Ren Fail. 2009;31:538-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Futrakul N, Butthep P, Chunhakan S, Banyatsuppasin W, Futrakul P. Vascular homeostasis in early (normo-albuminuric) type 2 diabetic nephropathy. Asian Biomed. 2010;4:987-990. [Cited in This Article: ] |
| 49. | Futrakul N, Futrakul P, Siriviriyakul P. Correction of peritubular capillary flow reduction with vasodilators restores function in focal segmental glomerulosclerotic nephrosis. Clin Hemorheol Microcirc. 2004;31:197-205. [PubMed] [Cited in This Article: ] |
| 50. | Futrakul N, Kulaputana O, Futrakul P, Chavanakul A, Deekajorndech T. Enhanced peritubular capillary flow and renal function can be accomplished in normoalbuminuric type 2 diabetic nephropathy. Ren Fail. 2011;33:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |