Peer-review started: May 24, 2020
First decision: June 15, 2020
Revised: June 29, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: September 25, 2020
High genetic variability of human immunodeficiency virus (HIV) has been a major intractable challenge to the practical design of vaccines. But a recent pioneer study published in PNAS Xenobots, is likely to revolutionize HIV prevention as it presented the world's first living robot made of cells. In the advent of this discovery, we herein discuss the possibility of using living biological cell robots to target HIV-infected T lymphocytes, and the prospects of this approach being a new HIV vaccine. We capture the current research status and trend of advances in biological cell robots' design as a new HIV vaccine. The key differences between this novel vaccine and other HIV vaccines are highlighted.
Core Tip: In February 2020, the birth of the world's first live-cell robot has brought hope for the artificial design of human live cells. Therefore, herein we propose a hypothesis: Can we artificially design a cell as an alternative target cell for human immunodeficiency virus (HIV) infection and use it as a new acquired immune deficiency syndrome vaccine to prevent HIV infection.
- Citation: Xie YY, Yang F, Liao XY. Hypothesis of design of biological cell robot as human immunodeficiency virus vaccine. World J Virol 2020; 9(3): 19-26
- URL: https://www.wjgnet.com/2220-3249/full/v9/i3/19.htm
- DOI: https://dx.doi.org/10.5501/wjv.v9.i3.19
While preventive human immunodeficiency virus (HIV) vaccine development has been a constant goal since the discovery of HIV, interest in a therapeutic vaccine for HIV-infected people has fluctuated. Many people thought that therapeutic vaccines were impossible because until recently there were no examples of such vaccines being used for other diseases. With the emergence of more capable, simple, and relatively non-toxic combination drug therapies, there have been fewer calls for the development of a therapeutic acquired immune deficiency syndrome (AIDS) vaccine. However, the world's first living somatic cell robot has rekindled interest in a therapeutic vaccine. The vaccine can not only be used as a stand-in for HIV target cells, through alternative ways to protect the body's healthy immune cells, but can also enhance immune-mediated clearance of virus-producing cells and/or assist in the destruction of the reservoir of latently infected cells that drug therapy alone does not seem to be able to eliminate[1]. So, herein we propose a hypothesis that artificial design of the live-cell robot can be considered as a new AIDS vaccine.
Since the discovery of AIDS in 1981, massive resources have been directed at research aimed at developing preventive and curative agents for affected patients. Nearly 40 years later, AIDS has become a global public health threat claiming many lives. A few years ago, a meeting was held in Keystone Symposia (March 21 to 26, 2012) to focus on basic aspects of immunology and HIV-1 virology to highlight issues that challenge the field. The genetic diversity of circulating HIV-1 variants puts extreme demands on the quality of the response that a prophylactic vaccine will need to elicit. The recent trials of therapeutic HIV vaccines were introduced and the results of therapeutic vaccines in non-human primate models were discussed. It is clear that therapeutic vaccine development trials and studies follow a standard preventive vaccine development path. After conceptualizing the product, 10 to 15 years of animal model testing are performed prior to 4 to 6 years of GMP product development, allowing another 5 to 10 years of phase I, phase II, and then phase III clinical trials for licensing and distribution of specific vaccine candidate products before they occur. We can see that this path is frustratingly slow and may not be the best way to address several of the key issues to be discussed in the development of therapeutic vaccines. It is risky for researchers to try to design a vaccine that solves such a complex problem by reasoning through aspects of the final product before testing it. At the end of a long trial, however, it is likely to fail completely because it does not contain a necessary ingredient or contains unnecessary ingredients that undermine the overall efficacy. We have long believed that it is postulated that the HIV vaccine is the most effective approach to control the AIDS pandemic. Although much progress has been made to achieve this goal[1,2], no licensed HIV vaccine has been put on the market to prevent HIV infections. It was clear that therapeutic HIV vaccine development requires addressing several very different issues including: (1) How to correctly understand the mechanism of HIV infection? (2) How does the vaccine respond to HIV mutations? and (3) How to choose the optimal way to block HIV infection?
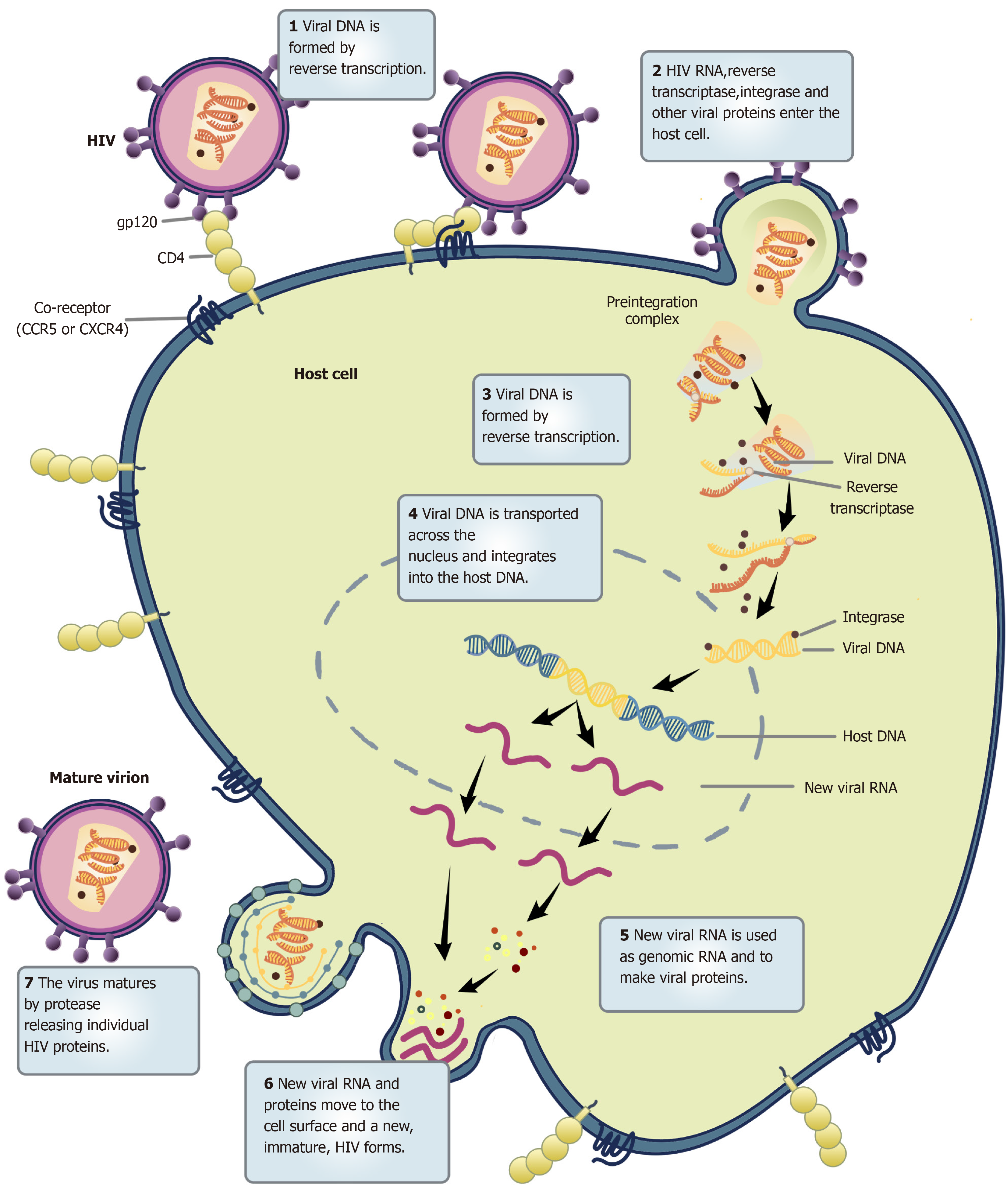
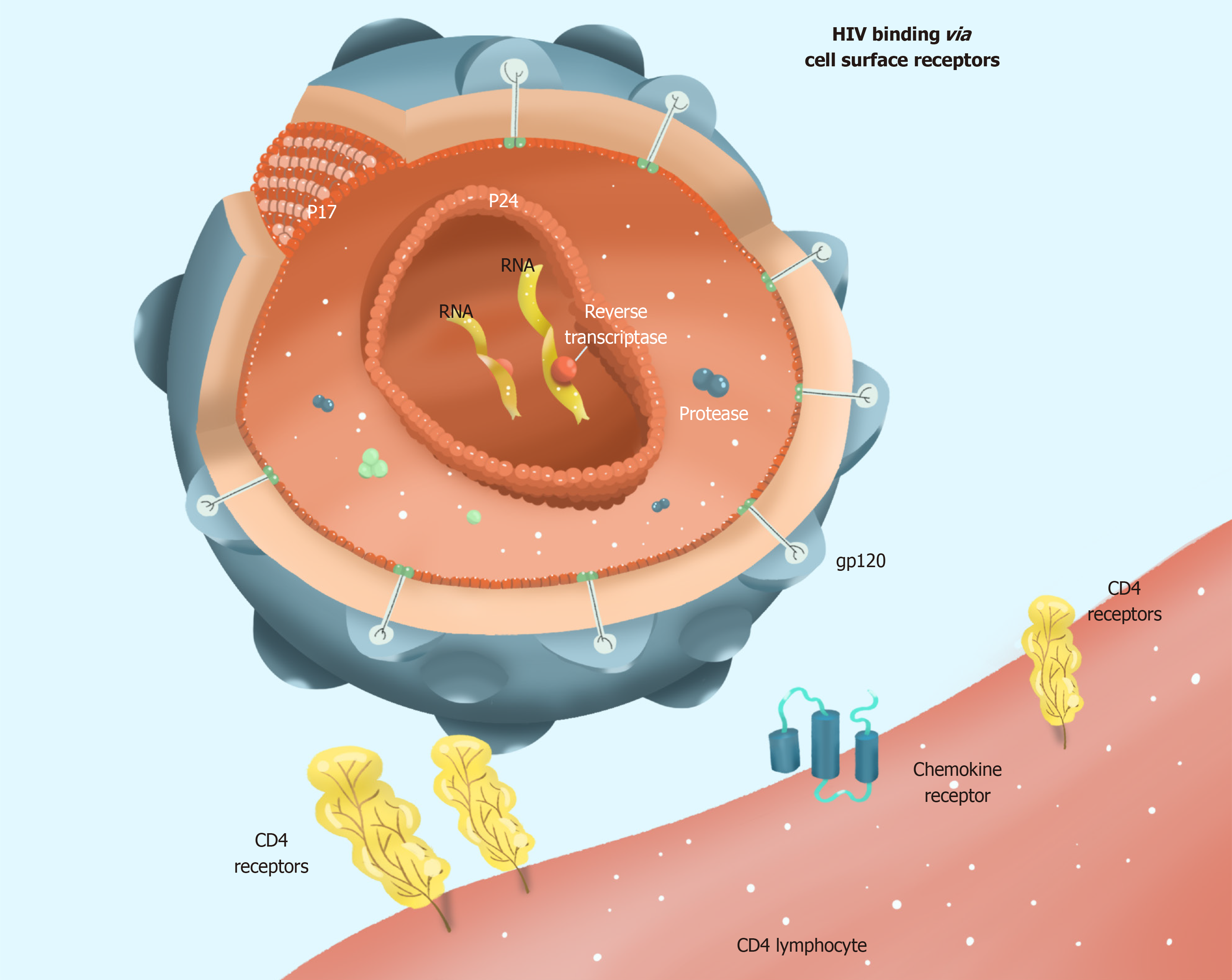
HIV selectively infects helper T lymphocytes, dendritic cells, and macrophages because these cells express CD4 molecules. The HIV infection process is complex, with several stages: Adsorption, entry, uncoating, reverse transcription, integration, replication, transcription, translation, assembly, and maturation (Figure 1). Current research provides evidence that HIV infection requires not only CD4 molecules and helper receptors (CXCR4 and CCR5), but also proteins encoded by HIV genes, such as gp120 and gp41. A detailed list of proteins encoded by genes associated with HIV infection in host cells is provided in Table 1[3]. When HIV enters the human bloodstream, it selectively invades host cells expressing CD4 molecules on their cell surface. HIV binds to the CD4 receptor on the surface of the host cell via its surface envelope protein gp120[4]. Upon binding, gp120 protein undergoes structural alterations exposing another envelope protein gp41. Meanwhile, the gp120-CD4 dimers formed to interact with the host's cell surface auxiliary receptor CXCR4/CCR5 to create three molecular complexes constituting gp120-CD4-CXCR4/CCR5. These complexes expose the host cell membrane and the envelope protein gp41, which is hydrophobic, enabling the HIV to be coated with the host cell membrane followed by HIV and host cell fusion. Finally, HIV nucleic acid is released into the host cell (Figure 2).
| Gene | Encoding protein | Protein function | Host cell-related proteins |
| Structural genes | |||
| gag | MA | Matrix proteins | Karyopherins, HO3, Calmodulin, VAN/NAF1, TRIM5α, CyPA |
| CA | Capsid protein | HP68/RNase L inhibitor, Actin | |
| NC | Nucleocapsid protein | ESCRT, Tsg-101, AIP-1, Nedd4, Ubiquitin | |
| p6 | Nucleocapsid protein | - | |
| pol | RT | A viral genome that can be transcribed and copied | - |
| PR | Cut polymerized protein | - | |
| IN | Integrate viral DNA with cellular DNA | INI1/hSNF5, LEDGF/p75, BAF, HMGal, ATR, | |
| ATM, Karyopherins, XRCC5 | |||
| env | gp120 | Attach the virus to the surface of the cell | CD4, CCR5. CXCR4, DC-SIGN, DC-SIGNR, MR, CD207 |
| gp41 | Fusion with host cells | - | |
| Necessary regulatory genes | |||
| tat | Tat | Trans-activated proteins that activate HIV gene transcription | NF-κB, cyclin T, CDK9, Med28 |
| rev | Rev | A regulator of viral protein expression that regulates mRNA splicing and promotes mRNA transport to the cytoplasm | TNPO3, importin β, Crm1, Ran GTPase, Sam68, p32 |
| Nonessential regulatory genes | |||
| nef | Nef | Negative regulatory factors, which change cell signals, reduce the expression of CD4 and MHC-I molecules, and reduce the killing of HIV infected cells by CTL, representing essential factors in the development of infection into AIDS | PACS-l, ASK1, PAK, PI3-K, Lck, VAN/NAFl |
| vif | Vif | Viral infectious factors that promote viral assembly and maturation | APOBEC3G |
| vpr | Vpr | Viral protein regulatory, which transports viral DMA to the nucleus and inhibits cell growth | Karyopherins, Uracil-DNA glycosylase, Weel |
| vpu | Vpu | Viral protein U that promotes the release of the virus | CD317 (Tetherin, BST-2) |
HIV is the most complex of all retroviruses. Its genetic material ranges between 9.2 and 9.8 kb of RNA. Structurally, it is not very stable and has a high mutation rate. Once HIV is coated and fused with the host cell membrane, it releases its capsid protein HIV p24, which is gradually degraded within the host cell to release HIV RNA and reverse transcriptase[5]. The reverse transcriptase uses the RNA as a template to synthesize viral DNA. The HIV DNA is integrated into the host cell nucleus chromosome by the integrase enzyme. Subsequently, the viral DNA uses existing host cell gene copies and protein replication machinery to synthesize its proteins. Activation of HIV-infected cells triggers the transcription of pre-viral DNA into viral RNA, further translated into structural proteins of HIV. These proteins assemble in the cytoplasm, forming several new viral particles. Eventually, viral particles are exported to the cell surface in the form of buds, which recognize and attack other target cells. Currently, it is known that HIV exhibits high RNA variability. More importantly, the reverse transcriptase of HIV is prone to the problem of base mismatch in the reverse transcriptase synthesis of DNA using RNA as a template, because the reverse transcriptase lacks base proofreading. This leads to a failure to remove the mis-introduced nucleotides in time for replication. An error occurs about once in every replication cycle, causing the virus to replicate with random mutations that are high-frequency and non-directional. The high genetic variability translates to high variability in the encoded proteins. A comparison of antigens extracted from wild type strains of HIV with those from AIDS patients in which HIV has already undergone many replications reveals that the structure and amino acid sequence of proteins from these two groups are different. It has been observed that the injection of vaccines based on HIV antigen into AIDS patients fails to induce the formation of immune cells and antibodies to neutralize HIV. This is because HIV surface antigen molecules undergo rapid mutations that help HIV-infected cells to escape immune recognition due to decreased affinity of produced antibodies for the mutant HIV antigens.
We analyzed the recently reported world's first living bio-robot, which we believe could be a new HIV vaccine. This vaccine differs significantly from conventional vaccines. We know that HIV has a high degree of antigenic variability. For this reason, HIV samples used for vaccine development contain mutated versions of HIV, hence such vaccines are not sufficient for controlling AIDS. This has been the biggest challenge hindering HIV vaccine research and development. Notably, we have observed that despite its many variants, HIV always targets cells expressing CD4 molecules, and the expression of the vast majority of CD4 particles is conserved; variations are rare. This means that human CD4 units are the primary receptors for HIV infection. Based on the recent publication by Kriegman et al[6], we believe that a living biological cell robot can be designed to target CD4 molecules.
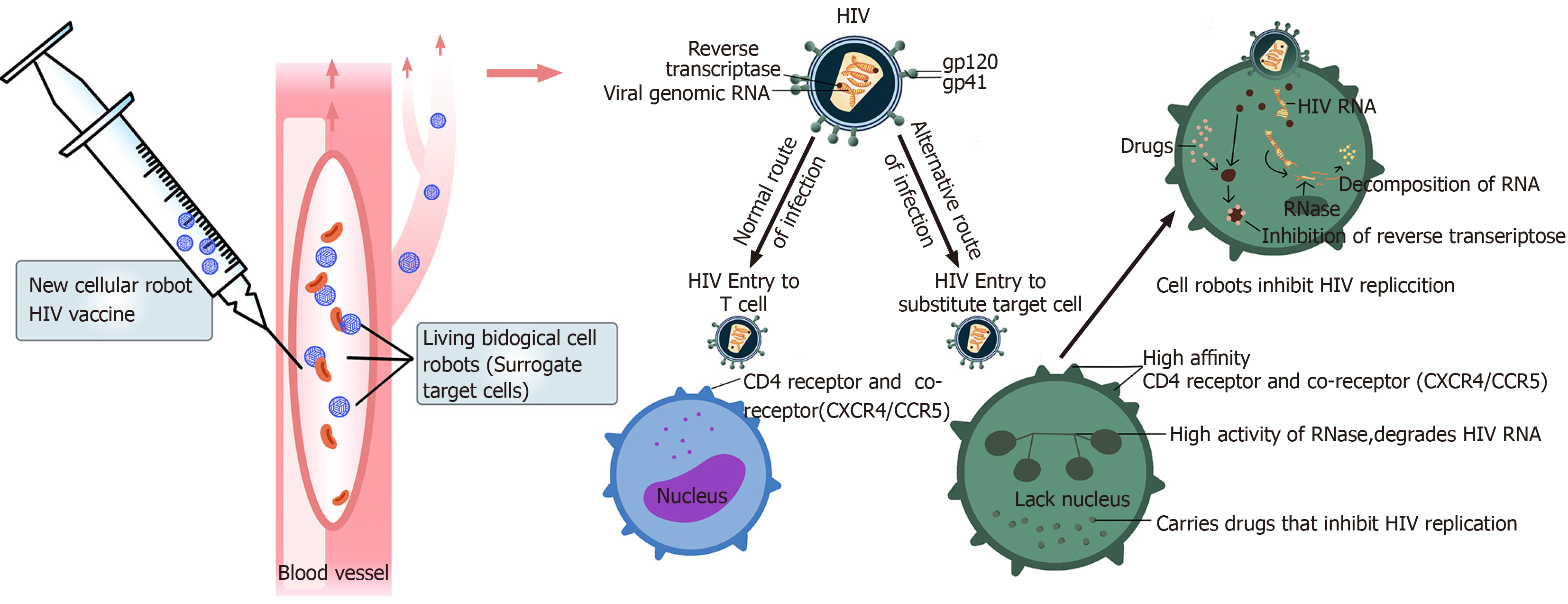
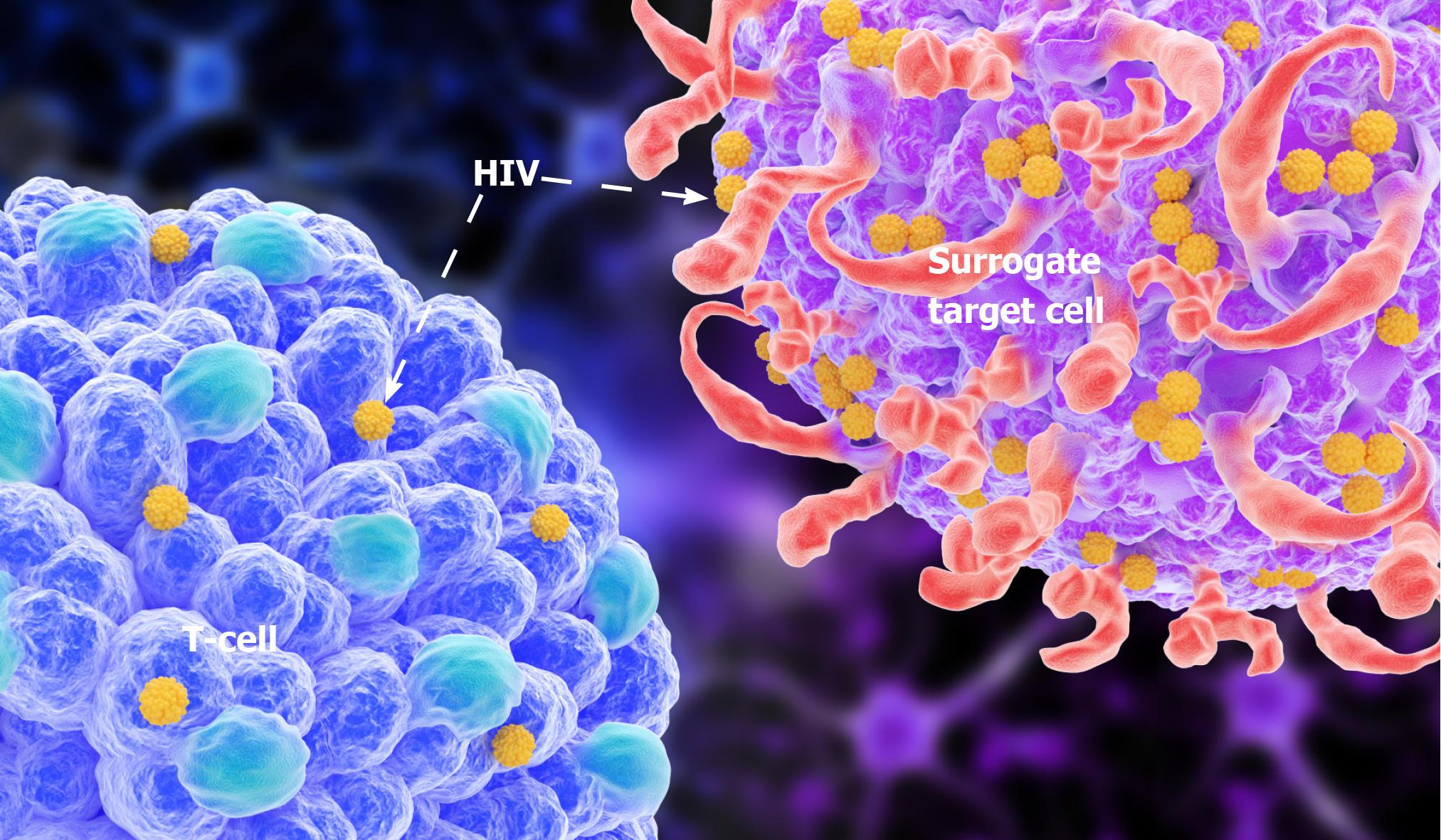
The surface molecules of this bio-bot are highly similar to the target cells, which not only express CD4 molecules but also other helper receptors needed for HIV infection (CXCR4/CCR5). We believe that this somatic cell robot can be an alternative target cell for HIV infection. This robot can be programmed using supercomputers and gene recombination technologies, to shorten its lifespan and make it undergo rapid apoptosis after HIV infection. Other cellular automata that are not infected with HIV will also degrade themselves within a short time, to avoid unnecessary immune responses. We can also enzymatically engineer the cell robot to synthesize HIV-RNase in the cell, degrade the HIV-RNA that enters the battery, or make the cell robot carry drugs that inhibit HIV replication (Figure 3). In this way, the reproduction of HIV in host cells can be immediately suppressed. To improve the binding rate of HIV to the cell robot, the target cells should be selectively and temporarily sealed with CD4 molecules in the original body using antibodies with radioactive isotopes. Blocking immune cells with CD4 molecules carrying the antibodies may reduce immunity in HIV-infected people, but only temporarily. A recent study showed that the use of live-cell robots as alternative target cells for HIV infection, combined with HIV fusion inhibitors, may reduce the use of CD4 blocking antibodies, which will indirectly enhance the new vaccine's effect without reducing the function of the patient's immune system. Besides, HIV fusion inhibitors can block the entry of HIV into human cells. This novel mechanism requires further research. Strategies that inhibit HIV fusion hold massive promise in solving the problem of HIV resistance. Such agents can be used as adjuvant treatments to the new vaccine, given after vaccination to reduce the adverse reactions of the new vaccine (Figure 4).
The new live-cell robot vaccine is still in the developmental stage and may remain an idea that requires further research. However, we note that it is a better alternative to other HIV vaccines because it overcomes the high variability of HIV. Traditional HIV vaccines currently being studied are primarily based on subunit vaccines, live attenuated vaccines, and inactivated vaccines. But these approaches are proving difficult due to the high antigenic variability of HIV, which hinders the identification of crucial representative genotypes or specific protein antigens. Live attenuated vaccines are associated with safety concerns. In a rhesus monkey model, the simian immunodeficiency virus (SIV) mutant strain lacking the nef gene prevents attack by pathogenic SIV. It protects monkeys from developing AIDS, but it cannot safeguard vaccinated monkeys against the over-infection of wild-type virus. Moreover, SIV without the nef gene can still cause AIDS, especially when given orally to young monkeys. Of note, genetic mutations or deletions in HIV may attenuate viral reproduction, but at the cost of reducing the vaccine's effectiveness. For inactivated vaccines, physical or chemical methods are needed to kill the virus, which requires that the antigenic nature of HIV be changed. These inactivated HIV antigens cannot effectively activate the body's immune system to produce immune responses, and the produced antibody titers are also very low[7]. This calls for collaborative research between computer science and biological science. In natural sciences, cellular simulations based on the ability of HIV to recognize and bind to CD4 receptors in host cells should be developed. Such simulations should consider some conserved proteins such as gp120 and gp41 to design alternative target cells for HIV infection. In computer science, supercomputers with well-designed evolutionary algorithms, through trial and error approaches, should be employed to program cell robots[5]. The development of "surrogate target cells" for HIV infections will lead to "HIV suicide" because they cannot replicate and reproduce[8].
However, we note that the new cell robot vaccine is still in the early stages of development, and many fundamental questions need to be addressed before it can be put into practical use. If cell robots can be made from patients' cells, the technology could be used for drug delivery in humans. Otherwise, it may elicit problematic immune responses. The most successful HIV vaccine is still in early trial stages at the population level. Although the results are promising, further large-scale clinical trials are needed before it can be deemed suitable for clinical application. The HIV vaccine induces antibody response in the body, but it does not show that it can effectively fight HIV and prevent AIDS. HIV mainly attacks the body's immune system, and the production of antibodies in traditional vaccines is inseparable from the immune system, which often causes failure of vaccination. So far, there has not been a single HIV self-healing case, suggesting that our immune system alone cannot suppress or eliminate HIV. More importantly, the primary defense against HIV in humans relies on cytotoxic T lymphocytes (CTL), which secrete a variety of cytokines involved in immune function. In the fight against HIV, the central role of CTL is to kill cells that have been invaded by the virus, thereby halting the reproduction of HIV. However, none of the vaccines developed so far are effective at activating CTL. Another challenge in vaccine development is the long incubation period of HIV/AIDS, which can last for years or decades. As a result, highly active antiretroviral therapy remains by far the most popular treatment for HIV, and we are still a long way from a truly widespread HIV vaccine.
The content of the information does not necessarily reflect the government's position or government policy, and no official endorsement should be inferred. We thank the FREE SCIENCE for the provided computational resources.
Manuscript source: Unsolicited manuscript
Specialty type: Virology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osna NA S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Shapiro SZ. A proposal to use iterative, small clinical trials to optimize therapeutic HIV vaccine immunogens to launch therapeutic HIV vaccine development. AIDS Res Hum Retroviruses. 2015;31:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Ncube B, Ansong J, Daniels K, Campbell-Stennett D, Jolly PE. Sexual risk behavior among HIV-positive persons in Jamaica. Afr Health Sci. 2017;17:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Chen M, Jiang SB, Liu SW. Host cell proteins associated with HIV infection. Xibao Yu Fenzi Mianyixue Zazhi. 2009;25:662-664. [DOI] [Cited in This Article: ] |
| 4. | Redd AD, Laeyendecker O, Kong X, Kiwanuka N, Lutalo T, Huang W, Gray RH, Wawer MJ, Serwadda D, Eshleman SH, Quinn TC; Rakai Health Sciences Program. Efficiency of CCR5 coreceptor utilization by the HIV quasispecies increases over time, but is not associated with disease progression. AIDS Res Hum Retroviruses. 2012;28:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Giraut A, Song XP, Froeyen M, Marlière P, Herdewijn P. Iminodiacetic-phosphoramidates as metabolic prototypes for diversifying nucleic acid polymerization in vivo. Nucleic Acids Res. 2010;38:2541-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kriegman S, Blackiston D, Levin M, Bongard J. A scalable pipeline for designing reconfigurable organisms. Proc Natl Acad Sci USA. 2020;117:1853-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 7. | Sun Y, Xu GL. Advances in HIV vaccine research. Guoji Shengwuzhipinxue Zazhi. 2008;4:167-171. [DOI] [Cited in This Article: ] |
| 8. | Yamamoto T, Kanuma T, Takahama S, Okamura T, Moriishi E, Ishii KJ, Terahara K, Yasutomi Y. STING agonists activate latently infected cells and enhance SIV-specific responses ex vivo in naturally SIV controlled cynomolgus macaques. Sci Rep. 2019;9:5917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |