THREATS OF YESTERDAY, TODAY AND TOMORROW
Many infectious diseases severely affect the health of humans, animals and plants. In humans, infectious diseases are responsible for 15 million of 57 million annual deaths in a global population of 6.2 billion people[1]. In the United States, each year, approximately 25% of physician visits are attributable to infectious diseases, with direct and indirect costs estimated at more than $120 billion[2]. Each year, about 2 million people die from acquired immune deficiency syndrome (AIDS) (caused by a retrovirus), 1.7 million people die from tuberculosis (caused by a mycobacterium, Mycobacterium tuberculosis), and more than 1.6 million people die from diarrheal disease caused by infectious pathogens.
Today, like yesterday, unknown pathogens are emerging in the world, while others, identified long-standing and hitherto regarded as controlled, have their importance increased. Diseases related to these emerging infectious agents can have major consequences in terms of public health, safety, balance of food chains and trade. They have already heavily influenced policy decisions at local, national or international levels while the strategies and specific tools adapted to these scourges are weak or absent, due to lack of integrated knowledge from multidisciplinary research.
Articles that address the emergence or re-emergence of infectious diseases are numerous, but their definition which applies to this concept often varies from one author to another. The definition of emerging infectious diseases (EIDs) in this review adopted by the Emerging Infectious Diseases Interdisciplinary Scientific Program of the French CNRS is as follows: EIDs belong to a nosological entity whose nature is proved infectious, regardless of the pathogen, or only suspected in case of novelty and until the agent is identified. It is understood that the identification of a “new” pathogen is compatible with a previously undisclosed preexisting one. By extension, it is assumed that the EIDs include infectious diseases known endemic showing an obvious resurgence. An EID can affect all types of eukaryotic organisms. The EIDs generally have a high social impact and economic consequences. An EID is obviously unusual; it is surrounded by uncertainty and anxiety, real or perceived, as to its evolutionary potential, its impact on health and the ability of leaders and stakeholders to control the phenomenon. These emerging diseases are therefore: (1) the development of a new disease, a consequence of a new pathogen (in its nature, its mode of transmission, its expression and/or its adaptation to host species); and (2) a disease previously identified but whose manifestations are new (associated with a sudden increase in the incidence, severity or geographical area within a time span of a few weeks/months to one or several decades). This definition therefore includes the resurgence of endemic diseases in more sensitive populations or of less controlled forms, and the emergence of pathogens resistant to treatment.
FACTORS FAVORING THE EMERGENCE OR RE-EMERGENCE OF PATHOGENS
The devastating plague of Athens, Greece, killed 70 000 Athenians in the years 430-426 BC; the Black death, or bubonic/pneumonic plague, killed approximately 34 million Europeans and 16 million Asians in the years 1347-1350; the French pox (French is called here because of a notorious outbreak in the French army), or syphilis, linked crewmen of Colombus’ voyages to the Naples outbreak of syphilis in 1494, and subsequently spread the disease to Europe (syphilis killed 5 million Europeans); the smallpox in 1520 was introduced in Central America by Spanish soldiers. It killed most of the Aztec army and 25% of the overall Aztec empire population; the American plague, or Yellow fever, in the years 1793-1798 killed more than 5000 of 50 000 Philadelphians; and the Spanish influenza in 1918-1919 turned out to be the deadliest emerging pandemic since it killed 50-100 million people. All these diseases are among the diseases that have marked the history of infectious diseases. They remain etched in the collective subconsciousness of the people as a threat for the survival of human societies. Sometimes the vector itself is the cause of phobias which are additional to the fear of disease, as is the case for rats [e.g. Black death, Argentine hemorrhagic fever (AHF)] and bats (e.g. Ebola hemorrhagic fever, Marburg hemorrhagic fever)[3,4]. At certain times of history, epidemics were even interpreted as divine punishment with all the irrational behavior; thus, when Black death appeared in Marseille (South of France) in 1347, and spread rapidly in France and Europe, it caused anti-Jewish riot up to massacre of people. Actually, plague remains a public health concern mainly in East Africa and Madagascar[5].
Scientific advances, however, have accomplished major progress in the fight against infectious diseases. The most important achievement was the eradication of smallpox by vaccination[6]. The last case of smallpox was declared in 1977 and, during 1980, the World Health Organization (WHO) announced the eradication of smallpox, a feat of human factors against the hostile factors of the environment. Compared with earlier generations of scientists, we possess an enormous scientific base, and the acquisition rate is high for new information about pathogens. Over the last 5 decades, numerous viruses responsible for new diseases or old diseases whose etiology is recently identified, have been discovered[2,7-10]. To highlight the importance and timeliness of EIDs without focusing only on the AIDS pandemic that killed more than 25 million people over the past 25 years, we should also keep in mind that severe acute respiratory syndrome (SARS), with 10 000 human cases and slightly less than 1000 deaths, had an estimated overall cost of over $100 billion for the global economy; a possible pandemic of bird flu transmission between humans also challenges the government and health authorities in the world. In addition, the zoonosis related to the H5N1 virus has already had devastating effects on certain economies. The epidemic of mad cow disease has caused 182 500 confirmed cases of bovine spongiform encephalopathy, or prion disease, in the UK and the ban on bovine products; the quite recent Chikungunya virus (CHIKV) outbreak in Reunion, with more than 230 000 human cases, had led to more than 180 deaths and also seriously affected the local tourist economy; the yellow mottle virus in rice, the main disease affecting irrigated rice in Africa and Madagascar, can destroy almost an entire harvest and have dramatic consequences on the survival of local populations.
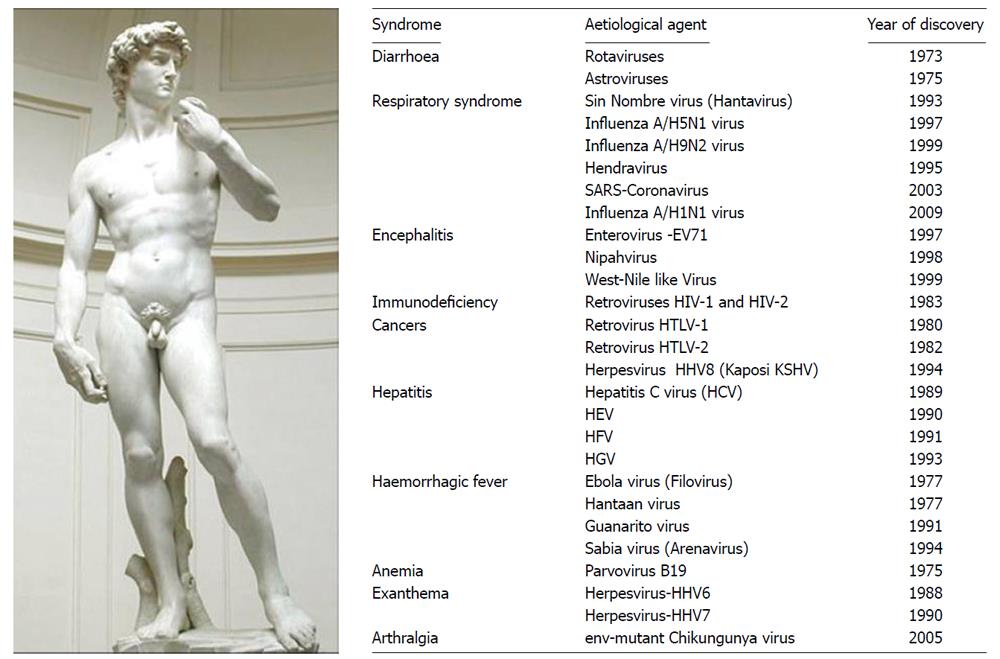
Just to cite some of the major epidemics to take stock of the situation, emergence of new pathogens affecting humans has been found true with the Marburg virus epidemic in 1967, the Lassa virus in 1969, the Rotavirus in 1973, the Ebola virus in 1976, the Hantaan virus in 1977, the influenza A/H5N1 virus in 1997, the SARS-Coronavirus in 2003, the influenza A/H1N1 virus in 2009. Pathogens identified as the causative agents of a disease can include the human T-cell lymphotropic virus 1 (HTLV-I) in 1981, the HTLV-II in 1982, the human immunodeficiency virus (HIV) in 1983, the hepatitis C virus in 1988, and the human herpes virus-8 (HHV-8 or Kaposi sarcoma-associated Herpesvirus KSHV) in 1995 (Figure 1).
Figure 1 Major emerging and re-emerging virus-associated diseases in human.
The etiological agents of infectious diseases identified since 1973, as newly discovered viruses of public health importance. It includes a few arboviruses that are transmitted to humans by arthropod vectors, upon biting. Among known arboviruses, some are transmitted by mosquitoes (Bunyaviridae: La Crosse encephalitis, California encephalitis, Rift Valley fever; Flaviviridae: Dengue fever, Yellow fever, Japanese encephalitis, West Nile; Togaviridae: Eastern equine encephalomyelitis, Western equine encephalomyelitis, Venezuelan equine encephalomyelitis; Ross River fever, O’Nyong-nyong fever, Chikungunya), others by ticks (Bunyaviridae: Crimean-Congo hemorrhagic fever; Flaviviridae: Tick-borne encephalitis; Omsk hemorrhagic fever; Reoviridae: Colorado tick fever). Most of the emerging pathogens in humans originate from zoonosis.
These cases may illustrate the diversity of situations encountered, such as (1) new diseases due entirely to a new virus as in the case of AIDS; (2) previously unrecognized diseases that are identified through quantitative or qualitative changes and due to improved diagnostic methods, such as CHIKV-caused disease that can be confused with Dengue (DEN) cases[11]; (3) diseases that appear in a new region, such as the epidemic West Nile virus introduced to the USA in 1999; or (4) diseases which existed previously in animals and are transmitted to humans, like avian influenza occurring since 1997. A pathogen such as influenza A/H1N1 virus is the result of a complex (triple) reassortment with portions of the genome that correspond to those of a swine virus, others are related to an avian virus and, finally match a human virus[12].
EMERGENCE LINKED TO CHANGES IN ECOSYSTEMS
An emerging disease may be caused by a disturbance of the ecosystem[13]. The exploitation of nature by man, with consequences such as deforestation and/or practice of hunting, may put men into contact with pathogens. This probably happened for the first human infections with simian immunodeficiency viruses (SIV), which have adapted to a new host, man. The combination of the density of people in ever-growing cities and unsafe sexual practices fostered the transmission of HIV among groups of individuals. Population flow, whether by migration or transportation, in particular the development of air transport, has been a source of rapid spread of HIV in the world. The fact that this virus is a Lentivirus[14], whose first symptoms appear only 7 years after the primary infection, has completely hidden epidemic in the face of the world. When it became clear, it was already too late. The spread of a pathogen can be even faster instead of sexual transmission, contamination may occur through aerosols. If we study what happened from November 2002 about the worldwide spread of SARS, a doctor who treated SARS patients in China’s Guangdong Province traveled to Hong Kong where he stayed in a hotel. SARS passed to a Hong Kong resident who visited the hotel, to a businessman guest who traveled to Vietnam, to a flight attendant guest who then flied to Singapore, and to a tourist guest who returned to Toronto, Canada. Then it was only a period of weeks that created a world crisis with more than 8400 infected individuals, leading to more than 900 casualties around the world in 2002-2003[15].
Another example of interest is that following intensive cultivation of corn in Argentina, there has been a very significant proliferation of rats that were at the origin of the AHF associated with Junin virus[16]. Since the first report in Argentina, this Arenavirus has caused annual outbreaks with more than 24 000 reported cases by 1993. The transmission of the pathogen resulted from the interaction between the virus, the animal that acted as a reservoir for the virus, and contact with an immunologically naive human population that was exposed to the virus due to environmental change. Among factors that may promote the emergence, it is not uncommon that they may be related to climate changes, leading to the installation of vectors within a new geographic area previously untouched by the disease. It can also result from the adaptation of viruses to vectors by genetic mutations. This last situation is perfectly illustrated by the example of the Chikungunya Alphavirus (CHIKV) epidemic in the Indian Ocean islands in 2006 and the first cases in Southern Europe. Also, the combination of different factors such as urbanization and deforestation is expected to have influenced the emergence or re-ermergence of vector-borne diseases such as Yellow fever. Yellow fever, which was originally maintained in forest cycles involving monkeys and canopy-dwelling mosquitoes, was under control in various places of the world such Cuba (1900-1901), Panama (1904), Brazil (1932), Americas (1954-1975), and West Africa (1950-1970), after successful vector-borne disease elimination programs. However, Yellow fever re-emerged in Africa in the 1990s in urbanized areas[13].
All these examples show how the medical and scientific world was wrong when in the late 1970s there was a talk of “the end of infectious diseases”. Fifteen years later, the infectious disease specialists needed to make it clear by admitting “the return of infectious diseases” and by proposing the concept of EIDs. The EID could thus be defined as exceeding the average level and retaining attention. There are different situations: (1) the disease caused by a newly identified pathogen did not exist previously (AIDS or SARS); (2) the disease existed before but that was hitherto unknown causative agent (hepatitis C); or, (3) the disease existed before and the causative agent was identified, but appeared for the first time in a geographic area where no cases had been diagnosed previously (West Nile Virus Epidemic in the USA).
ARBOVIRUSES: BEYOND THE THREAT, A GLOBAL PUBLIC HEALTH PROBLEM
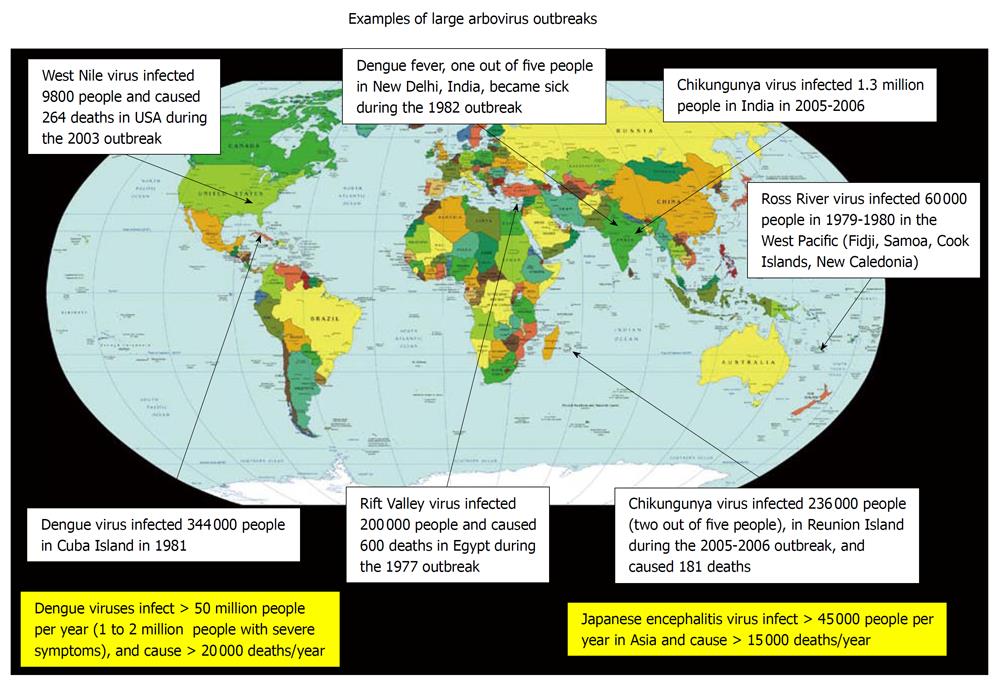
Each year, 800 000 people die of malaria and 20 000 of Dengue. While malaria, a human disease caused by a mosquito-borne infectious parasite of the genus Plasmodium, is the most important vector-borne disease because of its global distribution, the number of people affected (more than 220 million people) and the large number of casualties (about 800 000 deaths/year), the vector-borne viruses (arboviruses) are clearly the most numerous insect-borne pathogens (Figure 2).
Figure 2 Examples of large arbovirus outbreaks, from the literature.
This diagram illustrates several unexpected arbovirus outbreaks that have occurred in regions of the world which are currently the most vulnerable to arboviruses. It is likely that in the coming years other parts of the world will be affected, including regions that are outside of the tropical area.
Dengue, a disease provoked by a Flavivirus, is at the forefront of arbovirus-induced diseases with more than 80 million infected people a year. Dengue virus (DENV)-infection may be asymptomatic or may induce undifferentiated symptomatic febrile illness with headache, myalgias, arthralgias, rashes (eruptions may be observed on the neck and the face, followed by generalized rash on legs and arms) and leucopenia, most commonly affecting adolescents and adults. DENV-associated disease can also be much more severe (1-2 million people with severe symptoms), and sometime fatal[17], with the dengue hemorrhagic fever (DHF) and dengue shock syndrome (more than 20 000 deaths/year). DHF, which is most common in children of less than 15 years of age but also occurs in adults, is characterized by the acute onset of fever and associated with non-specific constitutional symptoms. There is a hemorrhagic diathesis and a tendency to develop fatal shock. Abnormal homeostasis, plasma leakage and week pulse, are the main patho-physiological changes constantly found. Although very common in the tropics, Dengue has also raged in Europe. The DEN epidemic occurred in Greece in 1927 and 1928 affecting more than 1 million people; this arbovirus outbreak killed 1500 infected persons far from a tropical area[18].
The globalization for arbovirus-induced diseases is a worrying situation because it is also accompanied by the resistance of vectors to insecticides, resistance of pathogens to drugs, unprecedented population growth in developing countries, uncontrolled urbanization in tropical areas where vector-borne diseases occur most frequently. Conditions necessary for arboviruses outbreaks include adequate populations of reservoir hosts (potentially humans in some settings), vector mosquitoes, and appropriate climatic conditions for transmission. The behavior of man on the environment, such as population pressure and agriculture, modifies the ecosystems (e.g. urbanization and deforestation). Anarchic urbanization in developing countries is often accompanied by habits of people who can facilitate the development of vectors. For example, automobile tires discarded in domestic environment where they collect rainwater, make ideal breeding sites for mosquitoes. In these facilities, mosquito larvae can reduce the risk of predation and can also survive in unfavorable climatic conditions. The same consequences can be the result of large water control projects such as irrigation systems and water reservoirs for intensive agricultural conditions.
RESURGENCE: LESSONS FROM CHIKV OUTBREAKS
When arboviruses emerge in endemic regions, they usually generate sporadic infections. However, when infections occur in naive human populations they usually induce severe epidemic bursts. To illustrate the resurgence of endemic diseases in more sensitive populations, examples will be given that are related to the situation encountered during the re-emergence of CHIKV. CHIKV belongs to the genus Alphavirus, family Togaviridae.
Choosing an arbovirus such as CHIKV to discuss the mechanisms of resurgence is not simply the result of chance. Indeed, these viruses illustrate not only the complexity of the interactions between an animal or a human and a virus-infected mosquito, the infection of another animal or a human bitten by an infected mosquito, but also the problems related to migration of human and animal populations, changes in hosts, transports, climate and global warming, water, etc., in other words, to an entire ecosystem apprehended in its entirety. We should also understand the dynamics of interaction at the molecular level as it is the case for the interaction between a CHIKV and membranes of an insect cell or a human cell, and subsequently, between viral proteins and cellular proteins.
As we will see later in this review, resurgence is exactly what happened in the Reunion Island during the CHIKV outbreak in 2006.
PATHOPHYSIOLOGY OF CHIKV-ASSOCIATED DISEASE
The CHIKV-induced DEN-like epidemic in the Reunion Island in 2005-2006 clearly corresponds to a disease previously identified but whose manifestations were associated with a sudden increase in the incidence, severity and change in geographical distribution. The symptoms[19] were most often clinically indistinguishable from those observed in Dengue, and evidence for dual infections has been reported[20]. CHIKV-induced disease appears after an average incubation period of 2-7 d. CHIKV fever is characterized by sudden onset, chills and fever between 39°C and 40°C, headache (70%), nausea/vomiting (60%), persistent myalgia/arthralgia (40%), and maculopapular rash (60%). Poly-arthralgia, the typical clinical sign of the disease, is very painful. The virus-associated symptoms, were also very similar to those previously observed in Australia during other unusual epidemic episodes caused by a related Ross River virus (RRV) in New South Wales in 1928[21], in Queensland in 1943[22], in the Schouten Islands near Papua New Guinea in 1946[23], and in the Muray Valley in 1956[24]. Until recently, CHIKV-associated syndrome differed from DENV-syndrome by the absence of fatalities and the clinician’s attention mainly focused on the rheumatologic consequences of CHIKV infection[25,26], and eventually on neurological and cardiac disorders, or hemorrhagic manifestations. CHIKV associated with a fatal hemorrhagic form was described once in India in 1964 and one case of a child who died from CHIKV infection in Ceylon was also documented in 1967. Yet, these cases were considered as exceptions and did not draw attention of the clinicians and biologists on the potential risks of CHIKV infection. Unfortunately, this lack of fatality can no longer be claimed, since 181 patients had died from CHIKV infection in the Reunion Island, indicating that CHIKV, like other Alphaviruses, is fatal for a low percent (around 0.5%-1%) of infected patients.
TEMPORAL AND SPATIAL DISTRIBUTION OF CHIKV OUTBREAKS
In the history of the disease linked to CHIKV, the first known viral isolate was from the serum of a febrile patient during a Dengue-like epidemic burst that occurred on the Makonde Plateau in Tanzania along the border between Tanzania and Mozambique in 1952[27,28]. Although the CHIKV epidemiological data are difficult to collect, it was reported that seroconversion was 31% in Bangkok in 1962[29], 15%-25% in Vellore, India in 1964[30], and 70% in Ibadan (Nigeria) in 1969. CHIKV has also been isolated from patients in Australia[31]. Since the first identification of CHIKV-infected humans, outbreaks of CHIKV have occurred throughout African and Asian countries where it is responsible for illnesses in hundreds to thousands of individuals. Comparative observations of Asian and African strains have been reported[32]. In both Africa and Asia, the re-emergence was unpredictable, with intervals of 7 to 20 years between consecutive epidemics. A representative example refers to the Indonesian outbreaks. The first cases of CHIKV were reported in Samarinda and Balikpapan, East Kalimantan, Indonesia in 1973. The disease then spread to Kuala Tungkai (Jambi Province) in 1980, and Yogyarkarta, Martapura and Ternate in 1983. After a hiatus of 20 years, 24 distinct outbreaks of probable CHIKV were reported between 2001 and 2003. Up to 8068 cases were reported in 29 cities of nine provinces in 2003, including Central Java, Yogyakarta, Banten, West Java, East Java, Bali, West Nusas Tenggara, East Nusa Tenggara and Lampung. In 2004, outbreaks were reported in Keagungan, West Jakarta (50 cases), Magetan, East Java (at least 168 cases), and Central Java (820 cases)[33].
In June 2004, a CHIKV outbreak was reported in Kenya[34]. The next outbreak emerged in the Indian Ocean Islands, during the first months of 2005[35]. More than 5000 cases were reported in Comoros from January to March 2005. A few months later, between March and June, the virus circulated in other islands, i.e. the Reunion, Mayotte, Seychelles, and Mauritius Islands. An outbreak of CHIKV was identified in the Reunion Island between April and June with a peak incidence in May which next decreased in winter season. The local sentinel network in the Reunion Island and the French health service administration (DRASS) reported 12 400 cases infected by CHIKV in the Reunion Island. Surprisingly, a second outbreak occurred at the end of December 2005 and the first couple of months of 2006, and a very fast increase in the number of infected persons of more than ten times (about 186 000 cases) was observed and DRASS announced 93 patients who died from CHIKV. In January 2006, it was also reported in Madagascar. By mid-March, about 214 000 persons had been infected with CHIKV in the Reunion Island, 148 of them died after infection. One month later, about 236 000 persons (up to one-third of the 770 000 people living on the Reunion Island), were infected and 181 of them died. Next, the Mauritius Island (more than 200 cases) and Seychelles (more than 1000 cases), and the French Island Mayotte (4308 cases, probably underestimated, of a population of about 180 000) which are also located in the Indian Ocean have also reported cases of CHIKV-infection in humans. The outbreak of CHIV which occurred in India between 2005 and 2007 has infected 1.5-6.5 million people[36]. Other outbreaks of CHIV took place in Cameroon in 2006 and in Gabon in 2007[37], where 11 500 cases were recorded between April and June.
Although the number of cases and the impact of the disease are not comparable to what has been described in Africa, India and the Indian Ocean area, Europe has not been spared by the CHIKV. In 2007, an infected traveller from India arrived in Italy and, within a few weeks, a small outbreak of more than 200 people was reported in this country where the local transmission was made possible by the enormous population of Aedes albopictus and the presence of a CHIKV-positive patient[38]. This European episode is very interesting because it helps understand the dynamics of virus outbreak, based on genetics characteristics of viruses, conditions encountered in interactions of virus with the vector, the geographical distribution of the vector and the climatic conditions favouring the development of an urban cycle of transmission. This will be commented in the subsequent section of this review.
SOUTH OF FRANCE: AN OBSERVATORY FOR INSECT-BORNE PATHOGEN DYNAMICS
In the coming decades, arboviruses will more frequently affect regions of the world that are outside of the tropical areas and it is very likely, for example, that Europe will face important arbovirus epidemics in the future[39]. This could occur through a combination of at least two major phenomena: First, the rapid global movement of people including those recently infected with arboviruses, as was observed in southern France in 2006, in Marseille, with people traveling from the Comorian Islands to France and carrying CHIKV; Secondly, because of changes in the implementation of vectors into new territories as in the cases of Aedes albopictus in southern France, perhaps linked to climate changes which likely determine the geographical and temporal distribution of arthropods as well as their life cycle, but most probably by accidental motorway transport. This adaptation of a mosquito is interesting and troubling because it opened the door to new epidemics. Interestingly, the most frequent vector of CHIKV, Aedes aegypti is not present in the South of France. In contrast, Aedes albopictus which can carry and transmit the CHIKV to humans, is now well established in the South East of France, in cities such as Nice and Marseille, and it has been reported further West towards Montpellier and further North to Valence and Grenoble[40]. In the event of contamination of mosquitoes by CHIKV, the spread of the disease will depend on many parameters including the abundance of mosquitoes in the area, the number of infected people moving around, the density of the population in the concerned region, the lifespan of infected mosquitoes, the replication time of the pathogen, the ability of mosquito to bite people, etc. The ecological conditions make an outbreak very likely, since some port cities of southern France are regularly visited by the people from infected areas and since Aedes albopictus tends to colonize urban areas (it is present in wet gardens and also in the water of flower pots at home), and preferably it bites man when it has the choice between man and animal.
Interestingly, two domestic cases of CHIKV and DENV transmission were reported in south of France in September 2010[41]. The two autochthonous cases of Dengue were diagnosed on September 10 and September 17, 2010 in people living in the city of Nice near an imported founder case (note that there were 173 cases of Dengue imported into France in 2010 with two-thirds coming from the Caribbean). The two autochthonous cases of CHIKV infections occurred in the town of Fréjus at monthly intervals, with the first case diagnosed on September 24, 2010 in a small area where resided an imported founder case of CHIKV. These CHIV isolates have been sequenced[42]. These infections have been made possible by the presence of mosquitoes Aedes albopictus (Figure 3), which are thought to have arrived in France with the motorway transport between Italy and France. Once installed in the city, mosquitoes tend to move slightly and bite people in the neighborhood.
Figure 3 Globalization of the distribution of pathogens.
Climate change, ecosystems evolution, anarchic urbanization, human behaviors, migration of humans and animals, development of air transport, extensive agriculture and water control projects, contribute to rapid spread of vectors and arbovirus-induced diseases in the world.
Moreover, this region of southern France is known for being a place of emergence of other arboviruses including outbreaks of West Nile virus. Initially reported in France in the 1960s, West Nile fever re-emerged in 2000 in the wetland of Camargue where it is episodically reintroduced by migratory birds and caused 76 equine clinical cases, 21 of which died[43]. West Nile virus is frequently associated with ornithophilic Culex spp. mosquitoes, which amplify the virus and transmit it to domestic and migratory birds, facilitating a wide geographical spread. In 2003, 7 human cases were reported in the Var district in Southeastern France. One may question what will be the impact of the introduction of Aedes albopictus in Southern France on the potential spread of West Nile virus. Indeed, the diet patterns of haematophagous arthropods are of major importance in the spread of arthropod-borne infectious pathogens. As recently studied[44], Aedes Albopictus acquired blood exclusively from human host. Accordingly, invasion of a geographic area by Aedes Albopictus could likely support local outbreak of Chikungunya and Dengue viruses induced diseases; in contrast, this mosquito could be expected to have negligible impact on the transmission of zoonotic agents, such as West Nile, since it avoids avian blood feeding in its diet.
It is also worth noting that, each year, there are a few thousand cases of imported malaria in France. The last known case of autochthonous malaria (Plasmodium vivax) was reported in Corsica, an Island at the South of France, in 2006[45]. The French Health Authorities have also identified other risks of arboviruses, in particular the Usutu virus, a Flaviviridae and the Toscana virus, a Bunyaviridae. Two autochtonus cases of Toscana virus-fever, a virus transmitted by Phlebotomus spp, were recently reported by the French Institute of Public Health, InVS, in 2011[46].
Vector control will be a requirement to interrupt the transmission of emergent/re-emergent vector-borne diseases.
FIGHT AGAINST HEALTH THREATS: ONE CANNOT BE EFFECTIVE AGAINST WHAT WE DO NOT KNOW WELL
It is of utmost importance to support and conduct research on basic and applied aspects of host, pathogen, and environmental factors that influence disease emergence and re-emergence as well as transmission. For example, CHIKV may survive in wildlife species through constant transmission cycles moving in epizootic waves (sylvatic cycle)[39]. Meteorologic factors such as temperature, rainfall, and humidity influence the transmission dynamic of vector-borne diseases, with increased viral spread following periods of heavy rainfall. Humans entering the areas where infected mosquitoes circulate may serve as incidental hosts for the mosquitoes and thus become infected. These people may then provide a source of virus to infect peridomestic mosquitoes, which next become involved in urban cycle of CHIKV transmission and epidemic episodes in the urban community.
Until recently, CHIKV episodes were restricted to diffuse epidemics in Africa where it was propagated by a variety of mosquitoes from the Aedes family, including Aedes furcifer, Aedes luteocephalus, Aedes taylori, Aedes africanus[47]. Then, the virus was imported to Thailand and India where it became an urban disease, transmitted largely by Aedes aegypti mosquitoes[39]. It is of particular interest that the CHIKV strain that has caused most of the epidemics in urban areas of the Indian Ocean is believed to have originated in Central/East Africa and transmitted by Aedes aegypti. Phylogenetic studies have shown that the earliest CHIKV isolated from the Reunion Island closely resembled those from East Africa, but as the epidemic accelerated, the CHIKV sequence of subsequent isolates changed, resulting in an amino acid substitution in the viral E1 envelope glycoprotein as a consequence of viral genome microevolution[48]. Simultaneously, this mutation appeared only in the CHIKV isolated from Aedes albopictus. Until very recently, CHIKV outbreaks in India were primarily associated with Aedes aegypti as it is in Africa. The Indian CHIKV-induced disease is recognized as an urban disease whereas the CHIKV African strains are expected to spread under sylvatic nature. Surprisingly, it was evidenced that CHIKV that circulated in India after the Reunion Island outbreak was genetically related to that circulating in the Indian Ocean Islands. This Indian CHIKV carries the mutation that allows a more efficient dissemination in Aedes albopictus[49]. CHIKV that were then circulated in Cameroon (2006), Gabon (2007) and Italy (2007), all bear the mutation which favors a transmission of the pathogen by Aedes albopictus. In Indian Ocean, Cameroon and Gabon, Aedes albopictus has effectively displaced Aedes aegypti through interspecific competition. Aedes albopictus appears to have adapted to activities of humans such as transportation and water programs, and it has colonized peridomestic storage of used car tires and transportation of plants. Aedes albopictus is now present in southern Europe. Although climate change has not yet been scientifically proven to have caused the emergence or re-emergence of any of the vector-borne diseases, a warming climate that would facilitate the introduction of mosquitoes in areas not yet infected, represents a serious hypothesis for the development of arboviruses in new regions of the world. Such considerations must be taken into account to anticipate changes that will undoubtedly lead to future epidemics. They have also important implications for the design of vector control strategies to fight against the virus in the regions at risk of Chikungunya fever.
African strains of CHIKV differ from the Reunion Island isolates of the virus regarding the sensitivity of Aedes albopictus for infection and replication of the viruses[50]. Acquisition of a single V226A mutation by the Reunion Island isolates coincides with the acquisition of a dependence on cholesterol in the target cell membrane[51] and their sensitivity to lysomotropic agents during infection of mammalian cells[52].
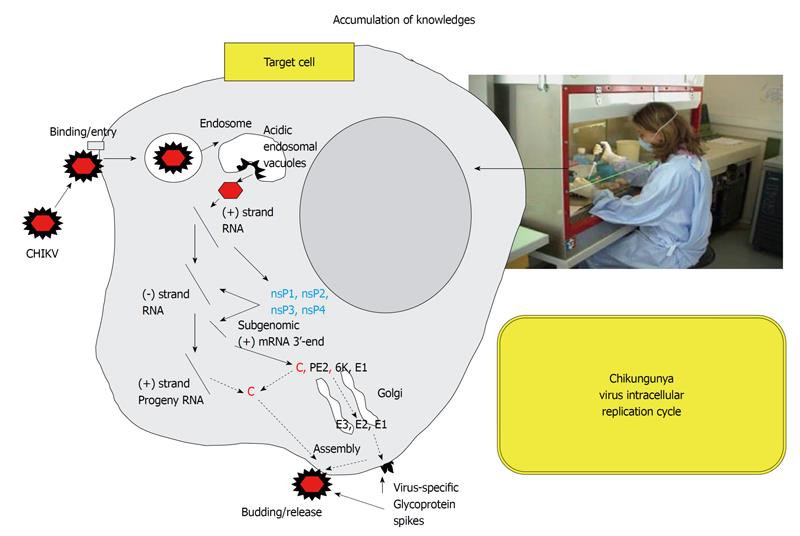
Research on vector control using environmentally safe insecticides will be required to interrupt the transmission of arboviruses. Other approaches could be to block the virus replication cycle. A better understanding of the replication cycle of CHIKV in both insect cells and mammalian cells should help move forward in this fight[53]. Insects cannot synthesize cholesterol de novo and depend on dietary cholesterol for their physiological requirements[54]. Under culture conditions that allows to question the requirement for membrane cholesterol in C6/36 Aedes albopictus cells infected by CHIKV, we found that cholesterol depletion reduced the infectivity of strains bearing an alanine residue at position 226 of E1 (African CHIKV strain), whereas strains bearing a valine at position 226 of E1 (the Reunion Island CHIKV strain) are markedly less sensitive, indicating that the presence of valine represents a selective advantage for the virus subjected to this selection pressure[55]. Yet, there are still many things to understand in the replication cycle of CHIKV (Figure 4). For example, we have recently demonstrated that entry of CHIKV into mammalian cells occurs by a pH-dependent pathway[52], and that entry of the virus into mosquito cells occurs by a pH-independent pathway[55]. In mammalian cells, CHIKV uses clathrin-independent, Eps15-dependent endocytosis as an entry pathway and requires functional early endosomes and low pH conditions in this compartment for productive infection[52]. The more we move forward in understanding intracellular cycle of the virus, the more chances we will have to design molecules capable of inhibiting the replication of the virus, thus developing them into efficient therapeutics.
Figure 4 Accumulation of knowledge.
We know relatively little about the cycle of CHIKV replication in mammalian cells and insect cells. It remains necessary to conduct experimental work to better understand the virus replication cycle, particularly in order to develop therapeutic agents. Understanding of each stage of the cycle, the molecular crosstalk between the pathogen and the host, and each molecular interaction, may offer a new avenue to fight the pathogen.
WORLD IS CHANGING, ECOSYSTEMS ARE CHANGING, BIOMONITORING MUST ADAPT
The few examples mentioned above illustrate the major development in the relations between pathogen, vector, animal and human target within ecosystems that have never changed so quickly. In this context, efforts should be made on the development of biosurveillance (Figure 5). This can be achieved, for example, by monitoring climate change, exposure to environmental hazards, vulnerability of populations, social behaviors, economic level of populations, hygiene of housing, demographic factors, and status of population health. It is also desirable to better inform people through communication among scholars, politicians and media, and probably consider warning signal coming from new sources, such as encouraging people to self-screen clinical signs of infection to accelerate the specialized care. This also involves a better knowledge of ecology of mosquitoes, the genetics of viruses, virus-target cells interaction in mosquitoes, animal reservoirs and humans, and replication cycle of the viruses[13,39,56]. Improvement of diagnostics and therapeutics is needed for early detection and control of infectious diseases (including preventive measures regarding blood transfusion in areas with a recent outbreak). This seems obvious, but one must know with certainty which pathogenic agent is causing the disease. In the past, similarities between symptoms of CHIKV-disease and DENV-disease, probably accounted for the frequent misclassification and some under-reports of CHIKV fever in areas with endemic DENV and, possibly, the incidence of CHIKV infection was much higher than reported[11]. On the basis of clinical symptoms, the infections by CHIKV may have been mistaken for infections caused by related viruses, such as RRV[57], or O’Nyong-nyong virus[58].
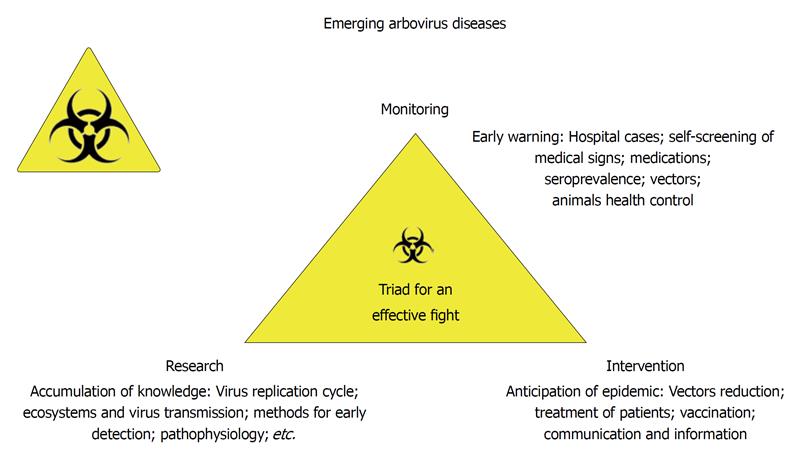
Figure 5 Emerging arbovirus diseases and the biosurveillance.
Biosurveillance is based on three main principles: research (accumulation of knowledge in all fields of science), large scale monitoring for early warning and intervention to prevent or reduce the epidemic risks.
Emerging infectious pathogens have significantly increased over the past few decades, prompting the need for more rapid outbreak detection and report. Of course, to prevent these health threats, many structures have been created that address this risk, such as the WHO, the Center for Disease Control (CDC, Atlanta), the European CDC, and others. An integrated global alert and response system for EID and other public health emergencies based on strong national public health systems and capacity, and an effective international system for coordinated response has been set up by the WHO[59]. The rapid expansion in Internet access and utilization has also provided a more open route for reporting that could push local governments toward greater transparency[10]. Additionally, NIAID and others (i.e. Welcome Trust, Gates Foundation), support training programs to maintain and develop the national and international scientific expertise required to respond to future health threats.
France obviously contributes to international efforts in the fight against emerging diseases. The country has specific structures such as the Institute for Public Health (InVS) with its division called the National Reference Center for arboviruses, and the International Network of Pasteur Institutes with many locations around the world. Facing the constant threat of emerging diseases, it is necessary to continue the development of a surveillance system of infectious diseases, to develop ways for quick diagnosis and prevention, and to expand international collaborations in case of emergence. But is this enough? Many French experts in the field of infectious diseases now consider the desirability of establishing Centers for Research and Biosurveillance, “Centre de Recherche et de Veille” (CRV), in five major regions of the world, to put themselves in situations dealing with the emergence of new pathogenic agents nearest to the place of emergence.
The need arose to establish a CRV when the crisis of CHIKV outbreak occurred in the Reunion Island. The main idea is simple: it is to set up transdisciplinary approaches to diseases of wildlife and domestic animals which have been highlighted as part of growing disciplines of conservation medicine. The purpose is to focus on more proactive approaches to surveillance, health assessment, and monitoring of wildlife populations as well as health and disease interactions with anthropogenic change and the ecological footprint. Indeed, the French Ministry of Health and the Ministry of Higher Education and Research quickly realized that despite the presence on this island of many specialists in biology, medicine, veterinary medicine and social sciences, the coordination between different institutions and different specialists of EID was poor. Decisions were made at the highest level of government to overcome these difficulties. As a consequence, the Indian Ocean CRV was created in 2007. CRVs should be valuable platforms to conduct biosurveillance under a close working relationship with neighboring countries’ governments and academic institutions. The synergy between this biosurveillance system near emergence sites and organizations of global health, is likely a formula for success. The CRV should also be centers where research teams can perform experiments under optimum quality and safety conditions. In addition, it will be necessary to establish quality controls to ensure that data produced by different research groups of the network of teams working in conjunction with the CRV, can be comparable and reproducible. The CRV may also help provide adequate scientific training to health professionals. Finally, the CRV could also be considered as a node of networks for international cohorts of patients or healthy individuals. The operation of these facilities should be provided through the contributions of individual institution partners, responding to international tenders to programs and the fund raising from international agencies and charitable organizations.
Ideally, in addition to the Indian Ocean CRV, it would be desirable to set up an African CRV, a Southeast Asia CRV, a Caribbean CRV, and a Mediterranean CRV. Of course, it depends largely on the geopolitical situation in these regions of the world, the state of diplomatic relations with the countries of the region, and the wishes expressed by our government in terms of scientific and health priorities.
ACKNOWLEDGMENTS
I am grateful to Dr. Isabelle Catala from the Emergency Department, Foch Hospital, Paris, for her helpful discussions. I thank Stephan Köhler (CPBS, UMR5236 CNRS-UM1-UM2, Montpellier) for his critical review of this manuscript.