Published online Jun 24, 2016. doi: 10.5500/wjt.v6.i2.336
Peer-review started: October 31, 2015
First decision: November 30, 2015
Revised: March 18, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: June 24, 2016
AIM: To study the impact of association between cytomegalovirus (CMV) pathogenesis with dendritic cell (DC) maturation and function was evaluated in CMV reactivated liver transplanted patients in comparing with non-reactivated ones, and healthy controls.
METHODS: Monocyte derived dendritic cells (MoDCs) was generated from collected ethylenediaminetetraacetic acid-treated blood samples from patient groups and controls. In these groups, expression rates and mean fluorescent intensity of DC markers were evaluated using flowcytometry technique. Secretion of cytokines including: interleukin (IL)-6, IL-12 and IL-23 were determined using enzyme-linked immunosorbent assay methods. The gene expression of toll-like receptor 2 (TLR2), TLR4 and IL-23 were analyzed using in-house real-time polymerase chain reaction protocols.
RESULTS: Results have been shown significant decreases in: Expression rates of MoDC markers including CD83, CD1a and human leukocyte antigen DR (HLA-DR), the mean fluorescence intensitys for CD1a and HLA-DR, and secretion of IL-12 in CMV reactivated compared with non-reactivated liver transplanted patients. On the other hand, significant increases have been shown in the secretions of IL-6 and IL-23 and gene expression levels of TLR2, TLR4 and IL-23 from MoDCs in CMV reactivated compared with non-reactivated liver transplanted recipients.
CONCLUSION: DC functional defects in CMV reactivated recipients, such as decrease in expression of DC maturation markers, increase in secretion of proinflammatory cytokines, and TLRs can emphasize on the importance of CMV infectivity in development of liver rejection in transplanted patients.
Core tip: Cytomegalovirus (CMV) can interfere with maturation and antigen-presenting function of dendritic cell (DC). This interference with DC function could promote viral spread by paralyzing the adaptive immune system. CMV with DC infection induces inflammatory cytokines and activation of the interferon pathway in transplanted patients. DCs undergo lytic viral cycles, can induce late gene expression of CMV, release of infectious virus, and stimulating of T-cell responses resulted to allograft rejection.
- Citation: Karimi MH, Shariat A, Yaghobi R, Mokhtariazad T, Moazzeni SM. Role of cytomegalovirus on the maturation and function of monocyte derived dendritic cells of liver transplant patients. World J Transplant 2016; 6(2): 336-346
- URL: https://www.wjgnet.com/2220-3230/full/v6/i2/336.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i2.336
Liver transplantation is the definitive treatment of choice for patients with end-stage liver disease[1]. Graft rejection and infection remains major complications post liver transplantation[2]. In liver recipients, cytomegalovirus (CMV) is the most determinative viral infectious pathogen cause of morbidity and mortality post-liver engraft and associate with diminished graft survival[3]. This ubiquitous viral infection in immunocompromised transplant patients belongs to family Herpesviridae, subfamily Betaherpesvirinae, genus human herpes virus 5, species Cytomegalovirus[4]. CMV primary infection results in life-long residence of the virus in the host, and reactivates in immunocompromised individuals frequently. Reactivation of CMV infection and development of related severe diseases and syndromes are common in solid organ recipients may lead to severe complications following transplant, such as acute rejection[5,6]. CMV may lead also to higher rates of bacterial and fungal infections in transplant recipients[7]. In transplant patients, CMV infection causes both direct effects, reflecting cell destruction and indirect effects, such as acute or chronic rejection[8]. Primary CMV infection induced immune related proinflammatory response that was maintained during latency. This continuous activation of the immune system may play a role in the acceleration of chronic diseases and pathogenesis of chronic allograft rejection[9,10].
CD14+ monocytes and/or myeloid progenitor cells are site of CMV latency and are capable of harboring quiescent viral genomes[11]. Monocyte represents a key cell type in the CMV pathogenesis, since mostly represent as an important cellular reservoir for latent virus[12,13]. A number of studies have shown that CMV infection in monocytes is non-permissive and cellular differentiation is prerequisite for CMV replication[11]. CMV replication can be reactivating in latently infected monocytes related to differentiation dependent manner[11]. The dendritic cells (DCs) generated from CMV infected monocytes. CMV infected monocyte derived DCs (CMV-MoDCs) have an altered phenotype and functional defects[14]. DCs are determinative initiators of cellular immunity against CMV infection[1]. DCs also act with superiority over other antigen-presenting cells (APCs) in stimulating T-lymphocyte responses and maintaining protective antiviral immunity[15].
CMV can interfere with maturation and antigen-presenting function of DCs and also disturb both innate and adaptive immunity[14,16]. This interference with DC function could promote viral spread by paralyzing the adaptive immune system[17]. CMV with DC infection induces many hallmarks of innate immunity, such as the production of inflammatory cytokines and activation of the interferon pathway in transplanted patients. This induction is rapid and can promote without requirement of CMV reactivation. DCs undergo lytic viral cycles, can induce late gene expression of CMV, release of infectious virus, and stimulating of T-cell responses resulted to allograft rejection[8].
Therefore, in this study the impact of association between CMV pathogenesis with DC maturation and function was evaluated in CMV reactivated liver transplant patients in comparing with non-reactivated ones and healthy controls.
Ten liver transplanted patients who admitted at Transplant Center of Namazi Hospital, Shiraz, Iran were enrolled in this study between years 2012 and 2014. These patients divided to two groups including: 5 patients with CMV reactivation and rest of them without CMV reactivation. Therefore, CMV reactivation was confirmed in these transplanted patients using antigenemia protocol. The CMV antigen positive cells were counted and positive results are reported as one or more CMV pp65 antigen infected cell per 50000 white blood cells (WBCs). In all 5 patients with CMV reactivation 5 to 7 CMV pp65 antigen infected cell was found per 50000 WBCs, with a mean of 6 ± 1.01 cells/50000 WBCs. Underlying diseases for studied liver transplanted patients have been shown in Tables 1 and 2. The 20 mL ethylenediaminetetraacetic acid (EDTA)-treated blood samples were collected from each evaluated transplant recipients.
| Underlying diseases | Patients (n = 10) |
| PSC | 2 |
| Hypercholesterolemia | 1 |
| Cryptogenic cirrhosis | 2 |
| Hepatitis C virus infection | 1 |
| Hepatitis B virus infection | 1 |
| Wilson disease | 1 |
| NASH | 1 |
| Autoimmune hepatitis | 1 |
| Surface marker | CMV reactivated patients | CMV non-reactivated patients | Healthy controls of MoDCs |
| MFI ± SE | MFI ± SE | MFI ± SE | |
| CD83 | 30.15 ± 1.06 | 32.3 ± 2.3 | 29.2 ± 2.5 |
| CD86 | 100.5 ± 3.1 | 103 ± 4.5 | 83 ± 6.7 |
| CD1a | 47 ± 1 | 80 ± 3 | 53 ± 13 |
| HLA-DR | 49.3 ± 5.4 | 73.6 ± 6.5 | 55.8 ± 4.9 |
Also, 20 mL EDTA-treated blood samples were collected from 5 healthy volunteers as healthy controls. The age range (20-50 years old) and male to female ratio were similar in studied transplanted patient groups and healthy controls. This study was in accordance with the ethical standards of Shiraz University of Medical Sciences Committee (the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki). The informed consent was obtained from each studied transplanted patients. The immunosuppressive conditioning regimen used in liver transplanted patients was previously described[18].
CMV antigenemia protocol was performed on the EDTA-treated blood samples to determine viral reactivation by evaluation of the presence of lower matrix pp65 antigen in polymorph nuclear cells using the CMV Brite Turbo kit (IQ Products, Groningen, Netherlands) according manufacturer instruction as previously described[19].
Leukocytes were isolated from 20 mL EDTA-treated blood samples collected from each liver transplanted patients with and without CMV reactivation using gradient centrifugation through Lymphodex (Inno-train, Germany), the cells from interphase were collected. CD14+ monocytes were isolated by positive selection using a MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer’s protocol.
Monocytes were cultured in six-well cell culture plates in RPMI medium (Invitrogen, United States) supplemented with 4 mmol/L L-glutamine (Life technologies, United States), 100 IU/mL penicillin (Life technologies, United States), 10% heat-inactivated fetal bovine serum (Life technologies, United States), 1% sodium pyruvate (Bioidea, Iran), 1% non-essential amino acid (Life technologies, United States), 1000 IU/mL recombinant human granulocyte macrophage-colony stimulating factor (R and D Systems, United Kingdom) and 500 IU/mL recombinant human interleukin-4 (IL-4; R and D Systems, United Kingdom) in a 37 °C 5% CO2 humidified incubator. Every 3 d 200 μL of the medium was exchanged with fresh medium and cytokines. For mature cells, maturation was induced on day 5 by adding 1000 IU/mL recombinant human tumor necrosis factor-α (TNF-α; R and D Systems, United Kingdom) and allowing maturation to proceed for 48 h.
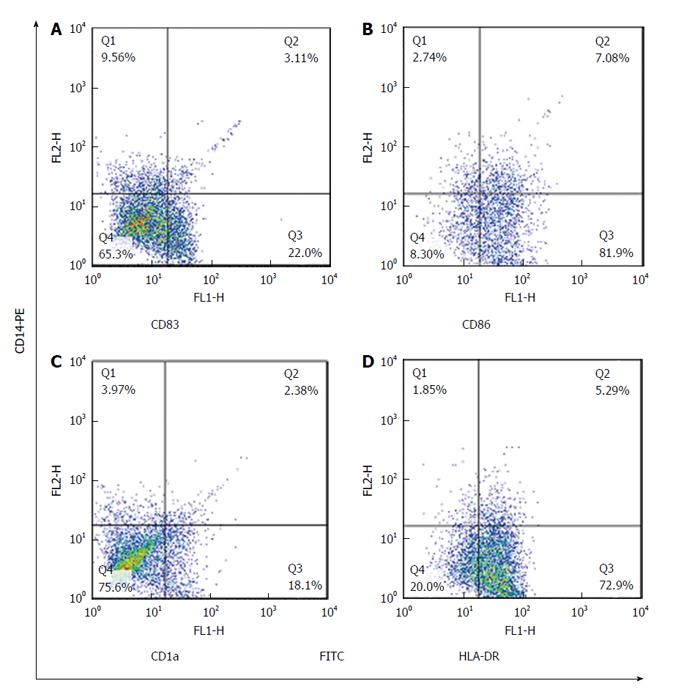
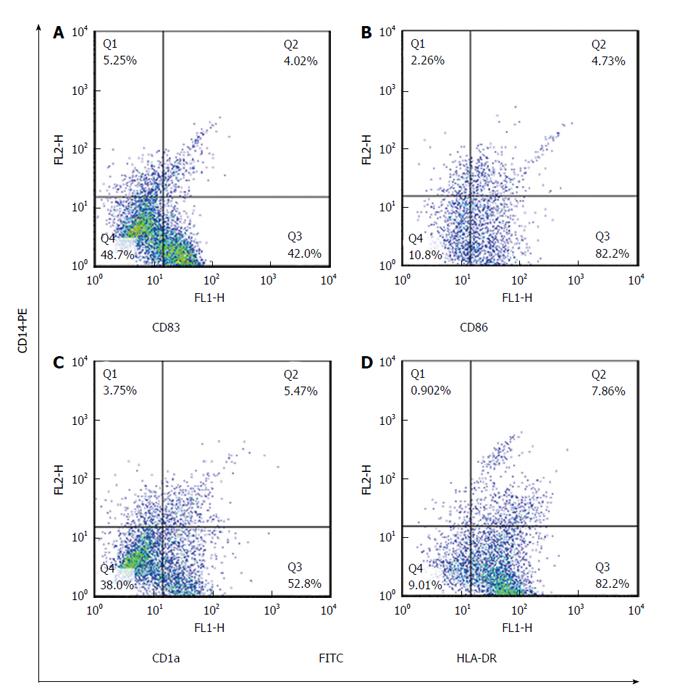
Maturated MoDCs were harvested and stained with fluorescently labeled monoclonal antibodies including: PE-anti-CD14, FITC-anti-CD83, FITC-anti-CD86, FITC-anti-CD1a and FITC-anti-HLADR (eBiosciences, United States). Cell suspension was mixed with antibody solution followed by incubation in the dark at 4 °C for 45 min. Cells were suspended in phosphate buffer saline and data acquisition for 10000 events was performed using flowcytometry (Becton Dickinson, San Jose, United States). FlowJo software (Flexera Company, United States) was used to analyze the expression rate and mean fluorescence intensity (MFI) of studied DC markers.
The IL-6, IL-12 and IL-23 cytokines released from MoDCs in culture supernatant were measured using the commercial human enzyme-linked immunosorbent assay Ready-SET-Go kits (eBioscience, United States) according to the manufacturer’s protocols.
Total RNA was extracted from MoDCs using RNX plus (CinnaGen, Iran). RNA samples were reverse transcribed using Reverse Transcriptase (Vivantis, Malaysia) and random hexamer as previously described[20]. An amount of 1 μg total RNA was used to produce cDNA. The primers that were used to analyze the gene transcripts including: TLR-2 (NM_003264.3), TLR-4 (NM_003266.3), IL-23 (NM_016584.2) and β-actin (NM_001101.3)[18]. The mRNA expression levels of the IL-23, TLR2 and TLR4 genes were finally determined in MoDCs of liver transplanted patients with and without CMV reactivation compared with healthy controls using in-house-real time polymerase chain reaction (PCR) protocols as previously described[18].
The PCR reaction was carried out in a final volume of 20 μL containing: 10 μL SYBR green Premix by Ex taq (Takara, Japan), 0.4 μL SYBR Green Dye, 0.8 μL forward and 0.8 μL reverse primers (8 pmol), 6 μL H2O and 2 μL cDNA template. The thermal cycling profile was the same for each primer set and consisted of an initial denaturation at 95 °C for 2 min, followed by 40 amplification cycles of 95 °C for 30 s and 65 °C for 20 s using Step One Plus Real-Time instrument (ABI, Step One Plus, United States). The mean Ct value of target genes in each sample was normalized using β-actin gene Ct value to give a ΔCt value. This was then normalized to healthy control (ΔΔCt), and finally the 2-ΔΔCt.
Statistical differences between studied groups were evaluated using non-parametric tests of version 15 of SPSS software (Chicago, United States). The sample analysis was also analyzed using version 5 of Graph Pad Prism software (United States). The 2-ΔΔCt value was calculated using Livak method for analysis of the expression level of studied genes. The P-value of < 0.05 was considered as significant.
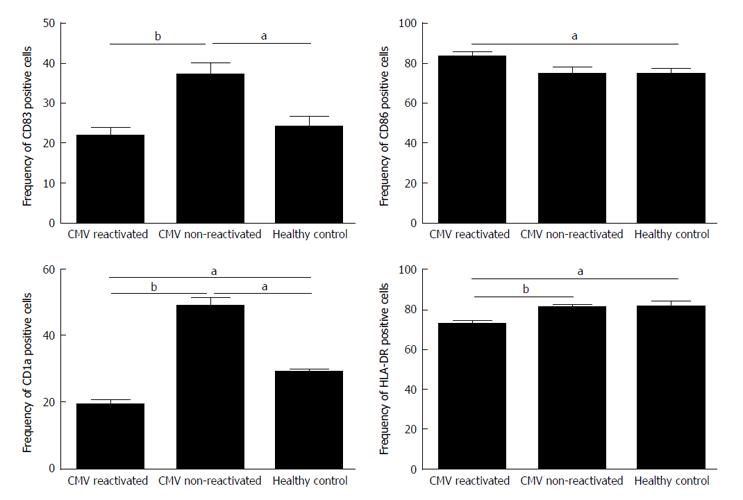
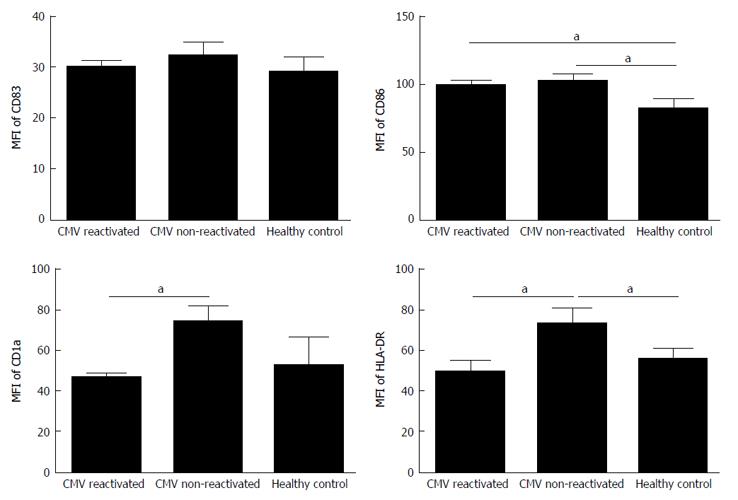
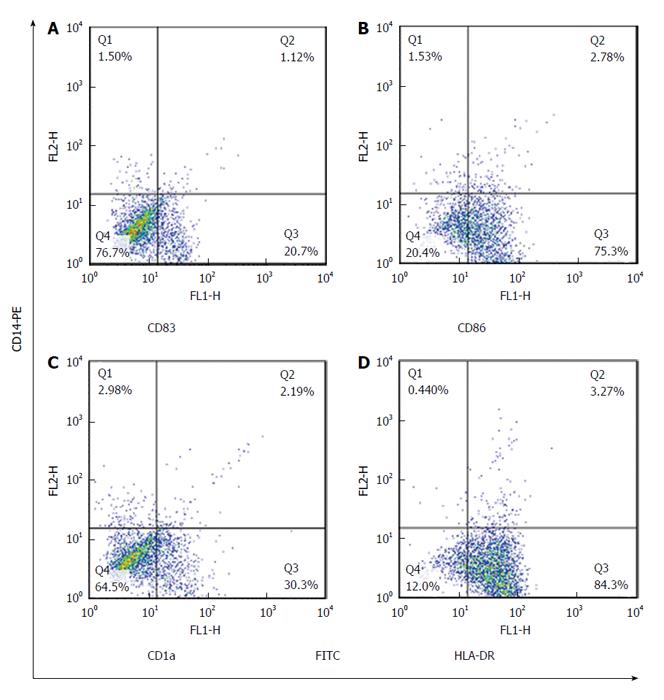
The expression rate (P = 0.02) and MFI (P = 0.04) of CD86 were both significantly increased in CMV reactivated patients in comparing with healthy controls with median value of 82% vs 75%, respectively (Figures 1 and 2). The expression rate of CD1a was significantly decreased in CMV reactivated patients in comparing with healthy controls with median value of 18% vs 30%, respectively (P = 0.01) (Figure 3). The expression rate of human leukocyte antigen DR (HLA-DR) was significantly decreased in CMV reactivated patients in comparing with healthy controls with median value of 72% vs 84%, respectively (P = 0.01) (Figure 4).
The expression rate of CD83 was significantly increased in CMV non-reactivated patients in comparing with healthy controls with median value of 40% vs 21%, respectively (P = 0.02) (Figure 1). The MFI of CD86 was significantly higher in CMV non-reactivated patients than that in healthy control (P = 0.04) (Figure 2). The expression rate of CD1a was significantly raised in CMV non-reactivated patients than that in healthy controls with median value of 50% vs 30%, respectively (P = 0.01) (Figure 1). The MFI of HLA-DR was significantly raised in CMV non-reactivated liver transplanted patients than that in healthy controls (P = 0.03) (Figure 5).
Expression rate of CD83 was significantly decreased in CMV reactivated compared with non-reactivated liver transplanted patients with median value of 22% vs 40%, respectively (P = 0.007) (Figure 1). The expression rate (P = 0.007) and MFI (P = 0.02) of CD1a was significantly lower in CMV reactivated compared with non-reactivated patients with median value of 18% vs 50%, respectively (Figures 1 and 2). The expression rate (P = 0.007) and MFI (P = 0.03) of HLA-DR was significantly decreased in CMV reactivated patients compared with non-reactivated recipients with median value 72% vs 80%, respectively (Figures 1 and 2).
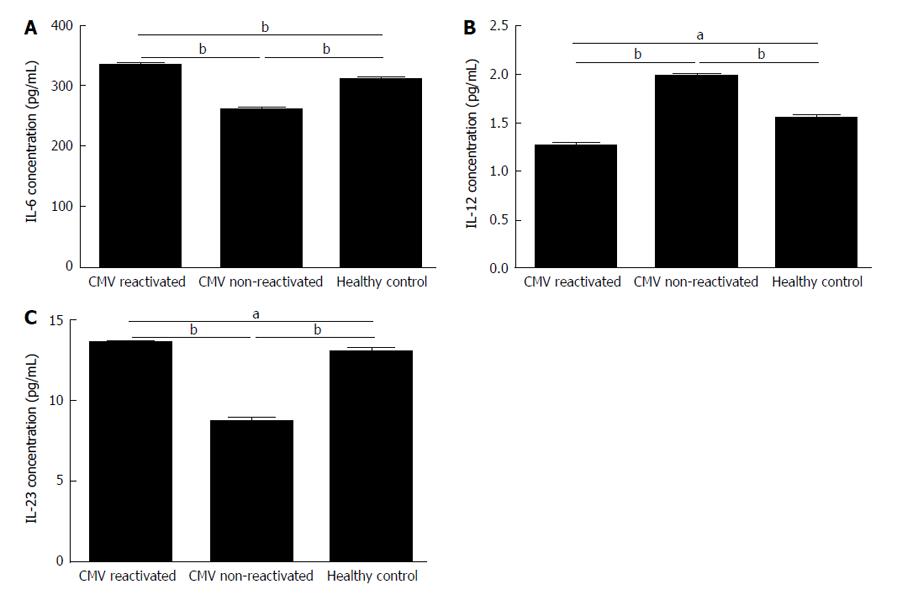
The secretion of IL-6 from MoDCs of CMV reactivated patients was significantly increased in comparing with healthy control with mean of 334.6 ± 2.2 pg/mL vs 312 ± 1.08 pg/mL (P = 0.009) (Figure 6A). The secretion of IL-12 from MoDCs of CMV reactivated patients was significantly decreased in comparing with healthy control with mean of 1.26 ± 0.04 pg/mL vs 1.54 ± 0.03 pg/mL (P = 0.01) (Figure 6B). The IL-23 secretion level from MoDCs of CMV reactivated patients was significantly increased in comparing with healthy control with mean of 13.53 ± 0.09 pg/mL vs 13.1 ± 0.1 pg/mL (P = 0.02) (Figure 6C).
The secretion of IL-6 from MoDCs of CMV non-reactivated patients was significantly decreased in comparing with healthy control with mean of 261.2 ± 3.72 pg/mL vs 312 ± 1.08 pg/mL (P = 0.006) (Figure 6A). Also, secretion of IL-12 from MoDCs of CMV non-reactivated patients was significantly increased in comparing with healthy control with mean of 1.98 ± 0.03 pg/mL vs 1.54 ± 0.03 pg/mL (P = 0.009) (Figure 6B). The secretion of IL-23 from MoDCs of CMV non-reactivated patients was significantly decreased in comparing with healthy control with mean of 8.77 ± 0.19 pg/mL vs 13.1 ± 0.1 pg/mL (P = 0.008) (Figure 6C).
The secretion of IL-6 from MoDCs was significantly higher in CMV reactivated patients than that in CMV non-reactivated ones with mean of 334.6 ± 2.2 pg/mL vs 261.2 ± 3.72 pg/mL (P = 0.005) (Figure 6A). The secretion of IL-12 from MoDCs was significantly lower in CMV reactivated patients compared with non-reactivated ones with mean of 1.26 ± 0.04 pg/mL vs 1.98 ± 0.03 pg/mL (P = 0.007) (Figure 6B). The secretion of IL-23 from MoDCs was significantly higher in CMV reactivated patients than that in CMV non-reactivated ones with mean of 13.53 ± 0.09 pg/mL vs 8.77 ± 0.19 pg/mL (P = 0.007) (Figure 6C).
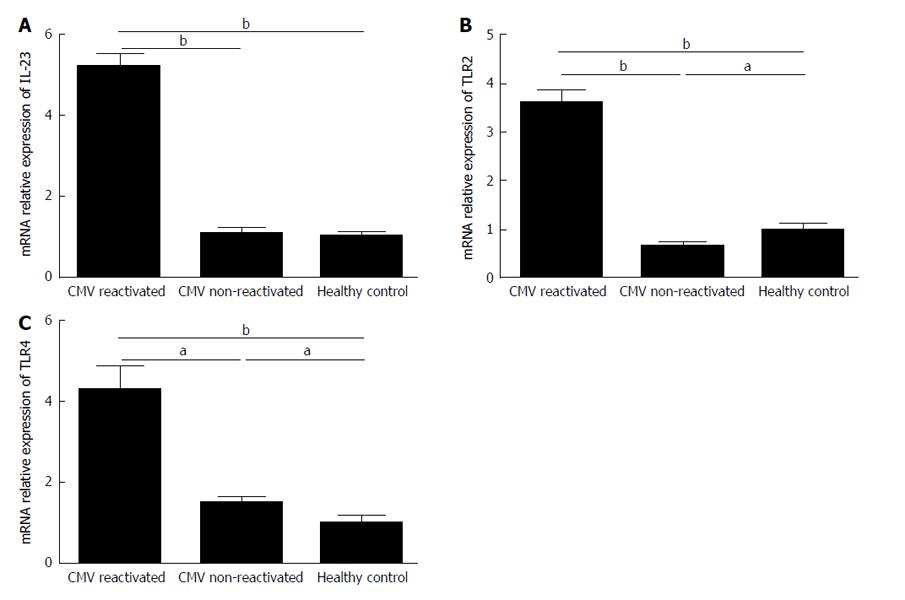
The IL-23, TLR2, and TLR4 mRNAs was expressed 5.2 (P = 0.005), 3.6 (P = 0.007), and 4.3 (P = 0.009) folds significantly more in CMV reactivated patients compared with healthy controls, respectively (Figure 7).
The gene expression level of IL-23 was the same in both CMV non-reactivated patients and healthy controls (P = 0.6) (Figure 7A). The TLR2 mRNA expression was significantly decreased in CMV non-reactivated patients in comparing with healthy controls (P = 0.02) (Figure 7B). The IL-23, TLR2, and TLR4 mRNAs was expressed 4.7 (P = 0.007), 5.4 (P = 0.005), and 2.8 (P = 0.01) folds significantly more in CMV reactivated compared with non-reactivated patients, respectively (Figure 7).
DCs have superiority over other APCs in viral infections to stimulate T-cell responses and maintaining protective antiviral immunity[15]. DCs are critical initiators of cellular immunity against viruses especially CMV[1]. CMV as a determinative human pathogen can cause fatal complications and promote rejection in transplanted recipients[21]. CMV infection can also increase the rate of immunosuppression with interfering the maturation and function of DCs post-transplantation[16,22]. Therefore, in this report, the effects of CMV reactivation compared to non-reactivation were elucidated on maturation and function of DCs in liver transplanted recipients and healthy controls.
CMV and DC maturation was interested of related researchers in earlier studies[16,22]. Down-regulation of major histocompatibility complex (MHC) class I and CD86 and CD83 costimulatory molecules on immature DCs and inhibition of DC maturation was indicated following in vitro CMV infection of DCs[16]. CMV targets DCs and alters their functions by interfering with MHC-II biosynthesis and maturation, as well as with the expression and function of related endocytic proteases[23]. CMV-infected DCs displayed abnormal phenotypic characteristic and stable expression of CD83 as a maturation marker[24,25]. Infectivity of CMV in MoDCs resulted in maturation of the surviving cells, up-regulation of the CD86, and down-regulation of MHC-I and II[21,26]. In this study, results also demonstrated the down-regulation of CD83, CD1a and HLA-DR molecules on MoDCs in CMV reactivated compared to non-reactivated liver transplanted patients. The MFIs of CD1a and HLA-DR were significantly down-regulated in CMV reactivated patients compared to non-reactivated ones. On the other hand CMV-mediated up-regulation of CD86 and down-regulation of CD1a and HLA-DR molecules were found in CMV reactivated patients compared to healthy controls. Therefore CMV interference with maturation of DCs promotes viral spread by paralyzing the adaptive immune system[17]. Especially, in transplanted recipients, CMV-infected DCs are less capable of developing antiviral activated APCs, this may lead to impaired immune responses not only against CMV, but most likely also against other invading microorganisms.
Stimulation of toll-like receptors (TLRs) on DCs activates signal transduction pathways lead to induction of a range of antimicrobial genes and inflammatory cytokines[11,27]. TLR signaling pathways trigger a series of interactions among specific intracellular mediators that ultimately result in the release of nuclear factor-κB (NF-κB) from its related endogenous inhibitors[11]. Earlier reports emphasized that markedly up-regulation of TLR2 and TLR4 responsible for early activation of alloimmune T-cells favoring to acute renal and also liver allograft rejection[28-30]. TLR2 was recently identified as a cell surface receptor activates secretion of inflammatory cytokine response to CMV infection[4,11]. In vitro stimulation of TLR2 by CMV resulted in NF-κB activation and cytokine secretion[31,32]. CMV was also able to activate TLR4 and mediate cytokine secretion in human monocytic cells[33]. Similarly, TLR2 and TLR4 gene expression by MoDCs was significantly increased in studied CMV reactivated liver transplanted patients compared to non-reactivated ones and healthy controls.
Pathological processes associated with CMV reactivation appear to be mediated by the release of inflammatory cytokines[11]. Following CMV infection DCs produce no IL-12 and only low levels of TNF-α[16]. Down-regulation of IL-12 production impairs the antiviral mechanisms of T cells and NK cells in patients with active CMV infection[34]. Similar to previous reports, results of the present study revealed that secretion of IL-12 by MoDCs was significantly decreased in CMV reactivated liver transplanted patients compared to CMV non-reactivated ones and healthy controls. But, Th17 cell linage and related cytokines like IL-17 and IL-23 have determinative role contribute to the mechanisms of allograft rejection[35-39]. IL-6 is also essential for differentiation of IL-17-producing human Th cells[40]. IL-6 and subsequent signaling pathways are important for activation and differentiating DCs. Activation and concomitant production of these cytokines also appear to be essential for reactivation and replication of CMV in infected patients such as transplant recipients[21]. Similarly, IL-6 and IL-23 secretion and expression by MoDCs are significantly higher in CMV reactivated in comparing with non-reactivated patients and healthy controls.
In conclusion, results of this study highlight the fact that, CMV and DCs contractions promote different pathways including: Interference with the maturation and expression of DC markers (CD83, CD1a and HLA-DR), IL-12 decreasing and IL-6 and IL-23 elevation from MoDCs and also increase of the mRNA expression levels of TLR2, TLR4 and IL-23 genes in MoDCs of CMV reactivated liver transplanted patients. These pathways can implicate in the development of acute or chronic allograft liver rejection.
The study was financially supported using a grant from Iran National Science Foundation. We also thanks to Transplant Research Center of Namazi Hospital, Shiraz, Iran for Lab support.
Cytomegalovirus (CMV) is the most determinative viral infectious pathogen cause of morbidity and mortality post-liver engraft and associate with diminished graft survival. CMV infected monocyte derived dendritic cells (CMV-MoDCs) have an altered phenotype and functional defects. DCs are determinative initiators of cellular immunity against CMV infection. CMV can interfere with maturation and antigen-presenting function of DCs and also disturb both innate and adaptive immunity. DCs undergo lytic viral cycles, can induce late gene expression of CMV, release of infectious virus, and stimulating of T-cell responses resulted to allograft rejection. However, association between CMV pathogenesis with DC maturation and function in CMV reactivated liver transplant patients was not yet evaluated.
CMV can interfere with maturation and antigen-presenting function of DCs and also disturb both innate and adaptive immunity. This interference can promote viral spread by paralyzing the adaptive immune system. CMV with DC infection induce the production of inflammatory cytokines and activation of the interferon pathway in transplanted patients.
This is the first study evaluating the interferance between CMV reactivation with maturation and antigen-presenting function of DCs in Iranian liver transplanted patients.
Interference of CMV and DCs can promote viral spread by paralyzing the adaptive immune system and induce the production of inflammatory cytokines and activation of the interferon pathway in transplanted patients. Results of this study highlight the fact that, CMV and DCs contractions promote different pathways including: Interference with the maturation and expression of DC markers and cytokines in MoDCs of CMV reactivated liver transplanted patients. These pathways can implicate in the development of acute or chronic allograft liver rejection.
CMV infected MoDCs have an altered phenotype and functional defects. DCs are determinative initiators of cellular immunity against CMV infection. DCs also act with superiority over other antigen-presenting cells in stimulating T-lymphocyte responses and maintaining protective anti-CMV immunity.
The manuscript entitled, “Role of cytomegalovirus on the maturation and function of monocyte derived dendritic cells of liver transplant patients”, by Karimi et al, demonstrated the functional defects of dendritic cells in CMV reactivated liver transplant recipients when compared to those without CMV reactivation or healthy norms demonstrating the differences in cytokine concentrations and expressions. This is very detailed investigation of cytokines of monocyte derived dendritic cells.
P- Reviewer: Boin IFSF, Sugawara Y, van Hoek B S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Sénéchal B, Boruchov AM, Reagan JL, Hart DN, Young JW. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103:4207-4215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | DeFilippis VR, Sali T, Alvarado D, White L, Bresnahan W, Früh KJ. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J Virol. 2010;84:8913-8925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76-98, Table of Contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 459] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 4. | Boehme KW, Singh J, Perry ST, Compton T. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J Virol. 2004;78:1202-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Söderberg-Nauclér C, Streblow DN, Fish KN, Allan-Yorke J, Smith PP, Nelson JA. Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent. J Virol. 2001;75:7543-7554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Sampathkumar P, Paya CV. Management of cytomegalovirus infection after liver transplantation. Liver Transpl. 2000;6:144-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Mardani M. The spectrum of CMV disease in solid organ transplant recipients. Iranian J Clin Infect Dis. 2009;4:125-127. [Cited in This Article: ] |
| 8. | Rossini G, Cerboni C, Santoni A, Landini MP, Landolfo S, Gatti D, Gribaudo G, Varani S. Interplay between human cytomegalovirus and intrinsic/innate host responses: a complex bidirectional relationship. Mediators Inflamm. 2012;2012:607276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | van de Berg PJ, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, Bemelman FJ, van Lier RA, ten Berge IJ. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis. 2010;202:690-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Gao LH, Zheng SS. Cytomegalovirus and chronic allograft rejection in liver transplantation. World J Gastroenterol. 2004;10:1857-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588-4596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 518] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest. 1997;100:3154-3163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Söderberg-Nauclér C, Nelson JY. Human cytomegalovirus latency and reactivation - a delicate balance between the virus and its host’s immune system. Intervirology. 1999;42:314-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Carlier J, Martin H, Mariamé B, Rauwel B, Mengelle C, Weclawiak H, Coaquette A, Vauchy C, Rohrlich P, Kamar N. Paracrine inhibition of GM-CSF signaling by human cytomegalovirus in monocytes differentiating to dendritic cells. Blood. 2011;118:6783-6792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Gredmark S, Söderberg-Nauclér C. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J Virol. 2003;77:10943-10956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99:2913-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Rölle A, Olweus J. Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures. APMIS. 2009;117:413-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Shariat A, Karimi MH, Mokhtariazad T, Moazzeni SM, Geramizadeh B, MalekHosseini SA, Yaghobi R. Maturation state and function of monocyte derived dendritic cells in liver transplant recipients. Iran J Immunol. 2014;11:153-165. [PubMed] [Cited in This Article: ] |
| 19. | Saadi MI, Yaghobi R, Karimi MH, Geramizadeh B, Ramzi M, Zakerinia M. Association of the costimulatory molecule gene polymorphisms and active cytomegalovirus infection in hematopoietic stem cell transplant patients. Mol Biol Rep. 2013;40:5833-5842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Afshari A, Yaghobi R, Karimi MH, Darbooie M, Azarpira N. Interleukin-17 gene expression and serum levels in acute rejected and non-rejected liver transplant patients. Iran J Immunol. 2014;11:29-39. [PubMed] [Cited in This Article: ] |
| 21. | Gredmark-Russ S, Söderberg-Nauclér C. Dendritic cell biology in human cytomegalovirus infection and the clinical consequences for host immunity and pathology. Virulence. 2012;3:621-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Hertel L. Human cytomegalovirus tropism for mucosal myeloid dendritic cells. Rev Med Virol. 2014;24:379-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kessler T, Reich M, Jahn G, Tolosa E, Beck A, Kalbacher H, Overkleeft H, Schempp S, Driessen C. Human cytomegalovirus infection interferes with major histocompatibility complex type II maturation and endocytic proteases in dendritic cells at multiple levels. J Gen Virol. 2008;89:2427-2436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schönrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836-4847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Huang MM, Kew VG, Jestice K, Wills MR, Reeves MB. Efficient human cytomegalovirus reactivation is maturation dependent in the Langerhans dendritic cell lineage and can be studied using a CD14+ experimental latency model. J Virol. 2012;86:8507-8515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240-273, Table of Contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1862] [Cited by in F6Publishing: 1968] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 28. | Hoffmann U, Bergler T, Rihm M, Pace C, Krüger B, Jung B, Reinhold SW, Farkas S, Rümmele P, Krämer BK. Impact of Toll-like receptor 2 expression in renal allograft rejection. Nephrol Dial Transplant. 2011;26:1080-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Testro AG, Visvanathan K, Skinner N, Markovska V, Crowley P, Angus PW, Gow PJ. Acute allograft rejection in human liver transplant recipients is associated with signaling through toll-like receptor 4. J Gastroenterol Hepatol. 2011;26:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Deng JF, Geng L, Qian YG, Li H, Wang Y, Xie HY, Feng XW, Zheng SS. The role of toll-like receptors 2 and 4 in acute allograft rejection after liver transplantation. Transplant Proc. 2007;39:3222-3224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094-7102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Brown RA, Gralewski JH, Razonable RR. The R753Q polymorphism abrogates toll-like receptor 2 signaling in response to human cytomegalovirus. Clin Infect Dis. 2009;49:e96-e99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Yew KH, Carpenter C, Duncan RS, Harrison CJ. Human cytomegalovirus induces TLR4 signaling components in monocytes altering TIRAP, TRAM and downstream interferon-beta and TNF-alpha expression. PLoS One. 2012;7:e44500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Nordøy I, Müller F, Nordal KP, Rollag H, Lien E, Aukrust P, Froland SS. The role of the tumor necrosis factor system and interleukin-10 during cytomegalovirus infection in renal transplant recipients. J Infect Dis. 2000;181:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1371] [Cited by in F6Publishing: 1352] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 36. | Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2961] [Cited by in F6Publishing: 3045] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 37. | Hoeve MA, Savage ND, de Boer T, Langenberg DM, de Waal Malefyt R, Ottenhoff TH, Verreck FA. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol. 2006;36:661-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Nakagiri T, Inoue M, Minami M, Shintani Y, Okumura M. Immunology mini-review: the basics of T(H)17 and interleukin-6 in transplantation. Transplant Proc. 2012;44:1035-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 20] [Reference Citation Analysis (0)] |
| 39. | Goriely S, Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr Opin Organ Transplant. 2008;13:4-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1385] [Cited by in F6Publishing: 1528] [Article Influence: 89.9] [Reference Citation Analysis (0)] |