Published online Sep 24, 2014. doi: 10.5500/wjt.v4.i3.153
Revised: June 16, 2014
Accepted: July 25, 2014
Published online: September 24, 2014
Donor human leukocyte antigen (HLA)-specific antibodies (DSA) play an important role in solid organ transplantation. Preexisting IgG isotype DSA are considered a risk factor for antibody mediated rejection, graft failure or graft loss. The post-transplant development of DSA depends on multiple factors including immunogenicity of mismatched antigens, HLA class II typing of the recipient, cytokine gene polymorphisms, and cellular immunoregulatory mechanisms. De novo developed antibodies require special attention because not all DSA have equal clinical significance. Therefore, it is important for transplant clinicians and transplant immunologists to accurately characterize DSA. In this review, the contemporary immunological techniques for detection and characterization of anti-HLA antibodies and their pitfalls are described.
Core tip: In solid organ transplantations the graft outcomes critically depend on the degree of human leukocyte antigen (HLA) matching between the donor and recipient. Although the cellular component of the allogeneic immune response to the transplanted tissue plays a key role, the contribution of antibodies should not be underestimated. The detection of anti-HLA class I and class II antibodies is an important component of the initial work-up of a potential transplant candidate. The introduction of new highly sensitive technologies such as solid-phase based technologies has had a tremendous effect on organ allocation and immunomodulation strategies.
- Citation: Lobashevsky AL. Methodological aspects of anti-human leukocyte antigen antibody analysis in solid organ transplantation. World J Transplant 2014; 4(3): 153-167
- URL: https://www.wjgnet.com/2220-3230/full/v4/i3/153.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i3.153
In most cases the development of alloantibodies against human leukocyte antigens (HLAs) is related to immunization via blood and/or blood product transfusions, pregnancy, and transplants. There are scattered reports in the literature indicating the production of HLA antibodies may be elicited by vaccinations and infections due to cross reactivity between viral/bacterial antigens and HLAs[1-4] or through the bystander effect[5-8]. Humoral or antibody-mediated immunity requires noncovalent contact between antigens and antibodies. The hyper variable regions of the light and heavy immunoglobulin chains are termed complementarity-determining regions and they are primarily involved in the interaction with antigens. Antibody effector functions are specified by the constant domains of the heavy chains. The most important function of these domains is the activation of the complement cascade, which is triggered by conformational changes in the hinge area after antigen binding. Complement activation results in the destruction of the cell membrane.
In solid organ transplantations of the kidney, heart, lung, and pancreas graft outcomes critically depend on the degree of HLA matching between the donor and recipient[9-17]. The cellular components of the allogeneic immune response to the transplanted tissue play a key role in this matching and the contribution of antibodies should not be underestimated[18-22]. The detection of anti-HLA class I and class II antibodies is an important component of the initial work-up of a potential transplant candidate (TC). The rationale for obtaining this information is related to clinical studies, which have universally demonstrated that pre-existing donor specific antibodies (DSAs) represent a significant risk factor for graft outcome[23-34]. The importance of the post-transplant monitoring of DSAs in kidney and cardiac transplants has been widely described[35-45]. The de novo development of DSAs strictly depends on the antigenicity and immunogenicity of mismatched HLAs. The substantial influence on antibody production involves other factors such as the HLA class II type of the responder, immunosuppressive medications, cytokine and chemokine genomic polymorphisms, and the hormonal background of the recipient[11,45-50].
It is generally accepted that de novo developed DSAs represent a risk factor for graft failure even at low concentrations. The early detection of DSAs considerably reduces the incidence of antibody-mediated rejection (AMR) and transplant glomerulopathy[23,51-58]. A post-transplant antibody analysis is a part of the routine monitoring of recipients. The introduction of new highly sensitive technologies such as solid-phase based technologies has had a tremendous effect on the clinical approach to anti-HLA antibody analysis[56-58]. The purpose of this review is to familiarize the reader with the methodological aspects and pitfalls of anti-HLA antibody analysis in solid-organ TC. Solid phase (SP) techniques will be specifically addressed.
Complement-dependent cytotoxicity: The long-established NIH complement-dependent cytotoxicity (CDC) method and its modifications are still widely used[59-64]. This assay allows the identification of high concentrations of antibodies to HLAs. There are two main purposes for applying the CDC method. This method can be used to estimate the percent of reactive antibodies (PRA) when the recipient serum is incubated with a panel of HLA typed T- or B-lymphocytes. If the serum contains antibodies against a particular HLA then the addition of rabbit complement causes cell death that is visualized by staining and microscopic examination. This test is also used for the cross-matching (CM) or detection of complement binding antibodies against the HLAs of a particular donor. Various modifications of the NIH CDC method including extended incubation, additional washings, and the addition of secondary antihuman light kappa chain specific antibodies have been used to increase the sensitivity of the assay. Notably, the NIH CDC assay detects anti-HLA antibodies of the IgG and IgM isotypes. However, the IgM isotype has considerably less clinical significance[20,64-68]. Donors with HLA recipient antibodies detected by CDC should be avoided due to the high risk of hyperacute or delayed hyperacute rejection. Currently, this assay is primarily used to determine the efficacies of the desensitization or immunomodulation of recipients with high concentrations of anti-HLAs and identify recipients that are CDC-CM positive for their donors. Numerous reports have demonstrated that changing CDC-CM from positive to negative via durable DSA removal significantly reduces the risk of graft loss. The short-term graft survival in such recipients is not significantly different from recipients without CDC-positive DSA[69-75].
In the early 1970s, Patel et al[76] reported a considerable rate of graft failure in recipients who were CDC-CM negative with their donors. These observations indicated that the sensitivities of the CDC assay and its modifications were insufficient to detect low concentrations of DSA and was deleterious for the transplanted organ. Methods for increasing the sensitivity of antibody detection by flow cytometry (FC) based techniques were introduced more than 30 years ago[77].
Although FC methods of analysis are more sensitive than the CDC method they are subject to the effects of non-HLA and autologous antibodies that complicate the interpretation of the results. This complication is particularly important in the case of B-lymphocytes because they express Fc receptors and various adhesion molecules on their surfaces that facilitate non-specific binding[67,78]. B-cell false positive FC CM results may erroneously preclude transplants that should have favorable outcomes. The non-specific antibody binding may be reduced by the incubation of the lymphocytes with pronase, enzyme destroying Fc receptors, and other members of the Ig superfamily[79-82]. FC cell-based assays can also be used for PRA analysis. In this case, pooled HLA-typed lymphocytes are incubated with the recipient serum, and the percentage of positively reactive cells is determined based on the median channel shift[83]. This method has limited applicability because further testing is required to determine the antibody specificity. Additionally, the absorption of anti-HLA class I is required when class II PRA is analyzed.
SP technologies use purified HLA class I and/or class II proteins that are attached to an artificial substrate or matrix. These assays offer significantly higher sensitivities and specificities than cellular methods[84-86].
Enzyme-linked immunosorbent assay (ELISA) was the first solid-phase analysis developed for antibody screening and specificity determination[87]. In this assay, HLA molecules are bound to the wells of plastic plates and positive reactions are measured by the color signal intensity produced by enzymes conjugated to anti-human antibodies following the addition of substrate to the wells. Purified pooled HLA-I and -II molecules bound to the wells are used for antibody screening/detection and PRA analysis. To define the antibody specificity, HLA proteins isolated from one individual are used to coat each well of the plate.
FC SP assays use microspheres that have been coated with soluble HLA proteins that are extracted from a single cell line for specificity analysis or mixed for PRA analysis (FlowPRA Specific, FlowPRA, One Lambda)[88]. After the addition of the fluorescence-conjugated anti-human secondary antibodies a light signal is generated that indicates a positive binding of the antibody to HLA molecule(s). As with ELISA, this method can be designed to detect antibodies of IgG and IgM isotypes depending on the specificity of the secondary antibodies[84,89,90].
Luminex-based technology has revolutionized the approach to anti-HLA antibody analysis and resolved ambiguities associated with the interpretation of CDC and FC results. This highly sensitive methodology has become an integral component of clinical decision-making and pathological diagnosis of transplanted organ injury. Luminex technology also incorporates microparticles (beads) that have been conjugated to varying amounts of two dyes, which enable the identification of 100 sets of beads. HLA-specific alloantibodies are detected via the addition to a reaction mixture of secondary phycoerythrin (PE)-conjugated anti-human antibodies. Each group of beads can be identified by the amount of conjugated fluorochromes and it is possible to identify which HLAs have bound antibodies. The light signal produced by bound antibody is proportional to its concentration and is expressed as the mean fluorescence intensity (MFI). The original assay was introduced as a combination of beads that were coated with HLA proteins extracted from individual cells. More recently, Luminex technology introduced a modification of the assay that included beads coated with a single class I or class II HLA. This methodological approach significantly improved antibody specificity analyses, particularly in highly sensitized patients[88,91-95]. The considerably higher surface density of HLAs on the microbeads compared to that on lymphocytes makes the Luminex single-antigen (SA) methodology extremely specific and highly sensitive. As a result, investigators can detect very low concentrations of HLA-directed antibodies. In the last decade, numerous reports have addressed the methodological aspects, clinical relevance, and standardization of the Luminex SP SA assay for the detection of antibodies[53,54,95-100]. The results of these studies have significantly expanded our understanding of anti-HLA antibody biology and the mechanism of the interaction between antibodies with antigens. This increased understanding includes improved information on isotypes and subtypes of antibodies, their abilities to bind complement, and fine epitope specificity. Fine epitope specificity is particularly important for the prediction of graft rejection[93,101-103]. There are also many new questions such as how does the signal produced by the DSAs detected in the SA Luminex assay correlate with positive FC CM, what is the clinical relevance of minimally reactive DSA, and how can the bead saturation effect be identified and overcome. Additionally, there are many other questions to address.
In this review, I describe the pitfalls, caveats, and limitations of the Luminex SP SA assay based on seven years of experience and clinical outcomes/observations at our transplant center.
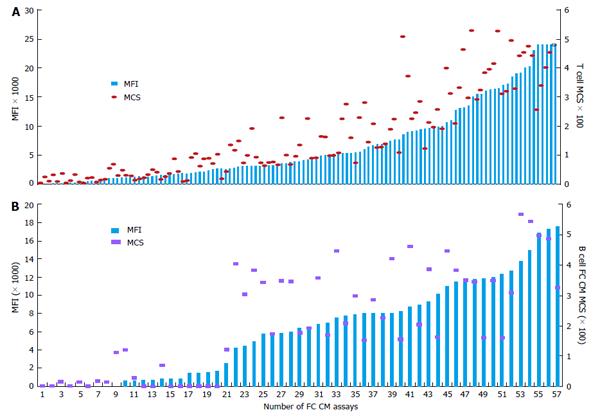
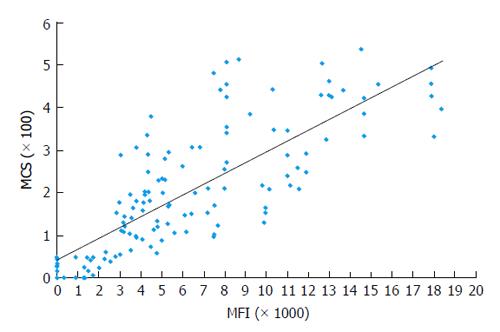
Correlation of DSA MFI values with the results of FC CM tests: It is generally accepted that preexisting DSA directed against HLA can cause allograft injury or loss. FC CM is the most sensitive immunological method and it allows for the detection of DSA in TC serum. Positive FC CM is associated with an elevated risk of AMR[12,16,17,21,32,104-107]. The accurate detection of the spectrum of anti-HLA antibodies is critical for organ allocation and the prediction of FC CM results. Comparisons of the antibody profiles in the sera of TCs with the HLA typing of the potential donors are called virtual cross matches (VCMs). VCMs are particularly important in cases of heart and lung allocation in which the cold ischemia time is limited. VCM has been widely used at our transplant center for several years. Although SP Luminex technology allows for the very specific detection of DSAs at relatively low concentrations, issues regarding how strong the DSAs must be to cause positive FC CM results have to be addressed. The results of a multicenter study performed by Reed et al[89] suggested optimal cutoffs from 1000 to 1500 MFIs for antibodies to the HLA-A, -B, -DRB1, and -DQB1 loci. DSAs with MFIs within the indicated range are considered weak, and those below this range are considered negative. The correlation analyses between MFI values of DSAs and the results of FC CM assays performed in our laboratory have demonstrated that MFIs ≥ 2600 produced by anti-HLA I antibodies (HLA-A and -B loci) most likely result in T cell-positive FC CM results (positive predictive value 97%). Furthermore, MFIs ≥ 3100 produced by anti-HLA II antibodies (HLA-DRB1 and -DQB1 loci) most likely generate B cell-positive FC CM results (positive predictive value 95%, R = 0.78)[93] (Figures 1 and 2). The positive cutoffs determined in this assay also predict positive FC CM results when the recipient has multi-specific DSAs.
The density of the HLA molecules on the beads is significantly higher than that on lymphocytes (5 × 104-105 molecules per cell) or endothelial cells. Therefore, even a minor admixture of anti-HLA antibodies or anti-idiotypic antibodies of the IgM isotype may cause false negative results. To overcome this obstacle, serum Dithiothreitol (DTT)-treatment is recommended[108-110].
We have observed strong positive T cell FC CM results and weakly reactive DSAs to HLA-B8 by SA Luminex. Subsequent DTT treatment of the serum resolved this discrepancy and revealed DSAs to the antigen with MFI values of 24000 (Table 1).
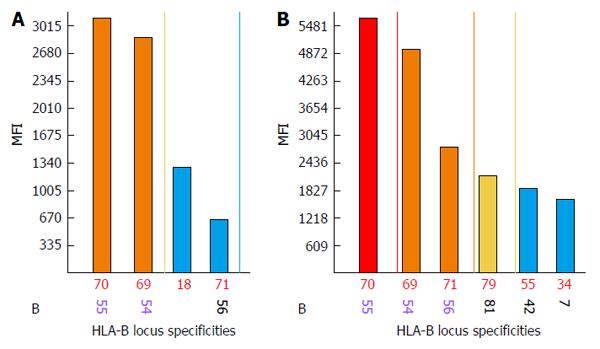
Discrepancies between negative FC CM results and strongly reactive DSAs are observed when biochemical modification (denaturing) of HLA proteins during the bead conjugation process occurs. Denatured HLAs have higher affinities for DSAs and when present in large amounts they produce strong false positive signals. An acid treatment is used to exclude antibodies against denatured HLAs. Antibodies against denatured HLA-B55 and HLA-B54 proteins are likely present when no differences or increased MFI values are observed between treated and untreated beads[110-113] (Figure 3). The acid treatment procedure is ineffective when analyzing anti-class II antibodies. In these types of situations, Luminex screening tests or Luminex class II phenotypic bead assays are performed.
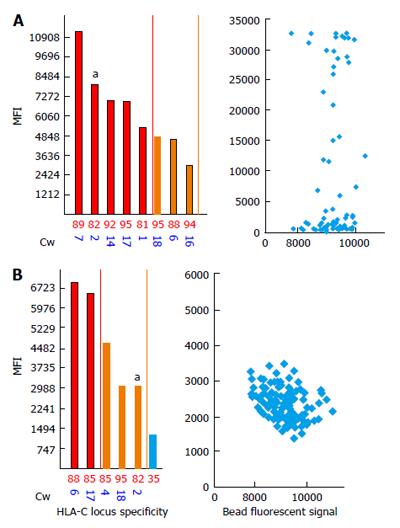
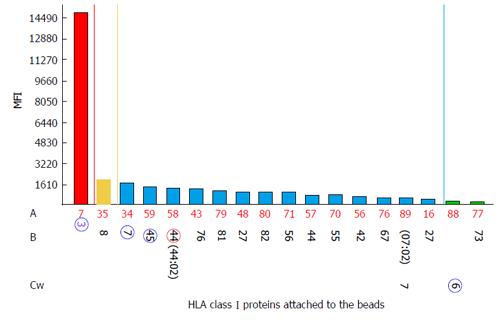
Strong DSAs to HLA-C2 in the recipient serum have been shown to yield negative FC CM results[114] (Figure 4). To investigate this discrepancy, we analyzed the bead counts and bead distributions on the SA dot blot histograms of the aforementioned bead sets. Figure 4A shows the MFI values of 13100 for the anti-HLA-C2 antibodies and an uneven bead distribution. As shown in the figure, a majority of the beads are located within the negative MFI range and only a few have MFI values of approximately 30000. These findings indicate that the MFIs observed on SA antibody panels represent an average number between the lowest and highest values. A repeat of the assay confirmed the absence of DSAs, and the SA dot blot C2 histogram formed single clusters of beads located in the negative area (Figure 4B).
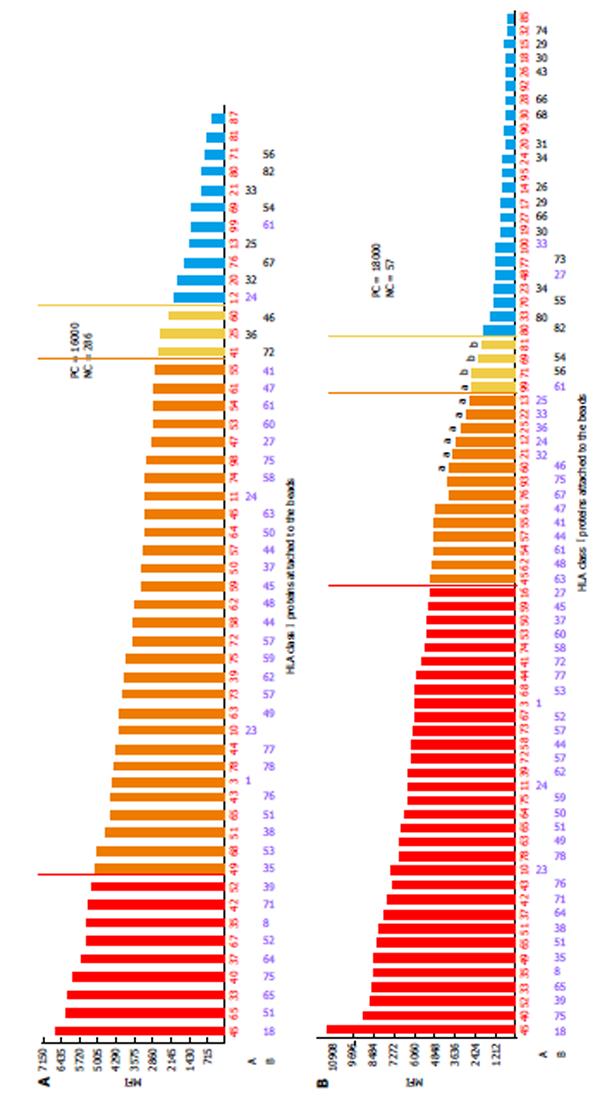
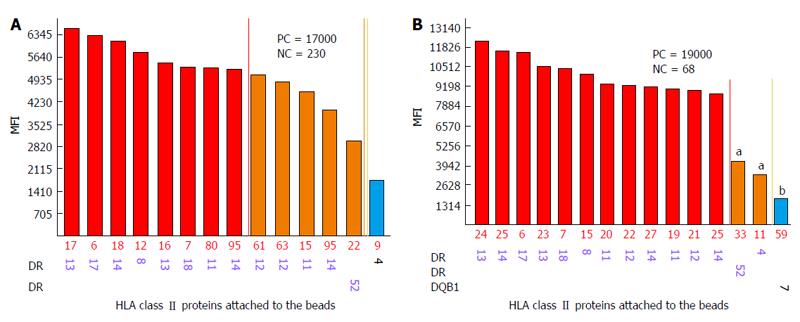
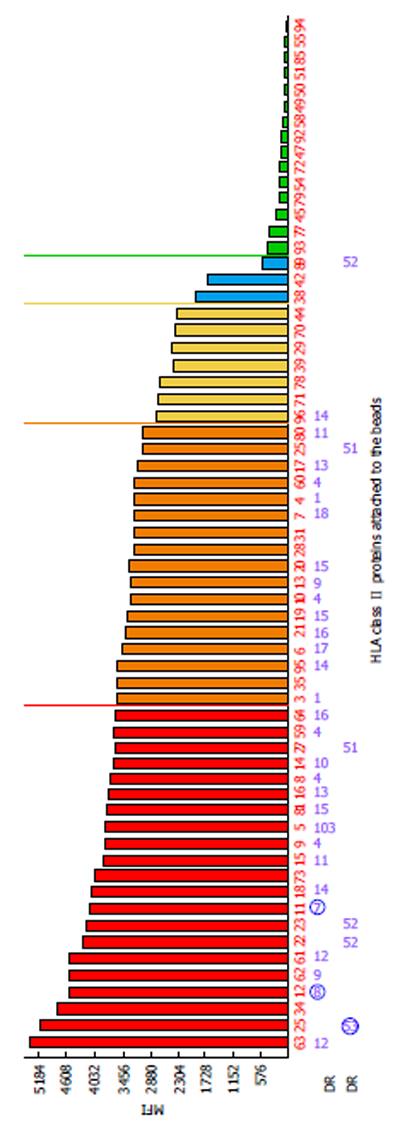
One of the limitations of SA SP Luminex technology detailed in the literature is non-specific reactivity[90,106]. The main reasons for high background levels may be related to antibodies reacting to latex, autoimmune disease(s), and some medications (IVIG)[75,115-117]. Non-specific antibody binding complicates antibody analysis due to the appearance of multiple false positive antibodies and may affect organ allocation and the interpretation of VCM results. The reagent Adsorb Out™ is manufactured by One Lambda and consists of microparticles designed for serum pre-incubation. This product reduces or removes strong background signals due to non-specific binding. Figures 5 and 6 demonstrate the additional seven anti-Class I and two anti-Class II antibody specificities that are associated with increasing the MFI (≥ 2600 for anti-Class I, and ≥ 3100 for Class II) and decreasing the negative control MFI values from 286 to 57 and from 230 to 60 for class I and class II antibodies, respectively. Additionally, the number of weakly reactive anti-Class I (MFI = 1500-2600) and anti-Class II (MFI = 1500-3100) antibodies also increased from nine to eleven and from two to three, respectively. Accounting for the MFI values of the antibodies to self-HLA also improves the TC antibody profiles. The high MFI values for the negative control (MFI > 100) usually indicate high background and require the test to be repeated. However, if serum samples taken at different time points consistently exhibit elevated non-specific reactivity after adsorption then antibody analysis can be performed appropriately[118]. An unusual anti-class II antibody profile was observed in one kidney TC. The serum of this patient contained pan-reactive anti-DRB1 antibodies including self-specificities (Figure 7; the self-antigens are circled in red). A subsequent autologous FC CM assay was B cell positive and Luminex SP screening PRA analysis did not detect any antibodies. The results of these tests led to the conclusion that the anti-DRB1 antibodies in the serum of this TC serum were clinically irrelevant.
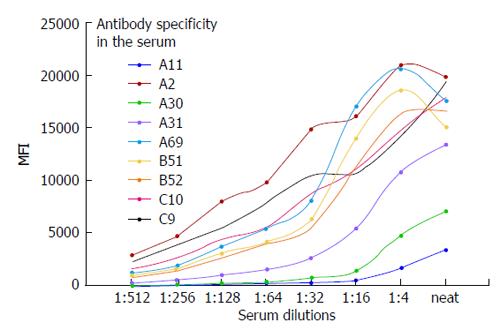
The sera from patients may contain strong high-titer antibodies that can cause a prozoning or oversaturation effect. Figure 8 shows the oversaturation of SA beads with antibodies to HLA-I. In our experience this phenomenon is suspected when the MFI values exceed 20000. As shown in Figure 8, the antibodies against A2, A69, B51, and B52 exhibited oversaturation that disappeared upon the dilution of the serum. This prozoning effect has been reported by others with the SA Luminex bead assay[119-123]. Dilution of the serum to exclude the oversaturation of antibodies is critical when the antibody analysis is performed on the sera of highly sensitized TCs who have been subjected to immunomodulation/desensitization.
The classical pathway of complement activation following antibody/HLA interactions is usually associated with graft cell damage and poor outcomes. Over the last decade it has been demonstrated that some of the DSAs detected with SA SP analysis but not with CDC (FC pos/CDC neg) can activate the complement system[124-128]. Numerous studies have demonstrated that inferior graft outcomes, graft loss, and C4d deposition are observed more frequently among recipients with DSAs that bind complement component 1 (C1q)[129-135]. The recently developed C1q SA SP assay (C1qScreen™) represents a reliable tool for distinguishing the binding IgG antibody from the complement-fixing antibodies of the IgG and IgM isotypes. The complement-fixing antibodies (C1q+) are detected using external C1q and anti-C1q antibodies conjugated to PE. The fluorescence intensity of the signal is proportional to the amount of bound C1q and is measured by MFI. The presence of C1q+ DSA is more strongly correlated with graft failure than the presence of antibodies that do not bind complement[126,136-139]. However, the absence of complement fixing antibodies has recently been reported in recipients with documented AMR. This result may indicate a low sensitivity of the C1q assay. The elegant studies of R. Liwski have demonstrated a good relationship between the anti-human globulin (AHG)-C1qScreen™ assay and CDC-AHG reactivity[128,129]. This highly sensitive modification of the original C1qScreen™ protocol would certainly be useful for risk assessment.
Each HLA protein represents a linear sequence of amino acid residues (AAR) or triplets, and the degree of mismatch is assessed as the number of triplets that are not shared between the donor and the recipient. There are two important points regarding this approach. First, only the AARs accessible to antibodies that reside in α-helical coils and β-loops are considered. In contrast, the triplets that are located in the β-pleated floor and beneath the α-chains are not available for antibody binding and are often not critical for antibody production because they are not immunogenic[140-143]. Second, alloantibodies can be produced only against non-self-mismatched triplets.
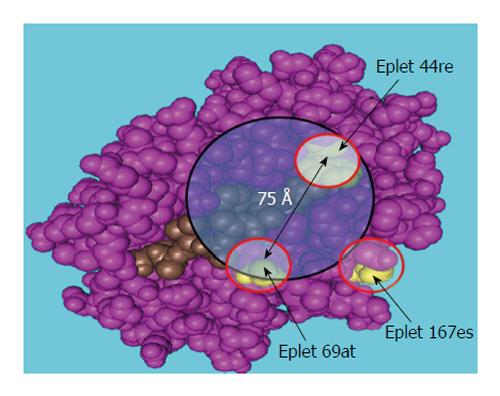
Furthermore, AAR triplet analysis (HLA Matchmaker computer algorithm) can explain or predict the development of post-transplant antibodies in kidney allograft recipients[46,50]. Subsequent analyses of patients’ antibodies and HLA-specific monoclonal antibodies have revealed that each HLA consists of structurally defined “eplets” that represent epitopes comprised of the AARs within a 3 Å-5 Å radius of the surface of the molecule[48,10,122]. An example of such analysis is presented in Figure 9, Figure 10 and Table 2. The HLA typing results of the recipient, previous donors, and current donors are given in Table 3. The pre-transplant evaluation of the second kidney TC revealed multiple weakly reactive (MFI cutoff ≥ 2600) antibodies including DSA B*44:02 (Figure 9) and strongly positive T cell FC CM results (ΔMCS = 129, positive cutoff = 50) (the autologous T cell FC CM results appeared to be negative). A HLAMatchmaker analysis of the HLA-B locus-specific antibodies determined that the antibody reactivity was restricted to two epitopes/eplets 44re and 167es on the immunizing HLA-B*45:01antigen. FC CM and SA bead assays revealed that the antigens targeted by the recipient antibodies shared the eplet pair 44re69at. Eplets that were mismatched with the immunizer were 44re and 167es, while 69at was shared by both the donor’s B7 and the patient’s A23 and A66 (Figure 10).
| HLA | MFI | Reactive eplets/pair of eplets | Number of mismatched eplets | Mismatched eplets | Comments |
| B*07:02a | 1689 | 44re | 5 | 44reb, 65qia, 70iaq, 113hd, 177dk | 44re + self 69at |
| B*45:01a | 1433 | 167es | 1 | 167esb | |
| B*44:03 | 1398 | 167es | 5 | 76ent, 79rt, 94ii, 167es, 199v | |
| B*82:01 | 1077 | 44re, 167es | 4 | 44re, 65qia, 70iaq, 167es | |
| B*44:02 | 841 | 167es | 5 | 76ent, 79rt, 94ii, 167es, 199v | |
| C*07:02 | 657 | 44re, 167es | 5 | 44re, 65qia, 70iaq, 94ii, 167es | |
| B*81:01 | 1222 | 44re | 4 | 44re, 65qia, 70iaq, 254ta | 44re + self 69at |
| B*27:08 | 1130 | 44re | 3 | 44re, 65qia, 71ka | 44re + self 69at |
| B*56:01 | 1059 | 44re | 3 | 44re, 65qia, 71ka | 44re + self 69at |
| B*55:01 | 837 | 44re | 3 | 44re, 65qia, 71ka | 44re + self 69at |
| B*42:01 | 761 | 44re | 3 | 44re, 65qia, 71ka | 44re + self 69at |
| B*67:01 | 681 | 44re | 4 | 44re, 65qia, 70iaq, 158t | 44re + self 69at |
| B*27:05 | 607 | 44re | 5 | 44re, 65qia, 71ka, 76 edt, 79rt | 44re + self 69at |
| B*73:01 | 327 | 44re | 6 | 44re, 65qia, 71ka, 103 m, 267qe, 275kp | 44re + self 69at |
| C*06:02a | 379 | 44re, 167es | 5 | 44re, 65qia, 70iaq, 94ii, 167es | |
| B*08:01 | 2046 | 44re | 1 | 44r | 44re + 69tn |
| B*15:12 (B76) | 1294 | NDc | 3 | 45rma, 113hd, 163lg | Unexplained |
| A locus | B locus | C locus | ||||
| Recipient | 23:01 | 66:01 | 41:01 | 49:01 | 07:01 | 17:01 |
| 1st donor (immunizer) | 03:01a | 26:01 | 07:02 | 45:01 | 06:02 | 07:01 |
| Current donor | 23:01 | 24:01 | 44:02 | 49:01 | 03:03 | 07:01 |
The identification of immunogenic epitopes significantly affects the prediction of post-transplant alloantibody specificities, donor selection, graft outcome, and organ allocation. The recent studies of Zeevi et al[142] and Duquesnoy et al[143] used monoclonal antibodies to demonstrate the anti-HLA antibody complement-fixing abilities strictly depend on the configuration of the critical contact eplet(s)[140,141]. The results of their studies indicated that complete complement cascade activation is determined by the energy produced from the antibody-HLA interaction. The amount of this energy should be sufficient to induce conformational changes of the constant region of the antibody to elicit C1q binding and subsequent component activation. The authors hypothesized that the binding energies of the SA C1q-negative antibodies are insufficient to induce conformational changes in the constant region. However, in the cases of the C1q+ and CDC+ antibodies this energy is sufficient to trigger complete complement activation and cell membrane damage[141,143].
The identification of anti-HLA antibodies in TC serum is a major task of HLA laboratories and transplant physicians and is important for graft failure risk assessment and donor selection. Furthermore, antibody detection is critical in highly sensitized TCs who have been subjected to desensitization (immunomodulation). SA SP analysis is a highly sensitive and highly specific method of antibody characterization that enables the detection of low concentrations of antibodies and their fine HLA specificities. However, this assay is not free from limitations including large variation in the numbers of HLA molecules per bead and the effects of manual (i.e., technologist-to-technologist) factors on assay variance. In this review, I attempted to share multiple years of experience performing SA SP assays for pre- and post-transplant antibody analyses in my laboratory and address some pitfalls and caveats of this assay. Evaluations of the clinical significance of anti-HLA antibodies should undeniably include their concentrations, isotypes, ability to fix the complement, and fine epitope specificity.
P- Reviewer: Garcia-Elorriaga G, Gheith O, Holan V, Torres MI S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Candon S, Thervet E, Lebon P, Suberbielle C, Zuber J, Lima C, Charron D, Legendre C, Chatenoud L. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant. 2009;9:2346-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Danziger-Isakov L, Cherkassky L, Siegel H, McManamon M, Kramer K, Budev M, Sawinski D, Augustine JJ, Hricik DE, Fairchild R. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 2010;89:838-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006;107:2806-2813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Kennedy RB, Ovsyannikova IG, Vierkant RA, Jacobson RM, Poland GA. Effect of human leukocyte antigen homozygosity on rubella vaccine-induced humoral and cell-mediated immune responses. Hum Immunol. 2010;71:128-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Benson MJ, Elgueta R, Schpero W, Molloy M, Zhang W, Usherwood E, Noelle RJ. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009;206:2013-2025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Dougan SK, Ashour J, Karssemeijer RA, Popp MW, Avalos AM, Barisa M, Altenburg AF, Ingram JR, Cragnolini JJ, Guo C. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503:406-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Kumar D, Campbell P, Humar A. Donor-specific alloantibody upregulation after influenza vaccination in transplant recipients. Am J Transplant. 2011;11:2538; author’s reply 2539-2540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kumar D, Danziger-Isakov L. Immunization against influenza: a balancing act. Am J Transplant. 2011;11:1561-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Abe M, Kawai T, Futatsuyama K, Tanabe K, Fuchinoue S, Teraoka S, Toma H, Ota K. Postoperative production of anti-donor antibody and chronic rejection in renal transplantation. Transplantation. 1997;63:1616-1619. [PubMed] [Cited in This Article: ] |
| 10. | Bas J, Mestre M, Grinyó JM, Massip E, Alsina J, Castelao AM, Corominas M, Buendia E. Peripheral blood lymphoid subsets and long-term clinical course of kidney recipients: a longitudinal study. Cytometry. 1998;34:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Cippà PE, Gaspert A, Etter C, Guenduez Z, Ferrari-Lacraz S, Rüsi B, Fehr T. Late antibody-mediated rejection by de novo donor HLA-DP-specific antibody after renal transplantation: a case report. Hum Immunol. 2014;75:462-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Claas FH, Dankers MK, Oudshoorn M, van Rood JJ, Mulder A, Roelen DL, Duquesnoy RJ, Doxiadis II. Differential immunogenicity of HLA mismatches in clinical transplantation. Transpl Immunol. 2005;14:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Deng CT, El-Awar N, Ozawa M, Cai J, Lachmann N, Terasaki PI. Human leukocyte antigen class II DQ alpha and beta epitopes identified from sera of kidney allograft recipients. Transplantation. 2008;86:452-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Scornik JC, LeFor WM, Cicciarelli JC, Brunson ME, Bogaard T, Howard RJ, Ackermann JR, Mendez R, Shires DL, Pfaff WW. Hyperacute and acute kidney graft rejection due to antibodies against B cells. Transplantation. 1992;54:61-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Takemoto S, Port FK, Claas FH, Duquesnoy RJ. HLA matching for kidney transplantation. Hum Immunol. 2004;65:1489-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005;17:541-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Arnold ML, Kloecker S, Roppelt D, Kalden JR. HLA DQ antibodies in patients awaiting kidney re-transplantation. Genes and Immunity. 2005;6:S68. [Cited in This Article: ] |
| 19. | Bartel G, Wahrmann M, Exner M, Regele H, Schillinger M, Hörl WH, Böhmig GA. Determinants of the complement-fixing ability of recipient presensitization against HLA antigens. Transplantation. 2007;83:727-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Sumitran-Holgersson S. HLA-specific alloantibodies and renal graft outcome. Nephrol Dial Transplant. 2001;16:897-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Vasilescu ER, Ho EK, de la Torre L, Itescu S, Marboe C, Cortesini R, Suciu-Foca N, Mancini D. Anti-HLA antibodies in heart transplantation. Transpl Immunol. 2004;12:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Stegall MD, Raghavaiah S, Gloor JM. The (re)emergence of B cells in organ transplantation. Curr Opin Organ Transplant. 2010;15:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Bartel G, Regele H, Wahrmann M, Huttary N, Exner M, Hörl WH, Böhmig GA. Posttransplant HLA alloreactivity in stable kidney transplant recipients-incidences and impact on long-term allograft outcomes. Am J Transplant. 2008;8:2652-2660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | O’Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, Knechtle SJ, McDiarmid SV, Shaked A, Terasaki PI. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Bishay ES, Cook DJ, El Fettouh H, Starling RC, Young JB, Smedira NG, McCarthy PM. The impact of HLA sensitization and donor cause of death in heart transplantation. Transplantation. 2000;70:220-222. [PubMed] [Cited in This Article: ] |
| 27. | Bishay ES, Smedira NG. Surgical management of massive atrial size mismatch in heart transplantation. Ann Thorac Surg. 2000;69:618-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Böhmig GA, Bartel G, Wahrmann M. Antibodies, isotypes and complement in allograft rejection. Curr Opin Organ Transplant. 2008;13:411-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Ghasemian SR, Light JA, Sasaki TA, Barhyte DY. Hyperacute rejection from antibody against class II HLA antigens. Clin Transplant. 1998;12:569-571. [PubMed] [Cited in This Article: ] |
| 31. | Grandtnerová B, Javorský P, Kolácný J, Hovoricová B, Dĕdic P, Laca L. Treatment of acute humoral rejection in kidney transplantation with plasmapheresis. Transplant Proc. 1995;27:934-935. [PubMed] [Cited in This Article: ] |
| 32. | Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Contrib Nephrol. 2009;162:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Lobo PI, Spencer CE, Stevenson WC, Pruett TL. Evidence demonstrating poor kidney graft survival when acute rejections are associated with IgG donor-specific lymphocytotoxin. Transplantation. 1995;59:357-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 35. | El Fettouh HA, Cook DJ, Bishay E, Flechner S, Goldfarb D, Modlin C, Dennis V, Novick AC. Association between a positive flow cytometry crossmatch and the development of chronic rejection in primary renal transplantation. Urology. 2000;56:369-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Banasik M, Boratyńska M, Kościelska-Kasprzak K, Mazanowska O, Bartoszek D, Zabińska M, Myszka M, Nowakowska B, Hałoń A, Szyber P. Long-term follow-up of non-HLA and anti-HLA antibodies: incidence and importance in renal transplantation. Transplant Proc. 2013;45:1462-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Banasik M, Boratyńska M, Kościelska-Kasprzak K, Mazanowska O, Krajewska M, Zabińska M, Bartoszek D, Myszka M, Nowakowska B, Dawiskiba T. The impact of de novo donor-specific anti-human leukocyte antigen antibodies on 5-year renal transplant outcome. Transplant Proc. 2013;45:1449-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Caro-Oleas JL, González-Escribano MF, Gentil-Govantes MÁ, Acevedo MJ, González-Roncero FM, Blanco GB, Núñez-Roldán A. Clinical relevance of anti-HLA donor-specific antibodies detected by Luminex assay in the development of rejection after renal transplantation. Transplantation. 2012;94:338-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Cooper JE, Gralla J, Chan L, Wiseman AC. Clinical significance of post kidney transplant de novo DSA in otherwise stable grafts. Clin Transpl. 2011;359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91:1103-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 42. | Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, Fontana I, Magnasco A, Nocco A, Tagliamacco A. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12:3355-3362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 43. | Huang Y, Ramon D, Luan FL, Sung R, Samaniego M. Incidences of preformed and de novo donor-specific HLA antibodies and their clinicohistological correlates in the early course of kidney transplantation. Clin Transpl. 2012;247-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Jimenez J, Young JB. Case 2: cardiogenic shock due to acute vascular rejection in a heart transplant recipient. J Heart Lung Transplant. 2000;19:817-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 45. | Mehra NK, Siddiqui J, Baranwal A, Goswami S, Kaur G. Clinical relevance of antibody development in renal transplantation. Ann N Y Acad Sci. 2013;1283:30-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Adeyi OA, Girnita AL, Howe J, Marrari M, Awadalla Y, Askar M, Martell J, Zeevi A, Shapiro R, Nalesnik M. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Fuller A, Profaizer T, Roberts L, Fuller TC. Repeat donor HLA-DR mismatches in renal transplantation: is the increased failure rate caused by noncytotoxic HLA-DR alloantibodies? Transplantation. 1999;68:589-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Lachmann N, El Awar N, Salama A, Terasaki PI, Schonemann C. Single amino acid mismatches determine immunogenic HLA epitopes. Tissue Antigens. 2008;72:253-260. [Cited in This Article: ] |
| 49. | Laux G, Mansmann U, Deufel A, Opelz G, Mytilineos J. A new epitope-based HLA-DPB matching approach for cadaver kidney retransplants. Transplantation. 2003;75:1527-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Lobashevsky AL, Senkbeil RW, Shoaf JL, Stephenson AK, Skelton SB, Burke RM, Deierhoi MH, Thomas JM. The number of amino acid residues mismatches correlates with flow cytometry crossmatching results in high PRA renal patients. Hum Immunol. 2002;63:364-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Lobashevsky A, Rosner K, Goggins W, Higgins N. Subtypes of immunoglobulin (Ig)-G antibodies against donor class II HLA and cross-match results in three kidney transplant candidates. Transpl Immunol. 2010;23:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Bosch A, Llorente S, Diaz JA, Salgado G, López M, Boix F, López-Hernández R, González-Soriano MJ, Campillo JA, Moya-Quiles MR. Low median fluorescence intensity could be a nonsafety concept of immunologic risk evaluation in patients with shared molecular eplets in kidney transplantation. Hum Immunol. 2012;73:522-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Caro-Oleas JL, González-Escribano MF, González-Roncero FM, Acevedo-Calado MJ, Cabello-Chaves V, Gentil-Govantes MÁ, Núñez-Roldán A. Clinical relevance of HLA donor-specific antibodies detected by single antigen assay in kidney transplantation. Nephrol Dial Transplant. 2012;27:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Dunn TB, Noreen H, Maurer D, Ozturk OG, Gillingham K, Matas AJ, Bray RA, Gebel HM. Impact of Low Level Donor Specific Antibodies in Renal Transplant Outcomes. Hum Immunol. 2010;71:S25. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 55. | Eng HS, Bennett G, Bardy P, Coghlan P, Russ GR, Coates PT. Clinical significance of anti-HLA antibodies detected by Luminex: enhancing the interpretation of CDC-BXM and important post-transplantation monitoring tools. Hum Immunol. 2009;70:595-599. [PubMed] [Cited in This Article: ] |
| 56. | Girnita AL, Brailey P, Alloway RR, Rike-Shields A, Sadaka B, Woodle ES. Early and Late Antibody Mediated Rejection Demonstrate Differential Responses to Proteasome Inhibitor-Based Therapy with Respect to Donor Specific Anti-HLA Antibody Specificity and Subtype. Am J of Transplant. 2011;11:171-172. [Cited in This Article: ] |
| 57. | Everly MJ. Summarizing the use of donor specific anti-HLA antibody monitoring in transplant patients. Clin Transpl. 2011;333-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Wu P, Everly MJ, Jin J, Mao Y, Chen J. Understanding the significance of low-level preformed donor-specific anti-HLA antibodies in renal transplant patients. Clin Transpl. 2011;365-368. [PubMed] [Cited in This Article: ] |
| 59. | Aurora D, Balazs I, Ebner D, Hariharan J, Jiang N, Mostecki J. Detection of complement activating anti-HLA class I and II antibodies using Lifecodes Single Antigen assay. Tissue Antigens. 2008;71:289-290. [Cited in This Article: ] |
| 60. | Jordan SC, Vo A, Bunnapradist S, Toyoda M, Peng A, Puliyanda D, Kamil E, Tyan D. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003;76:631-636. [PubMed] [Cited in This Article: ] |
| 61. | Jordan SC, Vo A, Tyan D, Toyota M. Desensitization therapy with intravenous gammaglobulin (IVIG): applications in solid organ transplantation. Trans Am Clin Climatol Assoc. 2006;117:199-211; discussion 211. [PubMed] [Cited in This Article: ] |
| 62. | Zachary AA, Montgomery RA, Ratner LE, Samaniego-Picota M, Haas M, Kopchaliiska D, Leffell MS. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519-1525. [PubMed] [Cited in This Article: ] |
| 63. | Cecka JM, Zhang Q, Reed EF. Preformed cytotoxic antibodies in potential allograft recipients: recent data. Hum Immunol. 2005;66:343-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Claas FH, Doxiadis II. Management of the highly sensitized patient. Curr Opin Immunol. 2009;21:569-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Leffell MS, Zachary AA. Anti-allograft antibodies: some are harmful, some can be overcome, and some may be beneficial. Discov Med. 2010;9:478-484. [PubMed] [Cited in This Article: ] |
| 66. | Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation. 2008;85:1705-1714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Jackson AM, Lucas DP, Melancon JK, Desai NM. Clinical relevance and IgG subclass determination of non-HLA antibodies identified using endothelial cell precursors isolated from donor blood. Transplantation. 2011;92:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Díaz I, Sánchez P, Alonso C, Valdés F. Immunological profile of patients awaiting a renal transplant. Clin Transplant. 2004;18:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Wasowska B, Baldwin WM, Howell DN, Sanfilippo F. The association of enhancement of renal allograft survival by donor-specific blood transfusion with host MHC-linked inhibition of IgG anti-donor class I alloantibody responses. Transplantation. 1993;56:672-680. [PubMed] [Cited in This Article: ] |
| 70. | Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006;6:459-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Morath C, Opelz G, Zeier M, Süsal C. Recent developments in desensitization of crossmatch-positive kidney transplant recipients. Transplant Proc. 2012;44:1648-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 426] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 74. | Song EY, Lee YJ, Hyun J, Kim YS, Ahn C, Ha J, Kim SJ, Park MH. Clinical relevance of pretransplant HLA class II donor-specific antibodies in renal transplantation patients with negative T-cell cytotoxicity crossmatches. Ann Lab Med. 2012;32:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Zachary AA, Eng HS. Desensitization: achieving immune detente. Tissue Antigens. 2011;77:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1129] [Cited by in F6Publishing: 1051] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 77. | O’Rourke RW, Osorio RW, Freise CE, Lou CD, Garovoy MR, Bacchetti P, Ascher NL, Melzer JS, Roberts JP, Stock PG. Flow cytometry crossmatching as a predictor of acute rejection in sensitized recipients of cadaveric renal transplants. Clin Transplant. 2000;14:167-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Dragun D, Hegner B. Non-HLA antibodies post-transplantation: clinical relevance and treatment in solid organ transplantation. Contrib Nephrol. 2009;162:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Hetrick SJ, Schillinger KP, Zachary AA, Jackson AM. Impact of pronase on flow cytometric crossmatch outcome. Hum Immunol. 2011;72:330-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Lobo PI, Isaacs RB, Spencer CE, Pruett TL, Sanfey HA, Sawyer RG, McCullough C. Improved specificity and sensitivity when using pronase-digested lymphocytes to perform flow-cytometric crossmatch prior to renal transplantation. Transpl Int. 2002;15:563-569. [PubMed] [Cited in This Article: ] |
| 81. | Lobo PI, Isaacs RB, Spencer CE, Pruett TL, Sanfey HA, Sawyer RG, McCullough C. Improved specificity and sensitivity when using pronase-digested lymphocytes to perform flow-cytometric crossmatch prior to renal transplantation. Transplant Int. 2002;15:563-569. [PubMed] [Cited in This Article: ] |
| 82. | Vaidya S, Cooper TY, Avandsalehi J, Barnes T, Brooks K, Hymel P, Noor M, Sellers R, Thomas A, Stewart D. Improved flow cytometric detection of HLA alloantibodies using pronase: potential implications in renal transplantation. Transplantation. 2001;71:422-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Shroyer TW, Deierhoi MH, Mink CA, Cagle LR, Hudson SL, Rhea SD, Diethelm AG. A rapid flow cytometry assay for HLA antibody detection using a pooled cell panel covering 14 serological crossreacting groups. Transplantation. 1995;59:626-630. [PubMed] [Cited in This Article: ] |
| 84. | Fuggle SV, Martin S. Tools for human leukocyte antigen antibody detection and their application to transplanting sensitized patients. Transplantation. 2008;86:384-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319-326. [PubMed] [Cited in This Article: ] |
| 86. | Smith J, Rose M. Transplantation Immunology: methods and Protocols. Detection and clinical relevance of Antibodies After Transplantation. New Jersey: Totowa, NJ 2009; 227-245. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 87. | Buelow R, Chiang TR, Monteiro F, Cornejo MC, Ellingson L, Claas F, Gaber O, Gelder F, Kotb M, Orosz C. Soluble HLA antigens and ELISA--a new technology for crossmatch testing. Transplantation. 1995;60:1594-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 306] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 89. | Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, Lunz J, Mohanakumar T, Nickerson P, Tambur AR. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA-drilling down on key sources of variation. Am J Transplant. 2013;13:3050-3051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 584] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 91. | Billen EV, Christiaans MH, van den Berg-Loonen EM. Clinical relevance of Luminex donor-specific crossmatches: data from 165 renal transplants. Tissue Antigens. 2009;74:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | El-Awar NR, Akaza T, Terasaki PI, Nguyen A. Human leukocyte antigen class I epitopes: update to 103 total epitopes, including the C locus. Transplantation. 2007;84:532-540. [PubMed] [Cited in This Article: ] |
| 93. | Lobashevsky AL, Higgins NG. Predictive Values (Pv) of Class I and Class Ii Antibodies (Ab) Detected by Luminex. Hum Immunol. 2009;70:S32. [Cited in This Article: ] |
| 94. | Mujtaba MA, Goggins W, Lobashevsky A, Sharfuddin AA, Yaqub MS, Mishler DP, Brahmi Z, Higgins N, Milgrom MM, Diez A. The strength of donor-specific antibody is a more reliable predictor of antibody-mediated rejection than flow cytometry crossmatch analysis in desensitized kidney recipients. Clin Transplant. 2011;25:E96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Zeevi A, Lunz J, Teuteberg J, Feingold B, Jelinek L, Michael S, Zaldonis D, Yoshiya T, Morrell M, Webber S. Does the Determination of Complement Binding Add to the Clinical Utility of Donor Specific Antibodies Detected by Luminex? J of Heart and Lung Transplant. 2011;30:S228. [Cited in This Article: ] |
| 96. | Amico P, Hönger G, Steiger J, Schaub S. Utility of the virtual crossmatch in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:656-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Tsapepas DS, Vasilescu R, Tanriover B, Coppleson Y, Rekhtman Y, Hardy MA, Dube G, Crew RJ, Ratner LE, Cohen DJ. Preformed donor-specific antibodies and risk of antibody-mediated rejection in repeat renal transplantation. Transplantation. 2014;97:642-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Brailey P, Woodle ES, Alloway RR, Tevar AD, Cardi M, Portwood E, Mogilishetty G, Govil A, Rike AH, Walsh RC. Preformed Low Reactive Anti-HLA Antibodies Are Associated with Majority of Early Antibody-Mediated Rejection in Renal Transplantation. Hum Immunol. 2010;71:S68. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 99. | Eng HS, Bennett G, Tsiopelas E, Lake M, Humphreys I, Bardy P, Coates PT, Russ G. Luminex R investigation of B-cell crossmatches: Low titer HLA donor-specific antibodies associated with inferior outcomes. Hum Immunol. 2007;68:S31. [Cited in This Article: ] |
| 100. | Leffell MS. Real benefits from virtual crossmatches. Transplantation. 2010;89:138-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Lobashevsky AL, Goggins W, Taber T, Higgins N, Duquesnoy R. Allelic mismatch at HLA-DRB3 locus resulted in production of a broad spectrum of alloantibodies. Tissue Antigens. 2011;77:377. [Cited in This Article: ] |
| 102. | Mostecki J, Hariharan J, Ebner D, Ray B, Balazs I. Detection of HLA Class I antibodies with recombinant Single Antigens using a Luminex-based assay. Tissue Antigens. 2006;67:553-555. [Cited in This Article: ] |
| 103. | Claas FH, Rahmel A, Doxiadis II. Enhanced kidney allocation to highly sensitized patients by the acceptable mismatch program. Transplantation. 2009;88:447-452. [PubMed] [Cited in This Article: ] |
| 104. | Arnold ML, Doxiadis IIN, Kalden JR, Spriewald BM. Crossmatch results with sera containing HLA class II antibodies depend on the donor cell source. Genes and Immunity. 2005;6:S67. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 105. | Lopez R, Al Zahrani SJ, Awaji MI, Housawi AA, Saghier M, Khan FM, Berka N. Flow Crossmatch Bimodal Distribution for T Cell Treated with Pronase. Hum Immunol. 2011;72:S154. [Cited in This Article: ] |
| 106. | Billen EV, Christiaans MH, Lee J, van den Berg-Loonen EM. Donor-directed HLA antibodies before and after transplantectomy detected by the luminex single antigen assay. Transplantation. 2009;87:563-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9:2561-2570. [PubMed] [Cited in This Article: ] |
| 108. | Vittoraki AG, Skoumi DA, Apostolaki MD, Athanasopoulou MG, Georgoulia G, Iniotaki AG, St Paterakis G. Predicting Flow Cytometry Crossmatches Based on Luminex MFI Values. Tissue Antigens. 2012;79:489-495. [Cited in This Article: ] |
| 109. | Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Hum Immunol. 2009;70:496-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 110. | Lobashevsky AL, Rosner K, Goggins W, Higgins NG. Do We Really Know Everything About Cross Match (Or Does IgG Subtype Matter)? Hum Immunol. 2010;71:S41. [Cited in This Article: ] |
| 111. | Gandhi MJ, Degoey S, Falbo D, Jenkins S, Stubbs JR, Noreen H, Lorentzen DF, Lee J, Stegall M. Inter and intra laboratory concordance of HLA antibody results obtained by single antigen bead based assay. Hum Immunol. 2013;74:310-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Pereira S, Perkins S, Lee JH, Shumway W, LeFor W, Lopez-Cepero M, Wong C, Connolly A, Tan JC, Grumet FC. Donor-specific antibody against denatured HLA-A1: clinically nonsignificant? Hum Immunol. 2011;72:492-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Grenzi PC, de Marco R, Silva RZ, Campos EF, Gerbase-DeLima M. Antibodies against denatured HLA class II molecules detected in luminex-single antigen assay. Hum Immunol. 2013;74:1300-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Poli F, Benazzi E, Innocente A, Nocco A, Cagni N, Gianatti A, Fiocchi R, Scalamogna M. Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02: 01: a case report. Hum Immunol. 2011;72:1045-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Susal C, Mahmoud K, Ovens J, Ruhenstroth A, Dohler B, Scherer S, Opelz G. Are Additional Antibodies Detected by Luminex Single Antigen Testing Clinically Relevant? A Collaborative Transplant Study Report. Transpl Int. 2010;23:36-37. [Cited in This Article: ] |
| 116. | Woodle ES, Girnita A, Brailey P, Sadaka B, Rike-Shields A, Alloway , RR , Wall G. Desensitization Results Are Influenced by the Nature of the HLA Ab Being Treated. Transpl Int. 2011;24:329. [Cited in This Article: ] |
| 117. | Kosmoliaptsis V, Chaudhry AN, Sharples LD, Halsall DJ, Dafforn TR, Bradley JA. Taylor CJ. HLA Class-I Alloantigen Immunogenicity Can be Predicted by Tertiary Structure and Electrostatic Charge Disparity. Transpl Int. 2009;22:69. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Kosmoliaptsis V, Bradley JA, Taylor CJ. Structural limitations to the mimetic HLA epitope hypothesis. Transplantation. 2009;87:1262-1263; author reply 1263. [PubMed] [Cited in This Article: ] |
| 119. | Kosmoliaptsis V, Bradley JA, Peacock S, Chaudhry AN, Taylor CJ. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation. 2009;87:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Taylor CJ, Kosmoliaptsis V, Summers DM, Bradley JA. Back to the future: application of contemporary technology to long-standing questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol. 2009;70:563-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, Haisch CE, Bolin P, Parker K. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 122. | Fontaine MJ, Kuo J, Chen G, Galel SA, Miller E, Sequeira F, Viele M, Goodnough LT, Tyan DB. Complement (C1q) fixing solid-phase screening for HLA antibodies increases the availability of compatible platelet components for refractory patients. Transfusion. 2011;51:2611-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 123. | Lunz JG, Webber S, Soltys K, Shapiro R, Teuteberg J, Feingold B, Morrell M, Jelinek L, Zeevi A. C1Q Binding Donor Specific Hla Antibody Monitoring by Luminex Single Antigen Assay Is Useful in Predicting Clinical Events. Hum Immunol. 2011;72:S50. [Cited in This Article: ] |
| 124. | Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91:342-347. [Cited in This Article: ] |
| 125. | Gebel HM, Liwski RS, Bray RA. Technical aspects of HLA antibody testing. Curr Opin Organ Transplant. 2013;18:455-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 126. | Liwski R, Lee S, Sperry R, Nickerson P, Bray R, Gebel H. The Anti-Human Globulin Enhanced C1Qscreen (Tm) Assay Improves the Detection of Complement Binding Donor Specific HLA Antibodies. Hum Immunol. 2013;74:48. [Cited in This Article: ] |
| 127. | Bohmig GA, Exner M, Watschinger B, Wenter C, Wahrmann M, Osterreicher C, Saemann MD, Mersich N, Horl WH, Zlabinger GJ. C4d deposits in renal allografts are associated with inferior graft outcome. Transplant Proc. 2001;33:1151-1152. [Cited in This Article: ] |
| 128. | Böhmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Säemann MD, Hörl WH, Watschinger B, Regele H. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091-1099. [PubMed] [Cited in This Article: ] |
| 129. | Collins AB, Chicano SL, Cornell LD, Tolkoff-Rubin N, Goes NB, Saidman SL, Farrell ML, Cosimi AB, Colvin RB. Putative antibody-mediated rejection with C4d deposition in HLA-identical, ABO-compatible renal allografts. Transplant Proc. 2006;38:3427-3429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Govil A, Arend LJ, Brailey P, Alloway RR, Mogilishetty G, Tevar AD, Roy-Chaudhury P, Woodle ES. DSA and C4d Deposition: Paradoxical Dissociation in Acute Pancreas Allograft Rejection. Am J of Transplant. 2010;10:89-91. [Cited in This Article: ] |
| 131. | Habicht A, Regele H, Exner M, Soleiman A, Horl WH, Watschinger B, Derfler K, Bohmig GA. A case of severe C4d-positive kidney allograft dysfunction in the absence of histomorphologic features of rejection. Wien Klin Wochenschr. 2002;114:945-948. [Cited in This Article: ] |
| 132. | Hönger G, Wahrmann M, Amico P, Hopfer H, Böhmig GA, Schaub S. C4d-fixing capability of low-level donor-specific HLA antibodies is not predictive for early antibody-mediated rejection. Transplantation. 2010;89:1471-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 133. | Wahrmann M, Bartel G, Exner M, Regele H, Körmöczi GF, Fischer GF, Böhmig GA. Clinical relevance of preformed C4d-fixing and non-C4d-fixing HLA single antigen reactivity in renal allograft recipients. Transpl Int. 2009;22:982-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 134. | Loupy A, Hill GS, Suberbielle C, Anglicheau D, Duong JP, Zuber J, Posson J, Mamzer MF, Martinez F, Thervet E. Significance of Minimal and Focal C4d in Stable DSA plus Kidney Transplant Recipients. Am J of Transplant. 2010;11:56-65. [Cited in This Article: ] |
| 135. | Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 136. | Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 137. | Duquesnoy RJ, Marrari M, Mulder A, Mostecki J, Claas FHJ, van Balazs I. Human monoclonal antibody reactivity with HLA class I epitopes defined by pairs of mismatched eplets and self-eplets. Tissue Antigens. 2011;77:376-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Mongkolsuk T, Ingsathit A, Worawichawong S, Jirasiritham S, Kitpoka P, Thammanichanond D. Shared molecular eplet stimulates acute antibody-mediated rejection in a kidney transplant recipient with low-level donor-specific antibodies: a case report. Transplant Proc. 2014;46:644-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 139. | Marrari M, Mostecki J, Mulder A, Claas F, Balazs I, Duquesnoy RJ. Human monoclonal antibody reactivity with human leukocyte antigen class I epitopes defined by pairs of mismatched eplets and self-eplets. Transplantation. 2010;90:1468-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 140. | Duquesnoy RJ, Marrari M, Mulder A, Claas FH, Mostecki J, Balazs I. Structural aspects of human leukocyte antigen class I epitopes detected by human monoclonal antibodies. Hum Immunol. 2012;73:267-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 141. | Duquesnoy RJ, Marrari M, Mostecki J, Mulder A, Balazs I. Human Monoclonal Antibody Reactivity with Hla Class I Epitopes Defined by Eplet Pairs. Hum Immunol. 2010;71:S77. [Cited in This Article: ] |
| 142. | Zeevi A, Marrari M, Feingold B, Webber S, Duquesnoy RJ. Human leukocyte antigen epitope analysis to assess complement- and non-complement-binding donor-specific antibody repertoire in a pediatric heart transplant recipient. Hum Immunol. 2012;73:48-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Duquesnoy RJ, Marrari M, Jelenik L, Zeevi A, Claas FH, Mulder A. Structural aspects of HLA class I epitopes reacting with human monoclonal antibodies in Ig-binding, C1q-binding and lymphocytotoxicity assays. Hum Immunol. 2013;74:1271-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |