Published online Aug 19, 2020. doi: 10.5498/wjp.v10.i8.187
Peer-review started: March 20, 2020
First decision: April 26, 2020
Revised: June 25, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 19, 2020
Schizophrenia spectrum disorders impact functioning, reduce quality of life and increase the risk of physical illness and premature mortality. Nutritional intervention studies aimed at decreasing body weight have demonstrated efficacy in improving metabolic outcomes; however, few studies have explored the impact of interventions designed to modify diet on mental health outcomes.
To synthesize the existing experimental studies of adjunctive diet modification as an intervention in the treatment of psychotic disorders, analyze findings related to effectiveness and safety, highlight knowledge gaps and limitations, and set forward recommendations for future research studies.
An extensive a priori search strategy was developed and the databases Embase, Embase Classic, Ovid MEDLINE were searched. Screening and data extraction were completed in duplicate. Studies included in this analysis were experimental studies of an adjunctive dietary intervention (overall dietary pattern or education on dietary change) for treatment of schizophrenia spectrum disorders. No restrictions were placed on control groups or blinding. The studies were required to report a mental health outcome.
Twenty-five clinical trials were identified, along with two additional protocols and two meta-analyses. Nineteen of the clinical trials reported improvement in one or more mental health domain including psychosis symptoms, cognition, and quality of life. A high level of heterogeneity was found with respect to patient population, intervention, and study design. All of the studies included lifestyle or psychosocial components in addition to dietary modification. The nutrition advice provided to participants was poorly described overall and compliance was not assessed. The studies that showed benefit tended to have a smaller sample size and were less likely to be randomized but were more likely to use a group delivery intervention.
Further research assessing effectiveness and efficacy of clearly reported dietary interventions is warranted, especially those using rigorous methodology, modifying diet in isolation and assessing participant compliance.
Core tip: While guidelines exist related to the role of nutrition interventions in the management of obesity and physical comorbidities in patient with schizophrenia spectrum disorders, there is limited clinical use in the management of mental health symptoms. This systematic review identified and analysed the findings of 25 clinical trials which modified participant diet and measured a mental health outcome. Nineteen reported improvement in one or more domain; however, many used additional interventions in combination with nutrition, and failed to report a detailed intervention protocol or assess compliance, limiting the conclusions that can be drawn. Further research is warranted using rigorous methodology.
- Citation: Aucoin M, LaChance L, Clouthier SN, Cooley K. Dietary modification in the treatment of schizophrenia spectrum disorders: A systematic review. World J Psychiatr 2020; 10(8): 187-201
- URL: https://www.wjgnet.com/2220-3206/full/v10/i8/187.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i8.187
Schizophrenia spectrum disorders (SSD) include schizophrenia, schizoaffective disorder, and other psychotic disorders. The etiology is largely unknown and likely multifactorial with genetic, biological, and social factors implicated. SSD symptoms include positive symptoms (including delusions and hallucinations), negative symptoms (including social withdrawal and apathy), depression, and cognitive symptoms. While many theories exist, these symptoms may be attributed to imbalances in neurotransmitters and their receptors, dysregulation of other neurochemicals such as brain derived neurotrophic factor (BDNF), immune dysregulation, neuro-inflammation, and mitochondrial and metabolic dysfunction[1-3]. Treatment of SSD includes a combination of pharmaceutical and psychosocial interventions including anti-psychotic medications.
Individuals with psychotic disorders are at significantly elevated risk of medical comorbidities, ultimately resulting in an estimated 8 to 20-year reduction in lifespan[4,5]. Several factors contribute to increased risk of mortality such as socioeconomic status, increased tobacco use, low quality diet, and metabolic complications caused by antipsychotic medication[6-10].
Guidelines and programs exist to support SSD patients in dietary choices to manage weight and physical illness; however, nutritional guidelines for the treatment of psychiatric symptoms do not exist. Nutritional psychiatry is a relatively recent field, studying the effects of nutrients on mental illness incidence and progression. An expanding body of evidence including observational[11], mechanistic, and intervention studies[12,13] demonstrates a role of dietary constituents and patterns in the prevention and treatment of mental illnesses, although research in SSD is relatively limited.
Health care providers often accurately report obstacles faced by patients with SSD in the implementation of dietary interventions. Obstacles include motivational and cognitive barriers, poverty, as well as psychotic symptoms[14]. However, dietary intervention trials designed to improve aspects of physical health have documented feasibility and acceptability among SSD patients. One diet intervention study demonstrated an increased vegetable intake and decreased intake of discretionary foods and calories in first episode psychosis patients[5]. Another study involving participants with severe mental illness demonstrated improvements in diet quality and cardiovascular disease risk factors following a Mediterranean diet intervention[15].
Although fewer in number, some intervention studies have assessed mental health symptoms as primary or secondary outcomes. The purpose of the present systematic review is to synthesize the existing experimental studies of dietary modification as adjunctive treatment for psychotic disorders. We sought to analyze findings related to mental health outcomes (efficacy) and safety, highlight knowledge gaps and limitations, and set forward recommendations for future research studies.
An extensive a priori search strategy was developed by an experienced medical information specialist through an iterative process, which included over 300 search terms. Supplementary material appendix 1 contains the full search strategy. The search was executed in Embase, Embase Classic, and Ovid MEDLINE on January 6, 2017 and updated on April 15, 2018 with the removal of a small fraction of search terms that had failed to yield useful results in the initial search (see appendix 1 for details). No limits were placed on language, publication status, or year of publication.
Eligible studies included experimental studies with an intervention that attempted to manipulate participants’ overall dietary pattern (e.g., “healthy diet”, diet counselling, nutrition education), not just an individual type of food, dietary constituent or isolated component (e.g., supplement studies, low carbohydrate diets, gluten-free diets). The studies included participants with psychotic disorders. No restrictions were placed on blinding or the presence of a control group. Studies were required to assess a mental health outcome; studies that measured only physical health outcomes were not eligible.
Title and abstract screening were completed, based on the above criteria, in duplicate using Abstrackr, an online open-source program that facilitates rapid screening decisions and concurrent tagging of results. Disagreement was resolved by consensus by the two principle investigators (Monique Aucoin and Laura LaChance). Studies that failed to meet inclusion criteria on full text review were excluded. Data was extracted in duplicate using a piloted template. The data extracted included: author name, year of publication, geographic location, presence of control group, use of randomization, use of blinding, study population, inclusion of individuals with additional psychiatric illnesses, sample size, intervention description, method of intervention delivery (group or individual), additional interventions provided simultaneously, mental health outcome reported, change in outcome, whether the mental health outcome was identified as the primary outcome, weight or other physical health outcome and adverse events. Because of the significant heterogeneity of the populations, interventions and outcomes, meta-analysis was not attempted. Studies were grouped based on whether or not a positive mental health outcome was reported, the primary outcome of interest. χ2 test was used to assess for significance in the differences between these groups of studies based on study design and factors which may contribute to bias.
The search strategy was initially designed for the conduct of a larger scoping review on the topic of dietary factors and psychosis which has been previously published[16].
On January 20, 2020, a search update was completed for any additional studies that met criteria for the present systematic review. Because this update was completed exclusively for the present review, a modified search string using the following terms was executed: (Diet or Dietary or Nutrition or food) and (psychosis or psychotic or schizophrenia).
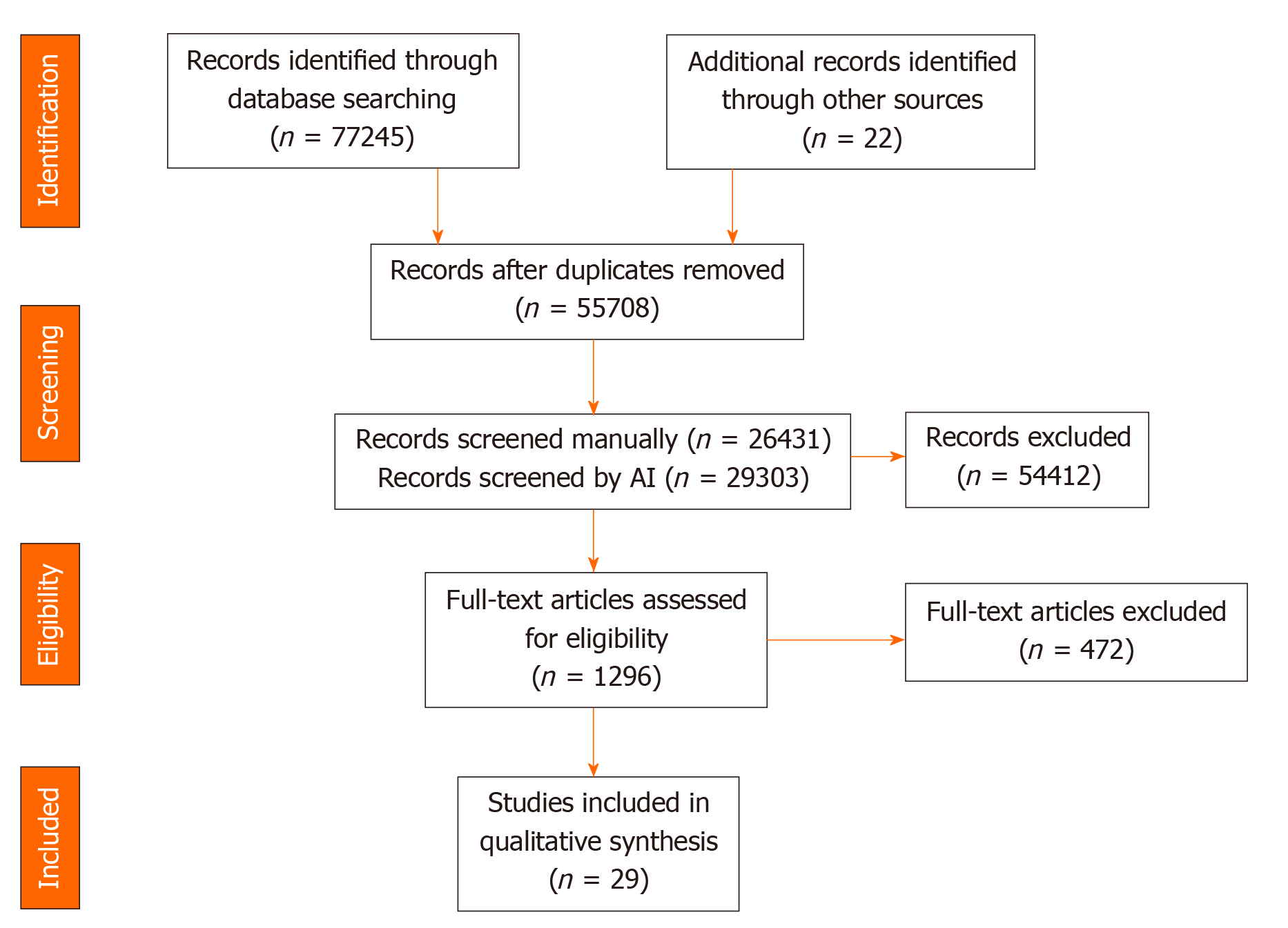
The initial search and updates identified 76889 results, which were reduced to 55330 after de-duplication. Using Abstrackr to facilitate screening allowed study authors to manually screen slightly less than 50% of results (26053), relying on the artificial intelligence feature of the program to screen the remaining articles. 1290 articles were assessed in full-text form with 822 meeting criteria for the scoping review. Of these, 27 manipulated the dietary pattern of participants and met criteria for inclusion in the present systematic review. The update of the search January 2020 yielded 356 results, of which two met criteria for inclusion (Figure 1).
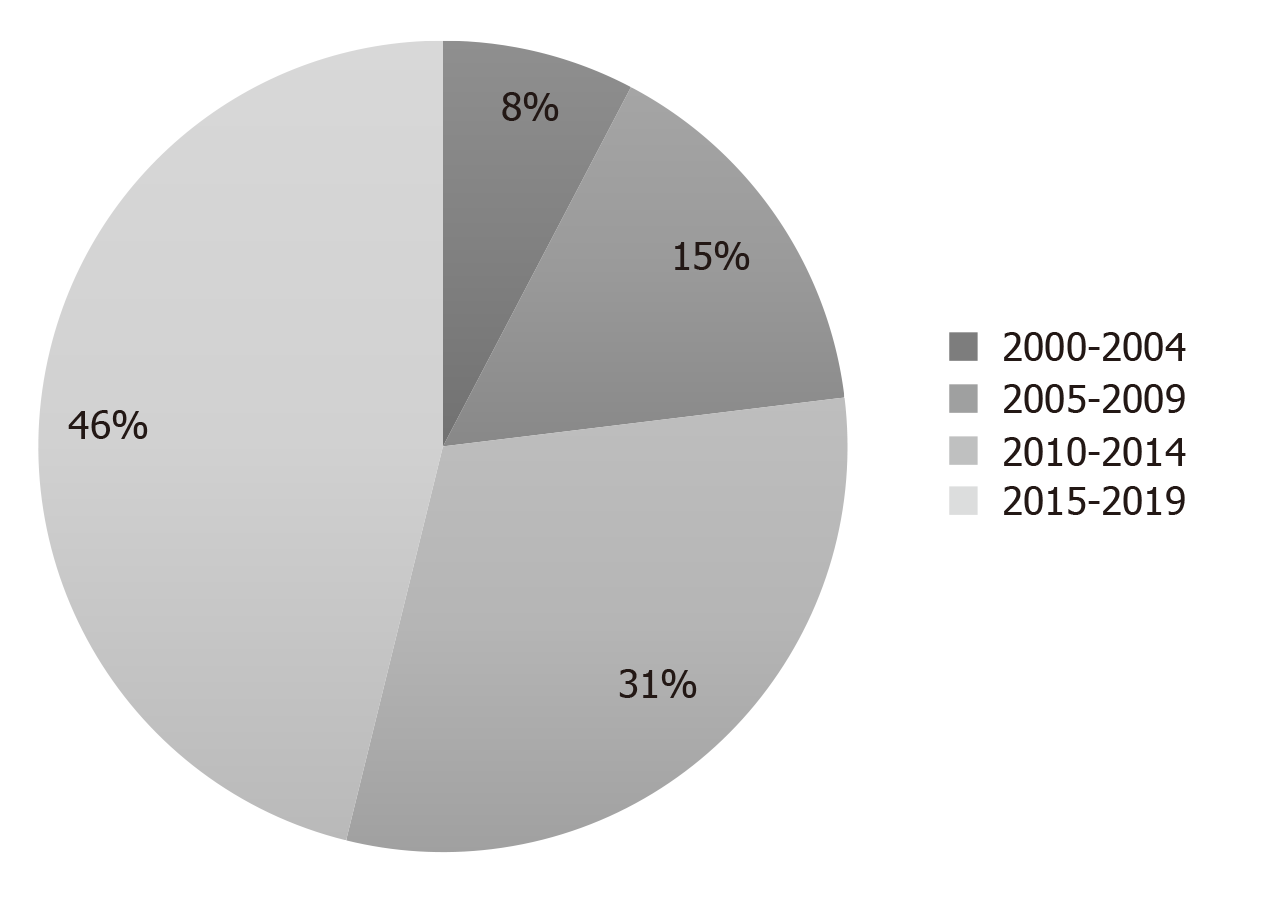
The majority of the studies were published between 2015 and 2019 (Figure 2).
Overall, most of the studies were published in the United Kingdom and Europe followed by North America with the remaining published in Australia, Asia, and South America.
Three publications described study protocols. The results of one of these protocols were described in another publication. Two studies were meta-analyses. The remaining twenty-five studies were completed clinical trials. Of those, four studies employed both blinding and randomization. An additional five studies used a control group and randomization but no blinding. Another six studies used a control group but lacked randomization and blinding. The remaining ten trials were completed with no control group (Table 1)[17-41].
| Ref. | Control | Random-ization and Blinding | Population | Sample size | Intervention | Mental health primary outcome | Duration (wk) | Symptom | Direction of Findings |
| Studies with one or more positive outcomes | |||||||||
| Usher et al[17], 2019 | TAU | None | Youth, early psychosis | 33 | Behaviour intervention teaching mindfulness meditation, cooking classes, supermarket trips, fast-food learning, nutrition education, exercise, moderated group discussion to facilitate healthier living | Yes | 12 | Positive symptoms (Quick Scale for the Assessment of Negative Symptoms) | Improvement |
| Osborn et al[18], 2018 | TAU | Randomized, single blind | Bipolar disorder (50%), schizophrenia (30%) or psychosis (20%); elevated cholesterol and 1 + modifiable risk factors for CVD | 327 | Manualized intervention to decrease cholesterol and CVD risk: Adherence to statins, improve diet and activity, reduce alcohol, quit smoking | No | 52 | Psychiatric In-patient costs | Improvement |
| QOL (Warwick-Edinburgh Mental Well-being Scale) | Null | ||||||||
| Hjorth et al[19], 2017 | None | None | Chronic schizophrenia | 45 | Lifestyle program including nutrition, meal planning, walking/running group, smoking cessation, MI. individual and group components | No | 120 | QOL (WHOQoL) | Null |
| Newly diagnosed schizophrenia | 123 | QOL (WHOQoL) | Improvement | ||||||
| Juel et al[20], 2017 | None | None | Psychotic disorder, affective disorder, anxiety disorder, or developmental disorder with comorbid substance use or dependence; 12.5% SSD | 64 | Nutrition, exercise and smoking cessation program. Includes health diet habits, reading nutrition labels, macronutrient recommendations. Includes individual and group components | No | 24 | QOL (WHOQoL) | Improvement |
| Medication Usage | Worsening | ||||||||
| Masa-Font et al[21], 2015 | TAU | None | Schizophrenic, schizoaffective or bipolar, treated with antipsychotic drug (67% SSD); BMI > 25 | 332 | Group nutrition education and exercise sessions | No | 12 | QOL (SF-36) | Improvement |
| Farhat et al[22], 2016 | None | None | Schizophrenia | 8 | Group educational program including nutritional balance, meal planning, budgeting, meal preparation, socialization | Yes | 16 | Negative (PANSS) | Improvement |
| Cognitive (BECS) | Null | ||||||||
| Provencher et al[23], 2016 | Waitlist | None | SMI including psychotic disorders; weight concern | 47 | Program teaching nutrition and cooking | No | 12 | QOL | Improvement |
| Sauvanaud et al[24], 2016 | None | None | Schizophrenia | 14 | Group educational program/ psychosocial program including schizophrenic disease, treatments, relationships to family, diet, social issues, relaxation | Yes | 15 | Functioning (GAF) | Improvement |
| QOL (SQoL) | Improvement | ||||||||
| Medication Adherence (MARS) | Improvement | ||||||||
| Insight (French IQ8) | Null | ||||||||
| Stiekema et al[25], 2014 | TAU | Treatment centers randomized | Patients with severe mental illness (primarily SSD), inpatient | 130 | Group diet and exercise intervention: Lifestyle intervention to change the obesogenic environment within residential setting (e.g., Whole grain bread available) | No | 12 | Depression | Improvement |
| Psychological functioning | Improvement | ||||||||
| QOL | Worsening | ||||||||
| Robertson et al[26], 2014 | None | None | Schizophrenia-spectrum | 25 | Group healthy lifestyle group, walking group, grocery shopping | Yes | 12 | Psychotic symptoms (PANSS) | Improvement |
| Bralet et al[27], 2013 | None | None | Schizophrenia | 10 | Group nutrition education, cognitive remediation, social skills, meal preparation | Yes | 17 | Psychotic symptoms (PANSS) | Improvement |
| Self Esteem | Improvement | ||||||||
| Cognition (BACS, STICCS) | Improvement | ||||||||
| Kuo et al[28], 2012 | TAU | None | Schizophrenia | 63 | Group weight reduction program including food recommendations (more vegetables and monounsat fats, less sugar and calories), exercise, behaviour therapy | Yes | 10 | Biologic (BDNF) | Improvement |
| Psychotic symptoms (BPRS) | Null | ||||||||
| Porsdal et al[29], 2010 | TAU | None | Schizophrenia or bipolar I, taking psychotropic medication | 373 | Group nutrition and exercise program | Yes | 12 | QOL (15D) | Null |
| Psychotic symptoms (CGI-S) | Improvement | ||||||||
| Van Citters et al[30], 2010 | None | None | DMS-IV Axis I or II diagnosis; 18% SSD | 76 | Individualized health promotion program: health eating, exercise social inclusion, decrease calories. Includes individual and group components | No | 36 | Mental health functioning (SF-12) | Improvement |
| Negative symptoms (SANS) | Improvement | ||||||||
| Gretchen-Doorly et al[31], 2009 | None | None | Schizophrenia | 9 | Nutrition (avoid high fat food, increase fiber, strategies for weight loss), exercise and stress management coaching | Yes | 6 | Self-efficacy for health practices, psychological wellbeing, health responsibility | Improvement |
| Castiglioni et al[32], 2008 | Monthly clinical exam | None | Psychotic disorders | 26 | Group health and Wellness program | No | 24 | QOL (Quality of Life Index) | Improvement |
| Guimarães et al[33], 2008 | TAU | None | Schizophrenia | 67 | Calorie-restricted diet; reduction in saturated fat and sugar, increase in fruit and vegetables. Individual delivery | No | 4 | BDNF | Improvement |
| Raine et al[34], 2003 | TAU | Randomized | Children aged 3-5 years, no diagnosis | 438 | Group nutrition, education and physical exercise program: structured nutrition program provided the children with milk, fruit juice, a hot meal of fish or chicken or mutton, and a salad each day | Yes | 104 | positive schizotypal personality (Schizotypal personality questionnaire) | Improvement |
| Cognition (Schizotypal personality questionnaire) | Improvement | ||||||||
| Aquila et al[35], 2000 | None | None | Inpatients with severe persistent mental illness taking atypical antipsychotics | 32 | Group diet intervention to decrease fat, calories, sugar, snacking | No | 78 | Negative (subjective assessment) | Improvement |
| Studies without positive outcomes | |||||||||
| Holt et al[36], 2019 | TAU | Randomization | Schizophrenia, schizoaffective disorder or first-episode psychosis, antipsychotic medication, BMI > 25 or concern about weight | 412 | Education on nutrition, physical activity and weight management. individual and group components | No | 40 | Depression | Null |
| Psychotic symptoms (BPRS) | Null | ||||||||
| Stiekema et al[37], 2018 | TAU | Randomized, Blinded | Psychotic disorder, mood disorder, personality disorder, anxiety, substance use disorder, developmental disorder or psychiatric comorbidity; 74% SSD; inpatient | 770 | Diet and exercise lifestyle intervention to adjust obesogenic environment (offer low-fat cheese, whole wheat alternatives, less sweets, smaller portions of snacks, fresh rather than canned vegetables) Includes individual and group components | Yes | 52 | Psychotic symptoms (PANSS) | Null |
| QOL (MANSA) | Worsening | ||||||||
| Depression (CDSS) | Null | ||||||||
| Naslundet al[38], 2017 | Gym membership | Randomized | Major depressive disorder, schizoaffective disorder, or schizophrenia; 46% SSD | 343 | Individual nutrition, MI, fitness goal setting | Yes | 52 | Depressive Symptoms (CESD) | Null |
| Detke et al[39], 2016 | Single 15-min session of basic information | None | Schizophrenia or Bipolar I | 203 | Individual intense behavioural weight counseling – nutrition education, pedometer, motivational enhancement, guidance on caloric restriction, low-fat, nutrient dense foods in moderate portion sizes | No | 52 | Psychotic symptoms (BPRS-C) | Null |
| Speyer et al[40], 2016 | TAU | Randomized, blinded | Schizophrenia, schizoaffective disorder or persistent delusional disorder, abdominal obesity | 428 | Individual lifestyle program teaching healthy diet, cooking, smoking cessation, physical activity, coordination of care for somatic health | No | 52 | Psychotic symptoms (SANS and SAPS) | Null |
| Perceived health and stress | Null | ||||||||
| Cognition (BACS) | Null | ||||||||
| QOL (MANSA/Euro QoL) | Null | ||||||||
| Jones et al[41], 2007 | None | None | Taking antipsychotic medication | 50 | Group psychoeducation and physical activity program including nutrition, budgeting, cooking, goal setting | No | 10 | QOL (SF-36) | Null |
The average sample size of the completed studies was 171.8; however, there was significant heterogeneity (SD = 191.5, range: 8 to 770).
The mean duration was 33.4 wk (SD = 31). Most studies used an intervention which lasted between four months and one year. Two studies lasted two years or longer.
The details of the interventions themselves were generally poorly described. Most of the interventions consisted of nutritional education that focused on healthy diet habits. Some programs reported more specific macronutrient recommendations while other emphasized general concepts like meal planning and goal setting. Three interventions involved following specific energy-restricted diets. An additional three interventions mentioned education about calories. The interventions were mainly group-based. Five trials implemented interventions that had both individual and group components and five trials consisted of individual interventions only. It was unclear if the interventions were individual or group-based in two of the studies. Nearly all studies failed to identify the qualifications or training of the individual(s) delivering the interventions.
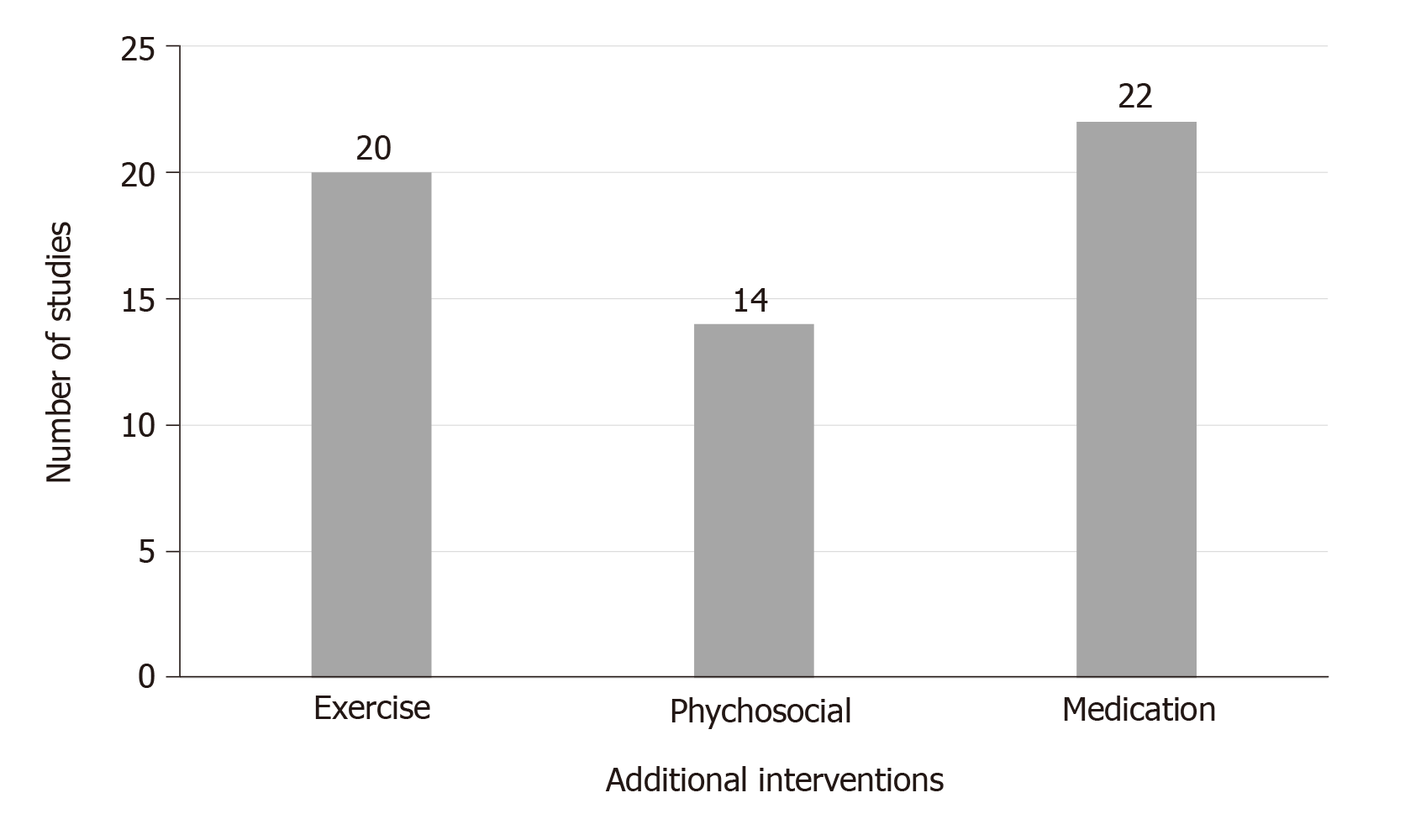
In most of the studies, the participants continued to take their psychiatric medications. All studies supplemented the dietary interventions with exercise and/or psychosocial interventions such as motivational interviewing, smoking cessation, or peer support. The additional interventions that were used alongside diet are displayed in Figure 3.
Seventeen of the twenty-five studies recruited patients with a diagnosis of schizophrenia, SSD, or psychosis. Two of the studies included participants who were taking an antipsychotic medication, regardless of their specific psychiatric diagnosis. Ten of the studies included participants diagnosed with schizophrenia in combination with other mental illnesses such as bipolar disorder, anxiety disorder, major depressive disorder, or personality disorders. Of those, one study required a co-morbid substance-use disorder diagnosis. One study was completed on healthy children who were followed to monitor the development of schizotypal personality traits or antisocial behaviour at ages 17 and 23.
Most of the interventions were carried out in an adult population. One study recruited teenagers (ages 13-17), one recruited youth experiencing early psychosis and one study included children (ages 3-5).
Eight of the studies required participants to have a weight concern such as recent weight gain or a body mass index (BMI) or waist circumference above a certain level. Three studies involved participants in a supportive housing or clinical care facility, the remaining studies were completed with community-dwelling participants.
Of the 27 study protocols described, twelve were designed with mental health symptoms as the primary outcome. The remaining studies described the mental health outcomes as secondary or exploratory.
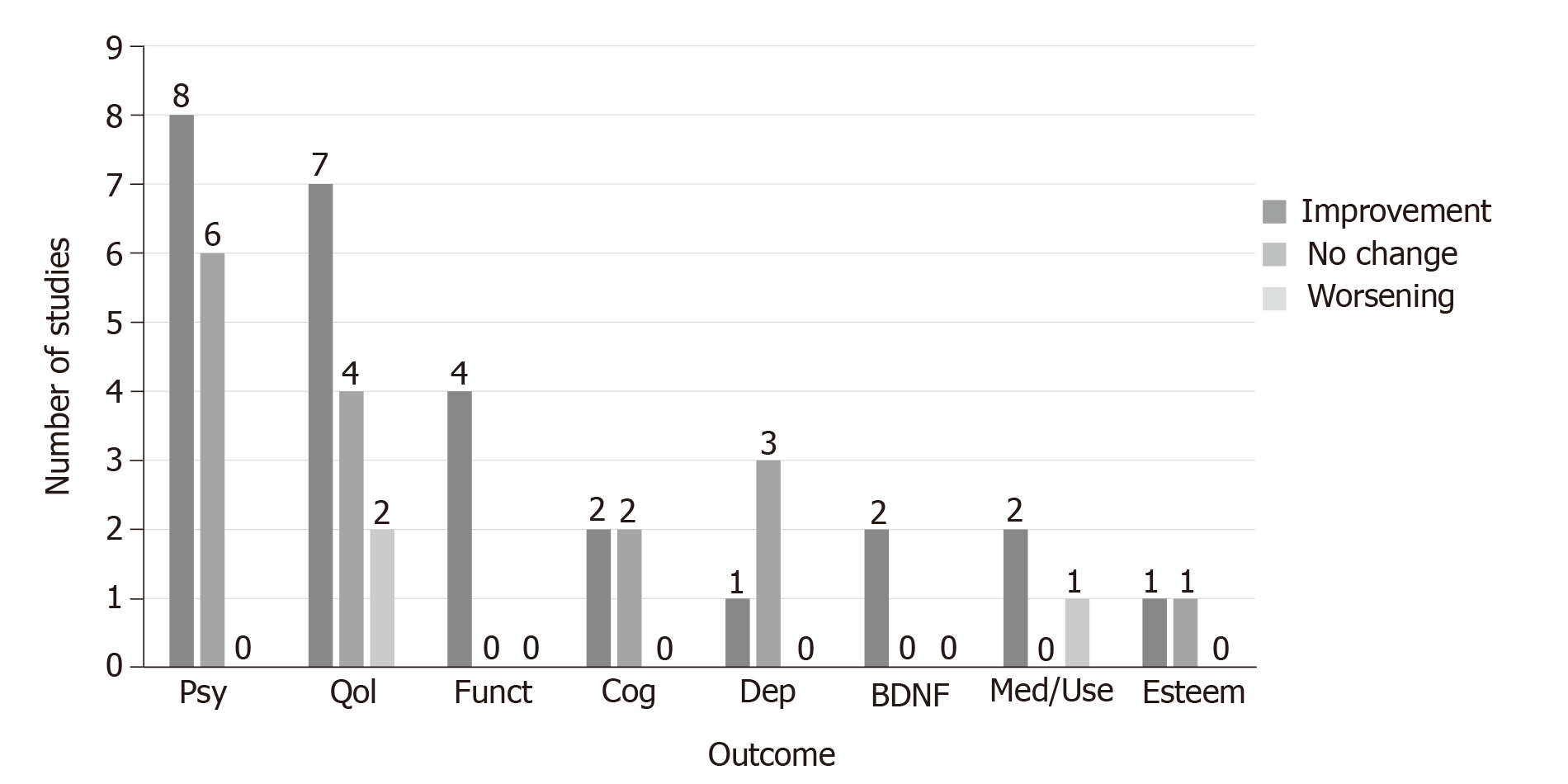
Outcome measures included quality of life, psychosis symptoms, cognitive symptoms, self-esteem, functioning, depression, mental healthcare service utilization and levels of the biomarker BDNF. Nineteen studies reported at least one positive mental health outcome (Figure 4, Table 1). While several studies reported changes in total psychosis symptoms, three reported improvements in negative symptoms and one in positive symptoms. The prospective intervention trial that assessed prevention of psychotic symptoms in children reported a decrease in positive symptoms at the later outcome assessment[34]; however, given that this study’s design was significantly different compared to the others in this review, these results may be considered separately from the other studies reviewed.
Studies that did not report a positive mental health outcome were more likely to include 200 or more participants, use a control group or randomization, or include participants with diagnoses other than schizophrenia. They were more likely to involve one-on-one intervention rather than group intervention. Compared with those showing benefit, they were less likely to cite mental health symptoms as a primary outcome and less likely to report positive findings in an outcome related to body weight. The differences between studies reporting a benefit to mental health outcomes and those that did not are reported in Table 2. The differences in sample size, group vs individual intervention format and, randomization achieved statistical significance.
| Number of studies reporting benefit (%) | Number of studies not reporting benefit (%) | P value | |
| Studies with greater than 200 participants | 4 of 19 (21) | 5 of 6 (83) | 0.00559 |
| Studies that included patients with non-SSD diagnoses (e.g., Depression, Bipolar, substance use disorder) | 8 of 19 (42) | 4 of 6 (67) | 0.29380 |
| Studies with an individual intervention component | 2 of 19 (11) | 3 of 6 (50) | 0.03509 |
| Studies designed with primary outcome related to mental health | 9 of 19 (47) | 2 of 6 (33) | 0.54599 |
| Studies that used a control | 10 of 19 (53) | 5 of 6 (83) | 0.18081 |
| Studies that used randomization | 3 of 19 (16) | 4 of 6 (67) | 0.01553 |
| Studies with positive finding in an outcome related to body weight | 7 of 10 (70) | 1 of 4 (25) | 0.12428 |
With respect to duration of the effect, one study reported that positive mental health and weight outcomes persisted at a six month follow up assessment[32], the remainder did not report on long term outcomes.
A meta-analysis of six lifestyle intervention studies, including 289 participants, evaluated changes in levels of peripheral serum and plasma BDNF in patients with SSD and reported some positive findings compared to a control group[42]. A meta-analysis of 25 lifestyle intervention studies, including 1518 SSD participants, reported improvement in depression symptoms[43].
In addition to assessing mental health outcomes, 18 studies assessed physical health outcomes such as body weight, blood pressure, serum cholesterol, waist circumference, oral glucose tolerance, serum cytokine levels, and glycosylated haemoglobin. Nine of 18 studies found improvements in at least one physical measure with body weight, BMI and waist circumference most likely to benefit. It should be noted, that this represents a subset of the research on this topic; studies assessing exclusively physical outcomes were excluded from this review.
Adverse events were not generally reported. One study commented on adverse events that were medication-induced and not attributed to the intervention[39]. Another trial noted an increase in psychotropic medication use over the course of the assessment period[20]. An additional trial commented on deterioration of mental well-being in the intervention group[37]. Finally, one of the studies found that there were three deaths in the intervention group and they were not considered a result of the intervention; any other adverse events were similar between the treatment and the control groups[36].
This systematic review identified the clinical trials which have attempted to modify dietary patterns in patients with SSD and reported mental health outcomes in response to treatment. The results suggest that these interventions may have promise for improving mental health symptoms in SSD patients. However, there are significant limitations to these studies and opportunities for further research.
Twenty-one of 27 primary and secondary research studies reported significant improvement in terms of quality of life, symptom improvement, increased functioning, decrease mental healthcare utilization and/or improved cognition. Studies with positive findings were generally less likely to include other patient populations (non-SSD), were more likely to have a group component; however, they tended to include smaller sample sizes and were less likely to be randomized.
Adverse events were often not reported and if they were reported, they were not attributed to the dietary intervention itself. Two studies reported worsening mental health in response to treatment. One study[20] noted that participants had to increase their use of medication during the trial. The medication that was typically increased was quetiapine (Seroquel), which is also used as a sleep aid. The authors theorized that the increase in medication may be due to sleep issues rather than increasing psychiatric symptoms as the participants’ sleep did worsen in the trial, possibly attributed to the various stages of withdrawal from substance use. The other study[37] noted that Quality of Life decreased in the intervention group. They attributed this to significant differences in levels of depression at baseline (non-optimal randomization) and a possible regression towards the mean. Furthermore, although the decrease in quality of life was statistically significant, it may not have been clinically relevant as it was a decrease of 1.5 points, on a scale with a range of 12-84 points.
Many of the studies utilized a group setting for the intervention. There is significant research suggesting a role of peer support and motivation in behavioural change[44,45]. In this review, studies using group interventions were more likely to report positive findings. Group delivery of interventions has the added benefit of cost effectiveness[46].
A significant limitation of this body of research is the generally small sample size and low methodological quality; few studies used randomization, blinding, or control groups. However, the most significant limitation is that all studies used multi-modal interventions rather than diet alone. These included exercise, psychosocial interventions, and smoking cessation in addition to medication. As the first two have significant evidence for a role in promoting improved mental health in SSD, it creates difficulty in understanding the exact role that diet modification may have played in achieving the outcomes reported.
Additionally, many of the studies were designed to test interventions for weight loss or other physical conditions such as hypertension, dyslipidemia and glucose dysregulation. Mental health outcomes were secondary or exploratory in most of the studies. As a result, studies may have been statistically powered to detect changes in the primary physical outcomes and not the mental health outcomes.
A significant limitation of this body of evidence is in the reporting of the diet interventions tested. Nineteen studies described the intervention as “a healthy diet” or “nutrition education”. While some studies provided details about the goals of the intervention (such as increased vegetable intake, decreased saturated fat intake), the majority of studies did not. Scientific understanding of a “healthy diet” has changed over time. For example, the American Dietary Guidelines removed cholesterol as a nutrient of concern in 2015 due to lack of evidence that dietary cholesterol impacts serum cholesterol levels[47]. However, previous guidelines had advised patients on limiting exposure to this nutrient. While certain dietary factors such as vegetables and fiber have been consistently viewed as healthy, fats and carbohydrates have experienced shifting attitudes over the previous decades[48]. As a result, studies designed at different times may have recommended significantly different macronutrient ratios. In addition to the role of macro- and micro-nutrients, a body of evidence has identified inflammation, oxidative stress, glycemic balance, food sensitivities, and the gut microbiome as intermediate factors between diet and mental health[49]. These various factors make it difficult to interpret the studies included in this review. It is possible that unique interventions with differing impacts on these pathophysiologic mechanisms contributed to the differing outcomes between studies. Future research may benefit from application of reporting checklists designed to improve the reporting of interventions[50]. A recent systematic review of methodology reporting in lifestyle interventions among people with psychosis also found generally incomplete reporting and put forward a recommendation for use of the CONSORT statement in future publications[51].
While it has been demonstrated that diet patterns in this patient population can be changed in response to nutrition education and counseling, it is widely acknowledged that this is challenging[14]. The lack of compliance data in the studies using educational programs and diet counselling creates significant difficulty in interpreting this body of evidence. It is possible that variability of compliance with the dietary interventions across studies could in part explain differing outcomes reported. Four studies included the provision of some or all of the participants’ food intake and thus had a higher degree of control. These included the prospective prevention study in children and three studies which took place in in-patient settings. Although these studies involved modification to the available food options, there was still a degree of control exercised by participants with respect to the food they consumed. Of note, three of these four studies reported benefit.
Further research should include assessment of compliance. In designing further studies, researchers could consider subjective assessment tools such as the Mediterranean Diet score or Alternative Healthy Eating Index[52] or objective assessment approaches such as plasma vitamin C and serum carotenoids[53] or metabolomic analysis[54]. While speculative, the non-significant association found between studies showing benefit in both mental health and physical health outcomes could be related to participant compliance. Alternatively, the improvement in mental health symptoms may be related to changes in weight, as a bidirectional relationship between the two is known[55]. In the absence of information related to intervention uptake, the studies showing a lack of change to mental health outcomes cannot conclude that diet does not impact psychosis and other mental health outcomes but rather that the educational intervention provided was not able to elicit these changes.
The results of this body of evidence suggest that diet may be a modifiable target for the treatment of psychotic disorders alongside treatment as usual. Dietary interventions were associated with improvements in psychosis symptoms, quality of life, and functioning in some studies. Improved nutrition has also been shown to improve the physical health in a population at high risk of medical illness[5]. There are studies reporting no effect of dietary intervention. These inconsistencies may be attributable to heterogeneity in dietary interventions (including the multi-modal nature), unclear participant compliance with educational interventions and heterogeneous participant populations.
Nutritional interventions have a few clear benefits. They are relatively low in cost, low in risk, and have the potential to benefit the highly prevalent and costly physical comorbidities of schizophrenia spectrum disorders. When interpreted in combination with the more advanced research in other psychiatric populations, such as randomized controlled trial of adjunctive dietary interventions in major depressive disorder, there is justification for more research in this area.
Future studies should aim to examine adjunctive dietary modification without manipulation of other health behaviours, assess compliance using subjective or objective methods, include more homogeneous patient populations, identify mental health symptoms as primary outcomes and be powered accordingly. In addition, high quality experimental studies with a control group, blinding, and randomization are needed in this area of research alongside continuing research exploring effectiveness in real world scenarios. Lastly, in reporting on these studies, clearer description of the experimental dietary interventions would assist in interpreting results, duplication of findings, and guiding further research.
Schizophrenia spectrum disorders (SSD) impact functioning, reduce quality of life and increase the risk of physical illness and premature mortality. Several observational and preclinical studies suggest a role of diet in the development and progression of SSD and rigorous clinical trials designed to assess the impact of adjunctive diet interventions in patients with major depression have reported benefit.
Nutritional intervention studies aimed at decreasing body weight have demonstrated efficacy in improving metabolic outcomes; however, few studies have explored the impact of interventions designed to modify diet on mental health outcomes. Systematic review of this body of literature has not been previously conducted.
This systematic review sought to synthesize the existing experimental studies of adjunctive diet modification as an intervention in the treatment of psychotic disorders, analyze findings related to effectiveness and safety, highlight knowledge gaps and limitations, and set forward recommendations for future research studies.
An extensive a priori search strategy was developed and the databases Embase, Embase Classic, Ovid MEDLINE were searched. Screening and data extraction were completed in duplicate. Studies included in this analysis were experimental studies of an adjunctive dietary intervention (overall dietary pattern or education on dietary change) for treatment of SSD. No restrictions were placed on control groups or blinding. The studies were required to report a mental health outcome.
Twenty-five clinical trials were identified, along with two additional protocols and two meta-analyses. Of the 25 clinical trials, four employed both blinding and randomization. Five studies used a control group and randomization but no blinding while another six studies used a control group but lacked randomization and blinding. The remaining ten trials were completed with no control group. The average sample size was 171 participants and the average duration was 33 wk. The interventions consisted primarily of nutritional education; however, the nutrition advice provided to participants was poorly described overall and compliance was not assessed. All of the studies included lifestyle or psychosocial components in addition to dietary modification. Of the 27 protocols, twelve were designed with mental health symptoms as the primary outcome. A high level of heterogeneity was found with respect to patient population, intervention, and study design. Nineteen of the clinical trials reported improvement in one or more mental health domain including psychosis symptoms, cognition, and quality of life. The studies that showed benefit tended to have a smaller sample size and were less likely to be randomized but were more likely to use a group delivery intervention. Adverse events were not generally reported.
The findings of this systematic review suggest that adjunctive diet interventions may be useful in the management of mental health symptoms in SSD; however, limitations in the existing research preclude clear conclusions.
Further research assessing effectiveness and efficacy of clearly reported dietary interventions is warranted, especially those identifying mental health symptoms as their primary outcome, using rigorous methodology (control group and blinding), modifying diet in isolation from other health behaviours and assessing participant compliance.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gregorio BM S-Editor: Zhang L L-Editor: A E-Editor: Li JH
| 1. | Agarwal SM, Caravaggio F, Costa-Dookhan KA, Castellani L, Kowalchuk C, Asgariroozbehani R, Graff-Guerrero A, Hahn M. Brain insulin action in schizophrenia: Something borrowed and something new. Neuropharmacology. 2020;163:107633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2016;176:23-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Gatov E, Rosella L, Chiu M, Kurdyak PA. Trends in standardized mortality among individuals with schizophrenia, 1993-2012: a population-based, repeated cross-sectional study. CMAJ. 2017;189:E1177-E1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Teasdale SB, Ward PB, Rosenbaum S, Watkins A, Curtis J, Kalucy M, Samaras K. A nutrition intervention is effective in improving dietary components linked to cardiometabolic risk in youth with first-episode psychosis. Br J Nutr. 2016;115:1987-1993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8:114-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 685] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 8. | Hansen T, Ingason A, Djurovic S, Melle I, Fenger M, Gustafsson O, Jakobsen KD, Rasmussen HB, Tosato S, Rietschel M, Frank J, Owen M, Bonetto C, Suvisaari J, Thygesen JH, Pétursson H, Lönnqvist J, Sigurdsson E, Giegling I, Craddock N, O'Donovan MC, Ruggeri M, Cichon S, Ophoff RA, Pietiläinen O, Peltonen L, Nöthen MM, Rujescu D, St Clair D, Collier DA, Andreassen OA, Werge T. At-risk variant in TCF7L2 for type II diabetes increases risk of schizophrenia. Biol Psychiatry. 2011;70:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M. Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. J Clin Psychiatry. 2012;73:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Stubbs B, Williams J, Gaughran F, Craig T. How sedentary are people with psychosis? A systematic review and meta-analysis. Schizophr Res. 2016;171:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 11. | Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017;253:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 12. | Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med. 2017;15:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 13. | Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, Itsiopoulos C, Niyonsenga T, Blunden S, Meyer B, Segal L, Baune BT, O'Dea K. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr Neurosci. 2019;22:474-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 265] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 14. | Teasdale SB, Samaras K, Wade T, Jarman R, Ward PB. A review of the nutritional challenges experienced by people living with severe mental illness: a role for dietitians in addressing physical health gaps. J Hum Nutr Diet. 2017;30:545-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Bogomolova S, Zarnowiecki D, Wilson A, Fielder A, Procter N, Itsiopoulos C, O'Dea K, Strachan J, Ballestrin M, Champion A, Parletta N. Dietary intervention for people with mental illness in South Australia. Health Promot Int. 2018;33:71-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Aucoin M, LaChance L, Cooley K, Kidd S. Diet and Psychosis: A Scoping Review. Neuropsychobiology. 2020;79:20-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Usher C, Thompson A, Griebeler M, Senders A, Seibel C, Ly R, Murchison C, Hagen K, Afong KA, Bourdette D, Ross R, Borgatti A, Shinto L. Meals, Mindfulness, & Moving Forward: A feasibility study to a multi-modal lifestyle approach in early psychosis. Early Interv Psychiatry. 2019;13:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, Blackburn R, Craig T, Gilbert H, Heinkel S, Holt R, King M, Michie S, Morris R, Morris S, Nazareth I, Omar R, Petersen I, Peveler R, Pinfold V, Walters K. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatry. 2018;5:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Hjorth P, Medici CR, Juel A, Madsen NJ, Vandborg K, Munk-Jørgensen P. Improving quality of life and physical health in patients with schizophrenia: A 30-month program carried out in a real-life setting. Int J Soc Psychiatry. 2017;63:287-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Juel A, Kristiansen CB, Madsen NJ, Munk-Jørgensen P, Hjorth P. Interventions to improve lifestyle and quality-of-life in patients with concurrent mental illness and substance use. Nord J Psychiatry. 2017;71:197-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Masa-Font R, Fernández-San-Martín MI, Martín López LM, Alba Muñoz AM, Oller Canet S, Martín Royo J, San Emeterio Echevarría L, Olona Tabueña N, Ibarra Jato M, Barroso García A, González Tejón S, Tajada Vitales C, Díaz Mújica B, Viñas Cabrera L, Sanchís Catalán R, Salvador Barbarroja T. The effectiveness of a program of physical activity and diet to modify cardiovascular risk factors in patients with severe mental illness after 3-month follow-up: CAPiCOR randomized clinical trial. Eur Psychiatry. 2015;30:1028-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Farhat SL, Hochard C, Orens S, Gautier C, Lambert T, Geret L, Bralet MC. [MODen: Psychoeducationnal therapeutic group program for schizophrenic patients, based on nutritional balance and pleasure, using cognitive functions: A pilot study]. Encephale. 2016;42:410-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Provencher MD, Bélanger MÈ, Shriqui C, Lachance I, Bonneville S. [Psychoeducation for overweight patients with psychiatric disorders: The Wellness program developed in Quebec]. Encephale. 2016;42:201-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Sauvanaud F, Kebir O, Vlasie M, Doste V, Amado I, Krebs MO. [Therapeutic benefit of a registered psychoeducation program on treatment adherence, objective and subjective quality of life: French pilot study for schizophrenia]. Encephale. 2017;43:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Stiekema APM, Looijmans A, van der Meer L, Bruggeman R, Schoevers RA, Corpeleijn E, Jörg F. Effects of a lifestyle intervention on psychosocial well-being of severe mentally ill residential patients: ELIPS, a cluster randomized controlled pragmatic trial. Schizophr Res. 2018;199:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Robertson S, Conrad G, Gibson M, Deslauriers C, Morris C, Daly J, Charlebois H, Mezzabotta L, Roy P, Kaluzienski M, Hoe W. The effect of a multi-modal, 12-week healthy lifestyle, grocery shopping and walking group intervention with and without switch to ziprasidone on metabolic parameters in a Canadian first episode psychosis population. Early Intervention in Psychiatry. 2014;8:31. [Cited in This Article: ] |
| 27. | Bralet MC, Hochard C, Kechid L, Gautier C, Lambert T, Orens S. MODEN: A french integrative rehabilitation program including cognitive remediation therapy, educational sessions and social skills for patients with schisophrenia. Schizophrenia Bulletin. 2013;39:S283. [Cited in This Article: ] |
| 28. | Kuo FC, Lee CH, Hsieh CH, Kuo P, Chen YC, Hung YJ. Lifestyle modification and behavior therapy effectively reduce body weight and increase serum level of brain-derived neurotrophic factor in obese non-diabetic patients with schizophrenia. Psychiatry Res. 2013;209:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Porsdal V, Beal C, Kleivenes OK, Martinsen EW, Lindström E, Nilsson H, Svanborg P. The Scandinavian Solutions for Wellness study - a two-arm observational study on the effectiveness of lifestyle intervention on subjective well-being and weight among persons with psychiatric disorders. BMC Psychiatry. 2010;10:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Van Citters AD, Pratt SI, Jue K, Williams G, Miller PT, Xie H, Bartels SJ. A pilot evaluation of the In SHAPE individualized health promotion intervention for adults with mental illness. Community Ment Health J. 2010;46:540-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Gretchen-Doorly D, Subotnik KL, Kite RE, Alarcon E, Nuechterlein KH. Development and evaluation of a health promotion group for individuals with severe psychiatric disabilities. Psychiatr Rehabil J. 2009;33:56-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Castiglioni FC, Valentino PA, Giovannelli I. Experimentation of a rehabilitative intervention in the management of body weight in psychotic patients treated with atypical antipsychotic drugs: One-year outcome. Quaderni Italiani di Psichiatria. 2008;27:146-8. [Cited in This Article: ] |
| 33. | Guimarães LR, Jacka FN, Gama CS, Berk M, Leitão-Azevedo CL, Belmonte de Abreu MG, Lobato MI, Andreazza AC, Ceresér KM, Kapczinski F, Belmonte-de-Abreu P. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1595-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Raine A, Mellingen K, Liu J, Venables P, Mednick SA. Effects of environmental enrichment at ages 3-5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. Am J Psychiatry. 2003;160:1627-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Aquila R, Emanuel M. Interventions for Weight Gain in Adults Treated With Novel Antipsychotics. Prim Care Companion J Clin Psychiatry. 2000;2:20-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Holt RIG, Gossage-Worrall R, Hind D, Bradburn MJ, McCrone P, Morris T, Edwardson C, Barnard K, Carey ME, Davies MJ, Dickens CM, Doherty Y, Etherington A, French P, Gaughran F, Greenwood KE, Kalidindi S, Khunti K, Laugharne R, Pendlebury J, Rathod S, Saxon D, Shiers D, Siddiqi N, Swaby EA, Waller G, Wright S. Structured lifestyle education for people with schizophrenia, schizoaffective disorder and first-episode psychosis (STEPWISE): randomised controlled trial. Br J Psychiatry. 2019;214:63-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Stiekema APM, Looijmans A, van der Meer L, Bruggeman R, Schoevers RA, Corpeleijn E, Jörg F. Effects of a lifestyle intervention on psychosocial well-being of severe mentally ill residential patients: ELIPS, a cluster randomized controlled pragmatic trial. Schizophr Res. 2018;199:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Naslund JA, Aschbrenner KA, Pratt SI, Lohman MC, Scherer EA, McHugo GJ, Marsch LA, Unützer J, Bartels SJ. Association Between Cardiovascular Risk and Depressive Symptoms Among People With Serious Mental Illness. J Nerv Ment Dis. 2017;205:634-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Detke HC, DelBello MP, Landry J, Hoffmann VP, Heinloth A, Dittmann RW. A 52-Week Study of Olanzapine with a Randomized Behavioral Weight Counseling Intervention in Adolescents with Schizophrenia or Bipolar I Disorder. J Child Adolesc Psychopharmacol. 2016;26:922-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Speyer H, Christian Brix Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, Hjorthøj C, Pisinger C, Gluud C, Mors O, Krogh J, Nordentoft M. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry. 2016;15:155-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 41. | Jones A, Benson A, Griffith S, Berk M, Dodd S. 'Mind and Body': a lifestyle programme for people on antipsychotic medication. J Eval Clin Pract. 2009;15:276-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Sanada K, Zorrilla I, Iwata Y, Bermúdez-Ampudia C, Graff-Guerrero A, Martínez-Cengotitabengoa M, González-Pinto A. The Efficacy of Non-Pharmacological Interventions on Brain-Derived Neurotrophic Factor in Schizophrenia: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Bruins J, Jörg F, Bruggeman R, Slooff C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: a meta-analysis. PLoS One. 2014;9:e112276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Lara J, Evans EH, O'Brien N, Moynihan PJ, Meyer TD, Adamson AJ, Errington L, Sniehotta FF, White M, Mathers JC. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med. 2014;12:177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr Rehabil J. 2004;27:392-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 46. | Rickheim PL, Weaver TW, Flader JL, Kendall DM. Assessment of group versus individual diabetes education: a randomized study. Diabetes Care. 2002;25:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 47. | Office of Disease Prevention and Health Promotion. 2015–2020 Dietary Guidelines for Americans 2015. Available from: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/. [Cited in This Article: ] |
| 48. | Temple NJ. Fat, Sugar, Whole Grains and Heart Disease: 50 Years of Confusion. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, Stubbs B, Schuch FB, Carvalho AF, Jacka F, Sarris J. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom Med. 2019;81:265-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 50. | Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5117] [Cited by in F6Publishing: 4899] [Article Influence: 489.9] [Reference Citation Analysis (1)] |
| 51. | Mucheru D, Hanlon MC, McEvoy M, MacDonald-Wicks L. An appraisal of methodology reporting in lifestyle interventions among people with psychosis: A systematic review. Health Promot J Austr. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101:587-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 53. | Woodside JV, Draper J, Lloyd A, McKinley MC. Use of biomarkers to assess fruit and vegetable intake. Proc Nutr Soc. 2017;76:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Macias S, Kirma J, Yilmaz A, Moore SE, McKinley MC, McKeown PP, Woodside JV, Graham SF, Green BD. Application of 1H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites. 2019;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Fuller NR, Burns J, Sainsbury A, Horsfield S, da Luz F, Zhang S, Denyer G, Markovic TP, Caterson ID. Examining the association between depression and obesity during a weight management programme. Clin Obes. 2017;7:354-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |