Published online May 23, 2015. doi: 10.5494/wjh.v5.i2.79
Peer-review started: October 1, 2014
First decision: October 28, 2014
Revised: November 11, 2014
Accepted: February 4, 2015
Article in press: February 6, 2015
Published online: May 23, 2015
Acute thoracic aortic dissection is part of the acute aortic syndrome triad. Caused by an intimal tear in the lumen of the aorta, it leads to the creation and propagation of a false lumen. In the acute setting this can lead to malignant hypertension, pain and end organ malperfusion. In the chronic setting it can lead to aneurysm formation and rupture. It remains the most common aortic emergency, affecting up to 4 per 100000 people per year in the United Kingdom and United States. Despite advances in treatment and centralisation of vascular services, it continues to be associated with a high pre-admission and in-hospital mortality. Dissection is classified in several ways according to anatomical extent, timing and underlying pathology, all of which guides clinical management. Traditionally, medical management has been the mainstay of treatment in patients with uncomplicated disease. Surgery has been used in symptomatic patients. With published information now available from several prospective international registries, we are beginning to see the advantages of newer surgical treatment options such as endovascular repair, in the acute setting. This review provides an update on diagnosis and management of aortic dissection, including new information that has become available in recent years.
Core tip: Aortic dissection remains the most common aortic emergency, affecting up to 4 per 100000 people per year in the United Kingdom and United States. Surgical management is indicated in dissection complicated by uncontrolled pain and hypertension, end-organ malperfusion and aneurysmal dilatation with risk of rupture. This update discusses results of thoracic stenting from more recently published prospective international registries, including risks and benefits to treated patients affected by this incredibly high risk condition.
- Citation: Benson RA, Patterson BO, Loftus IM. Diagnosis and management of thoracic aortic dissection: An update. World J Hypertens 2015; 5(2): 79-84
- URL: https://www.wjgnet.com/2220-3168/full/v5/i2/79.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i2.79
Aortic dissection is one of the conditions included in the term “acute aortic syndrome”. This collection includes true dissection, intramural haematoma and penetrating aortic ulcer[1]. Of these acute dissection is the most common, affecting up to 4 people in 100000 annually[2]. Despite advances in therapies, pre-hospital mortality remains high at 20%. Thirty percent of all dissections surviving to a vascular centre will die before discharge[2]. Mortality depends on dissection type, cause and treatment options. New information on the management of type B acute dissection has been published in recent years. This review will discuss all forms of thoracic aortic dissection, with a focus on the recent shifts towards use of surgical management of acute type B dissection using thoracic endovascular repair (TEVAR) rather than best medical therapy alone.
Dissection refers to the separation of the intima/inner media and outer media/adventitia of any artery, due to the tracking of blood into this potential space via a tear in the intima. The false passage can track both antegrade and retrograde[3]. Traditionally they are considered acute if within 14 d of onset and chronic after 14 d. However, publication of survival curves in patient presenting with dissection has shown that survival drops sharply around 30 d post-presentation[4,5]. Therefore the terms acute (< 2 wk), subacute (2-6 wk) and chronic (> 6 wk) have been suggested by a recent European panel[6].
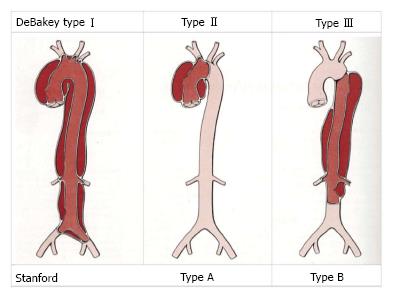
Three classification systems are in common use. The Stanford and DeBakey classification systems use anatomical markers to differentiate dissection type (Figure 1). Stanford type A dissections involve the ascending aorta, while type B originate anywhere distal to the origin of the left subclavian artery[7]. The DeBakey system has three groups. Type 1 involves ascending and descending aorta, type 2 ascending aorta only and type 3 descending aorta only[8]. The European Society of Cardiologists categorise dissection by aetiology using 5 classifications based on pathogenesis of the intimal injury. The advantage of this system, is that it can be used to guide clinical management toward medical or surgical therapy[1]. During this review, the authors will use the Stanford classification due to its wide use within the literature.
As with other aortic pathologies such as aneurysmal disease, those at greatest risk overall are white, male and over 60[8,9]. Type B dissection accounts for 25%-40% of all dissections[10] although recent literature suggests type B dissection is more common than type A amongst African American patients[11]. A study by the international Registry of Acute Aortic Dissection (IRAD) using data from 12 international centres showed that men accounted for 68% of acute presentation[9]. Hypertension, increasing age and pre-existing arterial disease were also common factors.
Systemic hypertension is present in up to 75% of patients at presentation. Physical exertion or a period of emotional stress may be identified as a trigger, likely due to it leading to an acute episode of hypertension[12]. Familial aneurysmal syndromes and connective tissue disease is an important factor in younger patients, more specifically Marfan’s syndrome with fibrillin-1 deficiency, Ehler-Dahnlos type IV (abnormal type III procollagen) and any other cause of cystic medial necrosis[13-15]. Other congenital defects related to younger presentation are a bicuspid aortic valve (likely due to associated aortic root abnormalities) and coarctation of the aorta (and its associated hypertension)[16]. Other causes of disease in the younger patient include pre-existing vasculitic disease, pregnancy and cocaine abuse[9,17]. Vascular interventions may also act as a trigger, for example following percutaneous cardiac catheterisation, coronary artery bypass grafting, or thoracic stenting procedures for aneurysmal disease.
Ninety percent of patients present with sudden onset pain in the chest. In type A dissection it may radiate to the neck, and in type B to the interscapular area[18]. Diabetes is thought to account for the remaining, asymptomatic dissections[19]. New aortic regurgitation is picked up in 31% of patients, and a radio-radio/radio-femoral delay in 15%[8]. Type A presents with hypotension in up to 25% of patients, whereas type B dissections tend to present with hypertension[8]. If both true and false lumens are perfused the aortic branches, and therefore end organs, will remain perfused. If this is not the case, dissection can present with neurological symptoms such as stroke, renal failure, bowel ischaemia or limb ischaemia[20]. These are considered high-risk features, and their effect on management is discussed below. On occasion, an asymptomatic dissection can lead to aortic dilatation and rupture, either acutely, or up to three years after the initial event[10].
Differential diagnoses include myocardial infarction, pulmonary embolus, perforated viscus, stroke or other neurological insult and embolic disease[21]. ECG and X-ray are not sensitive enough to diagnose dissection, but will identify concomitant acute coronary syndromes or act as indicators of alternative diagnoses[22]. CT angiography remains the recommended first line investigation in those suspected of having dissection[1]. It is also useful for planning surgical intervention. Other first line modalities include transoesophageal ECHO, which has the advantage of identifying new aortic regurgitation or pericardial effusion. However it cannot image the entire aorta, and is heavily operator dependent[1]. Magnetic resonance angiography lacks radiation exposure, and uses less nephrotoxic agent, which benefits patients with evidence of renal hypoperfusion. However availability is more limited and imaging takes longer, making it more suitable in the chronic setting or for patient follow-up. All three of these modalities have sensitivity and specificity of over 95% for diagnosis[23].
The mainstay of initial management is resuscitation and stabilisation, to allow transfer for diagnostic imaging and subsequent treatment. Large bore venous access and invasive monitoring including arterial line, cardiac monitoring and urinary catheterisation should be instigated. Close monitoring of end organ function will help identify any deterioration quickly. This includes cardiac monitoring as a proxy for coronary perfusion, cerebral perfusion, limb perfusion and urine output. Wherever possible, this should be in a high dependency setting.
In those patients presenting with hypertension, a target systolic blood pressure of between 100-120 mmHg and heart rate of 60-80 beats per minute should be sought[1,24]. The aim of this is to decrease shear force on the aortic wall and prevent further propagation of the dissection flap[25]. Systolic pressure control in the emergency setting is commonly in the form of a short acting intravenous beta-blocker such as Labetalol. This should be balanced against any deterioration of end organ perfusion. Once haemodynamically stable, the patient should be imaged without delay.
The mainstay of treatment for type A dissection is surgical. Left untreated, it carries a 50%-91% mortality at 7 d, due to rupture, stroke, visceral malperfusion, cardiac tamponade and heart failure[8]. Surgery involves open replacement of the aortic root and affected arch with a prosthetic graft. In extensive dissection involving the ascending and descending aorta, a portion of the graft can be sutured in a way that leaves a free section within native aorta. This provides a landing zone for the stent graft required to treat the rest of the diseased aorta and is known as a hybrid repair. The time lag between first and second stages of repair remains controversial[26]. In hospital mortality following a procedure such as this remains 24%[27]. Further surgical intervention in the form of aortic valve replacement or coronary artery bypass may also be indicated. Three and five year survival rates are 75% and 73% respectively[28].
Uncomplicated: Despite earlier trends towards stenting all acute type B dissection, international consensus is yet to publish recommendations for its use over medical management in uncomplicated disease. The VIRTUE registry’s intermediate findings indicate support for use of stenting in this setting, following favourable results for all-cause mortality (18%), dissection related mortality (12%) aortic rupture (2%), retrograde type A dissection (5%), and aortic reintervention rates (20%) at a follow up of three years[29]. One year results from the ADSORB trial have shown similar results to this. However the main advantage of stenting over medical management appears to be improved rates of false lumen thrombosis alone[10].
Medical management involves careful blood pressure control, to prevent further tearing or aortic dilatation. Beta blockers remain first line therapy, with follow-up shared between cardiology and the vascular surgeon[1,20]. Alternatives such as calcium channel blockers can be used in patients unable to tolerate first line therapy for any reason, e.g., chronic obstructive pulmonary disease. Survival rates of up to 78% at three years are reported[30] (Table 1).
| Registry | Authors | Design | Indication | Duration | Conclusion |
| Instead trial | Nienaber et al[37] | Prospective randomised trial | Comparison of TEVAR vs medical therapy in chronic type B dissection | 2 yr | TEVAR failed to improve survival or adverse events despite favourable aortic remodeling |
| Instead-XL | Nienaber et al[39] | Prospective randomised trial | Long-term outcomes of cohorts recruited to INSTEAD trial | 5 yr | TEVAR plus best medical therapy improved 5-yr aorta-specific survival |
| Mother registry | Patterson et al[36] | Collation of data from 5 clinical trials including VIRTUE and INSTEAD | Mid-term outcomes following endovascular repair using TEVAR for acute type B dissection | 5 yr | TEVAR provides good midterm protection from aortic-specific pathology High rates of re-intervention |
| Virtue registry | The Virtue registry investigators[29] | Prospective Multicentre Clinical trial | Safety, performance and health economic data in patients receiving the Valiant endograft | 3 yr (2006-2012) | TEVAR provides protection from aortic related death in midterm High rates of re-intervention |
| Adsorb trial | Hughes[10] | Multicentre randomised clinical trial | Comparison of best medical therapy vs medical therapy and TEVAR for acute type B dissection | 1 yr | TEVAR leads to improved aortic remodeling compared to medical therapy alone |
This group includes patients presenting with evidence of end-organ ischaemia, aortic rupture, pain or refractory hypertension, as well as those patients initially described as uncomplicated in whom disease has progressed despite optimal medical treatment[22,24]. These patients have a much poorer prognosis, with mortality approaching 50% in the untreated group[31]. Endovascular repair is the mainstay of treatment, with a 30 d mortality of 9.8%[32]. Even following surgery, 56% of cases will have ongoing false lumen perfusion, which can progress towards aortic expansion and rupture in 20%[33]. False lumen re-perfusion occurs in up to 16%, and this may require further surgery[34]. Over a 34 mo follow-up period data indicated 26% of patients required re-intervention for endoleak, distal fenestrations and concomitant pathology[35]. Retrograde type A dissection following TEVAR is a rare complication. Pooled data including the MOTHER registry found an incidence of 1.7% after TEVAR for all causes, with a mortality rate of 33.6%. Treatment for dissection was a significant risk factor, with an odds ratio of 10.0 (CI: 4.7-21.9) in acute disease and 3.4 (CI: 1.3-8.8) in chronic disease[36].
Medical management in chronic dissection has remained the mainstay of treatment. This follows results from randomised trials comparing optimal medical management alone to that in combination with thoracic stenting, the most significant being the INSTEAD trial[37]. This trial recruited patients with uncomplicated type B dissection in the sub-acute phase, and randomised 140 into one of the two groups described above. Follow up was 2 years, during which time endovascular repair failed to demonstrate a survival advantage for all cause mortality (88.9% ± 3.7% vs 95.6% ± 2.5% with optimal medical therapy) or aortic related mortality[38]. As with acute dissection, it did lead to higher rates of false lumen thrombosis (91.3% vs 19.4%).
A recently published analysis of the data from the same cohort, analysing outcomes from years 2 to 5 post randomisation (INSTEAD-XL) found a reduction in aorta-specific mortality in patients who underwent surgery (0% vs 3.6%, P = 0.001)[39]. By 5 years, there were significant differences in maximum aortic diameter (56.4 ± 6.8 mm vs 44.5 ± 11.5 mm medical management vs stenting respectively), false lumen diameter (37.1 ± 9.1 mm vs 10.4 ± 13.2 mm) and complete false lumen thrombosis (22% vs 90.6%). This appears to indicate that although there is little difference in survival between the two management strategies before two years, the advantages of stenting become apparent between 2 and five years post presentation. Despite this, two patients suffered from spinal cord ischaemia post TEVAR, and three patients required conversion to an open procedure following TEVAR within two years of randomisation.
Up to 15% of chronic dissection will be complicated by aneurysm formation; a survival analysis from the IRAD registry identified aortic growth and aneurysm formation to be the most common complication during follow-up[5]. Despite this, accurate prediction of the timing and course of progression remain elusive[6]. Once progression occurs, intervention should be planned. As with most surgery, TEVAR has an appreciable reduction in short-term morbidity and mortality in these patients, compared to an open operation (93% vs 79% respectively)[24,31]. At 10 years, survival following open surgery has been reported at 35%, while equivalent data for endovascular management is still unknown[31].
It is clear that dissection carries significant risk of disease progression despite optimal treatment and irrespective of aetiology. In those with hereditary aortic wall structure defects, mortality from rupture in an aorta measuring greater than 6 cm is 12%, with women at higher risk than men[40]. Therefore lifelong surveillance is mandatory, with axial imaging in the very least being used for routine imaging. MRA reduces the contrast and radiation exposure over a patient’s lifetime compared to CTA[41]. Imaging at 1, 3, 6 and 12 mo followed by annual review is recommended by the European Society of Cardiology[1]. Intervals should be altered depending on aortic size. All patients should receive life-long blood pressure management, and treatment should involve cardiology and vascular surgical input at all stages[42].
Optimal management of all type A dissections and uncomplicated or chronic type B dissections has changed little in recent years. However with the publication of results from multi-centre randomised controlled trials now becoming available, we are seeing the potential advantages in early use of endovascular repair on both short and longer-term mortality, progressive aortic dilatation and aortic remodeling. Throughout all of this, the message persists; aortic dissection remains a disease with a high mortality and need for life-long follow-up.
P- Reviewer: Athanasios G, Cebi N, Falconi M, Ueda H S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, Rakowski H, Struyven J, Radegran K, Sechtem U. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 893] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 2. | Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 562] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Srichai MB, Lieber ML, Lytle BW, Kasper JM, White RD. Acute dissection of the descending aorta: noncommunicating versus communicating forms. Ann Thorac Surg. 2004;77:2012-220; discussion 2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, Froehlich JB, Ehrlich MP, Oh JK, Januzzi JL. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19-730.e24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, Eagle KA, Isselbacher EM, Nienaber CA. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6:876-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | Fattori R, Bacchi-Reggiani L, Bertaccini P, Napoli G, Fusco F, Longo M, Pierangeli A, Gavelli G. Evolution of aortic dissection after surgical repair. Am J Cardiol. 2000;86:868-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Crawford ES, Svensson LG, Coselli JS, Safi HJ, Hess KR. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg. 1989;98:659-673; discussion 673-674. [PubMed] [Cited in This Article: ] |
| 8. | DeBakey ME, McCollum CH, Crawford ES, Morris GC, Howell J, Noon GP, Lawrie G. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982;92:1118-1134. [PubMed] [Cited in This Article: ] |
| 9. | Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, Sumiyoshi T, Bossone E, Trimarchi S, Cooper JV. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108 Suppl 1:II312-II317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Hughes GC. Management of acute type B aortic dissection; ADSORB trial. J Thorac Cardiovasc Surg. 2015;149:S158-S162. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Bossone E, Pyeritz RE, O’Gara P, Harris KM, Braverman AC, Pape L, Russo MJ, Hughes GC, Tsai TT, Montgomery DG. Acute aortic dissection in blacks: insights from the International Registry of Acute Aortic Dissection. Am J Med. 2013;126:909-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Hinchliffe RJ, Halawa M, Holt PJ, Morgan R, Loftus I, Thompson MM. Aortic dissection and its endovascular management. J Cardiovasc Surg (Torino). 2008;49:449-460. [PubMed] [Cited in This Article: ] |
| 13. | Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 767] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 14. | Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 929] [Cited by in F6Publishing: 794] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 15. | He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, Eagle KA, Mehta RH, Nienaber CA, Pape LA. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43:665-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 418] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Karthikesalingam A, Holt PJ, Hinchliffe RJ, Thompson MM, Loftus IM. The diagnosis and management of aortic dissection. Vasc Endovascular Surg. 2010;44:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977-2982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 256] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-e369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 952] [Cited by in F6Publishing: 1161] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 21. | Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Hinchliffe RJ, Loftus IM, Thompson MM. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42:632-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 23. | Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest. 1993;104:1248-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM. The diagnosis and management of aortic dissection. BMJ. 2012;344:d8290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Chavan A, Karck M, Hagl C, Winterhalter M, Baus S, Galanski M, Haverich A. Hybrid endograft for one-step treatment of multisegment disease of the thoracic aorta. J Vasc Interv Radiol. 2005;16:823-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, Myrmel T, Sangiorgi GM, De Vincentiis C, Cooper JV. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Ince H, Nienaber CA. Diagnosis and management of patients with aortic dissection. Heart. 2007;93:266-270. [PubMed] [Cited in This Article: ] |
| 29. | VIRTUE Registry Investigators. Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37:149-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 31. | Estrera AL, Miller CC, Goodrick J, Porat EE, Achouh PE, Dhareshwar J, Meada R, Azizzadeh A, Safi HJ. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83:S842-S845; discussion S842-S845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Eggebrecht H, Nienaber CA, Neuhäuser M, Baumgart D, Kische S, Schmermund A, Herold U, Rehders TC, Jakob HG, Erbel R. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Böckler D, Schumacher H, Ganten M, von Tengg-Kobligk H, Schwarzbach M, Fink C, Kauczor HU, Bardenheuer H, Allenberg JR. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg. 2006;132:361-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Moon MC, Pablo Morales J, Greenberg RK. Complicated acute type B dissection and endovascular repair: indications and pitfalls. Perspect Vasc Surg Endovasc Ther. 2007;19:146-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Patterson B, De Bruin JL, Brownrigg JR, Holt PJ, Loftus IM, Thompson MM, Hinchliffe RJ. Current endovascular management of acute type B aortic dissection - whom should we treat and when? J Cardiovasc Surg (Torino). 2014;55:491-496. [PubMed] [Cited in This Article: ] |
| 37. | Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-2528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 502] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 38. | Kwolek CJ, Watkins MT. The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial: the need for ongoing analysis. Circulation. 2009;120:2513-2514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 711] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 40. | Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17-27; discussion 27-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 725] [Cited by in F6Publishing: 649] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 41. | Mastracci TM, Greenberg RK. Follow-up paradigms for stable aortic dissection. Semin Vasc Surg. 2009;22:69-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 773] [Cited by in F6Publishing: 628] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 43. | Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108:628-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |