Published online Nov 20, 2013. doi: 10.5493/wjem.v3.i4.74
Revised: September 27, 2013
Accepted: November 1, 2013
Published online: November 20, 2013
Mechanotransduction has been proven to be one of the most significant variables in bone remodeling and its alterations have been shown to result in a variety of bone diseases. Osteoporosis, Paget’s disease, orthopedic disorders, osteopetrosis as well as hyperparathyroidism and hyperthyroidism all comprise conditions which have been linked with deregulated bone remodeling. Although the significance of mechanotransduction for bone health and disease is unquestionable, the mechanisms behind this important process have not been fully understood. This review will discuss the molecules that have been found to be implicated in mechanotransduction, as well as the mechanisms underlying bone health and disease, emphasizing on what is already known as well as new molecules potentially taking part in conveying mechanical signals from the cell surface towards the nucleus under physiological or pathologic conditions. It will also focus on the model systems currently used in mechanotransduction studies, like osteoblast-like cells as well as three-dimensional constructs and their applications among others. It will also examine the role of mechanostimulatory techniques in preventing and treating bone degenerative diseases and consider their applications in osteoporosis, craniofacial development, skeletal deregulations, fracture treatment, neurologic injuries following stroke or spinal cord injury, dentistry, hearing problems and bone implant integration in the near future.
Core tip: Mechanotransduction has been shown to be of major significance in modulating bone remodeling under physiological and pathological conditions. Therefore the study of the underlying mechanisms is of major importance and necessary step towards the better understanding of bone biology as well as the development of therapeutic strategies against conditions characterised by deregulated mechanotransduction. This review will consider the molecular mechanisms behind mechanotransduction as well as the scientific models currently used for its better understanding. It will also focus on mechanostimulatory techniques that could be used against a variety of deregulated mechanotransduction-related diseases.
- Citation: Spyropoulou A, Basdra EK. Mechanotransduction in bone: Intervening in health and disease. World J Exp Med 2013; 3(4): 74-86
- URL: https://www.wjgnet.com/2220-315X/full/v3/i4/74.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i4.74
The importance of bones for a living organism is undeniable and goes far from just providing structural support for the body, protecting vital organs and exchanging minerals. Bones also comprise a multi-functional system that interacts with other systems and abnormalities in bone tissues may result in mild or severe diseases.
Bone tissue is composed of the bone matrix and five different cell types. The bone matrix contains an inorganic (carbonated hydroxyapatite) and an organic phase (mainly type I collagen and several growth factors) whilst the cellular content of the bone tissue comprises of osteoprogenitors, osteoblasts, osteocytes, osteoclasts and lining cells[1]. Osteoprogenitor cells comprise pluripotent cells of mesenchymal origin, localised on bone surfaces[1] which have the ability, under the appropriate conditions, to commit and differentiate towards osteoblasts[1]. On the same bone osteoblasts, the bone forming cells, are cited. They are responsible for the protein synthesis of the bone matrix as well as its calcification[1]. The cavities of the calcified bone matrix bear osteocytes which comprise entrapped inactive osteoblasts forming a net of communicating cells inside the calcified matrix[1]. Osteoclasts are large multinucleate cells of blood monocyte origin, settled inside bone resorption lacunae and they are responsible for bone resorption in bone remodeling areas[1]. Lining cells comprise inactive osteoblasts with the ability to protect bone surfaces from bone resorption[1].
Runt-related transcription factor 2 (Runx2) or core-binding factor subunit alpha-1 (Cbfα1), the major osteo-specific transcription factor[2] is responsible for the regulation of osteoblast differentiation as well as for hypertrophic cartilage synthesis[2,3]. Its expression is necessary and sufficient for the commitment of mesenchymal cells towards the osteoblastic cell line[4].
Abnormalities in Runx2 expression are indicative of its importance in bone biology. When Runx2 is expressed ectopically it has been shown to lead to increased expression of osteocalcin, alkaline phosphatase, collagenase-3, bone sialoprotein and collagen type Iα1[5]. Osteoblast maturation in mice bearing a mutant runx2 gene is inhibited and thus so are the procedures of intramembranous and endochondral ossification[6,7]. Furthermore, it has been shown that differentiation of stem cells in adipocytes and chondrocytes in runx2 knockout mice has not been impaired. In addition, heterozygous mice (runx2-/+) developed characteristic skeletal abnormalities similar to human heritable skeletal disorder cleidocranial dysplasia (CCD) abnormalities[8]. On the other hand, tissue-specific Runx2 over-expression in transgenic mice results in decreased bone density, bone fractures and osteopenia[7,9,10].
Bone remodeling, the continuous bone reconstruction is of major importance for conserving bone structural integrity as well as for the bone to perform its metabolic role by modulating calcium and phosphorus levels in the body[1].
Shortly, bone remodeling activation depends mostly on local factors and their effects on mesenchymal progenitor cells. Bone reconstruction initiates with osteoclasts performing bone resorption and forming cavities inside the bone. At the end of this phase, osteoclasts produce the appropriate signals for the initiation of bone synthesis[1]. Osteoblasts quickly cover the cavity surfaces and synthesize new bone. Those two bone remodeling phases, bone formation and resorption are closely correlated and interconnected. This means that under normal conditions, the newly formed and the reabsorbed bone quantities are equal[11]. Impaired bone remodeling may lead in pathophysiological bone conditions like osteoporosis, Paget’s disease, orthopedic disorders and osteopetrosis among others[1].
Research has shown that the GH-IGF-1 axis may also be of significance in the modulation of bone mass quantity and quality. More specifically, growth hormone (GH) is suggested to potentially play a role on bone remodeling[12]. However, the exact mechanisms through which GH acts on osteoblast biology have not been elucidated[12].
The receptor activator for nuclear factor κb (RANK)/ receptor activator for nuclear factor κb ligand (RANKL)/ osteoprotegerin (OPG) system comprises the main modulator of bone remodeling[13]. More specifically, pre-osteoclasts express RANK in their surface. Its ligand, RANKL, is produced in osteoblasts, stromal cells as well as activated T cells[14]. In osteoblasts and under steady-state conditions, vitamin D, parathyroid hormone and prostaglandins lead in induced RANKL expression. The binding of RANK and RANKL leads in osteoclast differentiation[15,16]. More specifically, during normal bone remodeling, RANKL is produced by cells of the bone marrow- supporting tissue and osteoblasts. RANKL binds to RANK on pre- osteoclasts resulting in their maturation and activation. Nuclear-factor κB (NF-κB), which is of importance in inflammation response, also plays a central role in osteoclast activation. NF-κB performs both aforementioned functions through regulation from interleukin-6 (IL-6). Pro-inflammatory cytokines play an important role in bone remodeling as indicated by the presence of interleukin-1 (IL-1), IL-6 and tumor necrosis factor-α (TNF-α) receptors on pre-and mature osteoclasts[17]. OPG is produced by osteoblasts and has the ability to bind to RANKL and block its functions resulting in decreased bone resorption[17,18].
Bone remodeling is a strictly regulated process, largely modulated by the application of different mechanical stimuli or by metabolic stress on the bone[3].
More specifically, local mechanical stress leads in bone resorption as an initial response[19]. The nature of the mechanical stimulus is of importance in the regulation of bone remodeling since different types of mechanical stimuli result in different responses. For example, constant repetitive application of mechanical force inducing high stress levels or unusual load distribution result s in elevated bone synthesis and high bone mass. Furthermore, short pauses between long periods of mechanical loading have been shown to enhance bone strength and structure[20]. However, static load, slow rates of pressure rotation as well as “predictable” pressure application, lead in decreased bone synthesis, enhanced bone resorption and thus low bone mass[21,22].
Bone remodeling and mechanostimulation have been shown to roughly follow these rules: Bone synthesis is promoted by dynamic and not static loading application. Short-term load applications are sufficient for adaptive response initiation and lead in increased bone formation whereas long-term load applications result in decreased bone synthesis and enhanced resorption[23,24]. In addition, the repentance of the same mechanical stimulus results in decreased response due to signaling prediction[25]. The application of these rules is evident in the effects of space microgravity, osteoporosis or paralysis on bone tissues, where bone loss is observed[20,26], as well as in the effects of tennis at a professional level on bone tissues, where bone growth is observed[27].
Signals of mechanical nature induce in osteoblasts and osteocytes the production and secretion of different types of molecules, which modulate osteoblast differentiation and proliferation[3]. Such mechanical stimuli can include flow of fluids, strain of the substrate, membrane deformation or stimulation of integrins, vibration, altered gravity forces and compressive loading[3]. Bone remodeling functions, after the application of different mechanical stimuli, are locally regulated by cytokines and growth factors among other molecules. More specifically, IL-1β, TNF-α, prostaglandin E2 (PGE2)[26,28], IL-6, IL-8, RANKL, OPG[27,29-31], insulin-like growth factor (IGF), transforming growth factor β-1 (TGFβ-1) and fibroblast growth factor (FGF)[32,33] have been demonstrated to be induced after application of mechanical stimuli. Additionally, it has been shown that mechanical stimulation in osteoblasts results in increased mRNA levels of osteopontin, osteocalcin, platelet derived growth factor and collagen types I and III[34,35].
Although some of the molecules taking part in mechanotransduction are known, the mechanisms behind it have not been fully elucidated.
The stage of osteoblast differentiation is shown to be of importance in osteoblast proliferation, apoptosis and translation of mechanical cues[36]. Furthermore, it has been shown that undifferentiated mesenchymal stem cells seem to respond more successfully to load application than mesenchymal stem cells that have already started to differentiate[37].
A diversity of molecules have been considered to play the role of mechano-sensors in differentiated osteoblasts: mechanical stimulation has been shown to lead in enhanced sensitivity and elevated open cation channels number[38,39], increased communication through gap junctions between osteoblasts as well as increased integrin production in osteoblasts[39]. Actin cytoskeleton abnormalities have been shown to prevent mechanical signaling and therefore the integrin network has been considered as the main candidate for transduction of mechanical signals[39]. On the other hand, a considerable number of research groups argue that cytoskeletal components involved in mechanotransduction differ depending on different types of stress or the response under study[39].
Integrins comprise transmembrane receptors connecting the extracellular matrix to the cytoskeleton[40]. Under mechanical signal application, integrins form complexes with molecules of the cytoskeleton with the help of the Rho family of Ras-related GTPases[40]. Rho family members also induce multiple kinase cascades and particularly mitogen-activated protein kinase (MAPK) cascades[40]. Rho and other Ras-related GTPases have been shown to play a role in osteoblast response after application of mechanical pressure[41]. More specifically, it has been shown that the continuous application of mechanical forces leads in deregulation of Rab and Rho GTPases activity in osteoblast-like cells[41].
Recently, another molecule, Polycystin-1 (PC1), was suggested to provide a link between environmental mechanical signals and their transformation towards biochemical signals. It has been shown that PC1 not only functions as a mechanosensor but that also conveys mechanical signals through the calcineurin/nuclear factor of activated T-cells (NFAT) signaling pathway and thereby regulates osteoblast- specific gene transcription as well as osteoblast differentiation[42].
The primary cilium, a cellular sensory system, has also been demonstrated to be of importance in the transfer of mechanical signals as well as in mesenchymal stem cell differentiation. Additionally it was shown that the cilium modulates fluid flow mechanotransduction in human mesenchymal stem cells by maintaining fluid flow-induced osteogenic gene expression elevation and preventing fluid flow-induced increased proliferation[43].
Following the reception of mechanical cues, the signal conveying the mechanical conditions of the extracellular environment is carried towards the nucleus through MAPK kinases and more importantly through extracellular signal-regulated kinases (ERKs) and c-Jun N-terminal kinases (JNKs)[44,45]. ERKs, which in human osteoblasts seem to be induced by growth factors, estrogen and fluoride among others[45], have been shown to play a significant role in osteoblast maturation and in osteoblast biology in general[45-49]. Furthermore, duration and strength of JNK/ERK signaling is indicated to be significant in gene expression[50].
Following ERK/JNK activation, the signal is transmitted to transcription factors that alter gene expression, like Jun and Fos family members[51]. In their turn, c-Jun and Fos family members interact to form activator protein-1 (AP-1) transcription factor, which has been shown to be of major importance in osteoblast differentiation[52] since it regulates the expression of collagen type I, osteocalcin, osteopontin and osteonectin[52].
Application of continuous mechanical pressure in osteoblast-like cells as well as osteoblasts resulted in increased production of AP-1 components through activation of MAPK cascades[41,53,54]. However, data on c-Jun expression after mechanical stimulation are inconclusive with some research groups arguing that human osteoblast-like cells after mechanical loading over-express c-Jun[53] whereas others have opposing results[55,56]. However, the above mentioned differences could be attributed to application of different stress type or usage of different cell system. Finally, different types of mechanical pressure applied on osteoblasts seem to result in different composition AP-1 and therefore regulate gene transcription accordingly depending on the extracellular signal applied[57].
Application of short-term mechanical pressure activates both JNK2 and ERK2, with following activation of downstream molecules, like c-Jun, which alter the expression of osteoblastic genes[54]. More specifically, it has been demonstrated that short-term continuous mechanical stimuli of physiological intensity in osteoblast-like cells activates JNK and ERK members resulting in enhanced AP-1 DNA binding activity on the human L/B/K ALP gene and thus osteoblast differentiation[54]. This is further evidenced by the observation that osteoblast-like cells receiving mechanical stimuli synthesized increased quantities of type 1 collagen and osteocalcin, markers of early osteoblast differentiation[58].
PGE2 production has been shown to be induced in osteoblast-like cells after mechanical stimulation[59] and in osteoblasts under the effect of physiological stress, growth factors, hormones, trauma or inflammatory cytokines and its production leads in cAMP-dependent IGF-1 induction in osteoblasts[3]. IGF-1 and IGF-2, in turn, induce osterix (Osx) transcription factor expression in osteoblasts[60], induce osteoblast function in vitro as well as lead in increased bone mass in vivo[61]. PGE2 is also shown to lead in increased Runx2 expression in vivo[62]. Downstream of PGE2, TGF-β expression, which leads in proliferation of osteoblasts and extracellular matrix synthesis[63], has been found increased in human osteoblast-like cells under mechanical stimulation. Furthermore, TGF-β receptor 1 comprises a Runx2 target in osteoblasts[64]. Those two observations combined explain why Runx2 knockout mice demonstrate characteristic abnormal extracellular matrix formation due to decreased number of mature osteoblasts[65,66].
Nitric oxide (NO) production in osteoblasts is another response to mechanical stimulation. NO functions through the MEK/ERK cascade by binding to a regulatory site on Ras leading in cell proliferation and extracellular matrix production[67]. Following, cyclooxygenase 1 (Cox1), Cox2, ERK1 and ERK2 are activated and result in bone matrix formation[68].
Additionally, signals of mechanical nature have been shown to promote vascular endothelial growth factor-, bone morphogenetic protein 2 (BMP-2)- and BMP-4- dependent and PGE2- independent increased expression of IGF-1[69]. BMPs result in bone synthesis in osteoblasts[70] and BMP-2 expression promotes Runx2, Osx and Dlx5 expression[71].
Mechanical cues also promote the expression of genes that encode for c-Fos, early growth response factor 1 (Egr-1) and basic fibroblast growth factor (bFGF) which have been shown to promote cell growth in MC3T3-E1 osteoblasts[22].
The nature the mechanical signal determines whether bone or cartilage formation will occur[72]. More specifically, application of pressure of high frequency and low intensity in bone cells in vitro, results in elevated extracellular matrix (ECM) disposition and thus increased bone formation[73]. On the contrary, mechanical loading of high intensity on osteoblasts leads in BMP extracellular antagonists expression and therefore results in inhibition of osteoblast development[74]. In addition, the application of continuous mechanical forces on osteoblastic cells in vitro promotes inflammatory cytokines and their receptors expression[75]. More specifically, IL-1b production is found elevated under such mechanical stimuli, and is accompanied by RANK-RANKL signaling pathway activation and thus bone resorption[76]. Stimuli from short periods of fluid flow or cyclic substrate tension at physiological intensity levels promote osteoblast proliferation and survival[77]. Mechanical signals of physiological intensity levels are associated with survival of human osteoblasts and several studies suggest that pro-survival proteins promote the production of survival factors like IGF-1 or IGF-2 and activate estrogen receptor[78]. It has also been shown that gravitational force maintains osteoblast survival whereas when gravitational force is not taking place, osteoblasts are led to apoptosis through reduced DNA binding of an important for survival transcriptional factor[18]. In vivo, the absence of mechanical signals promotes osteoblast apoptosis and thus osteoporosis[72]. The application of excessive mechanical force in vitro leads in cell detachment from their adhering surface[79] as well as in a form of programmed cell death called anoikis[80].
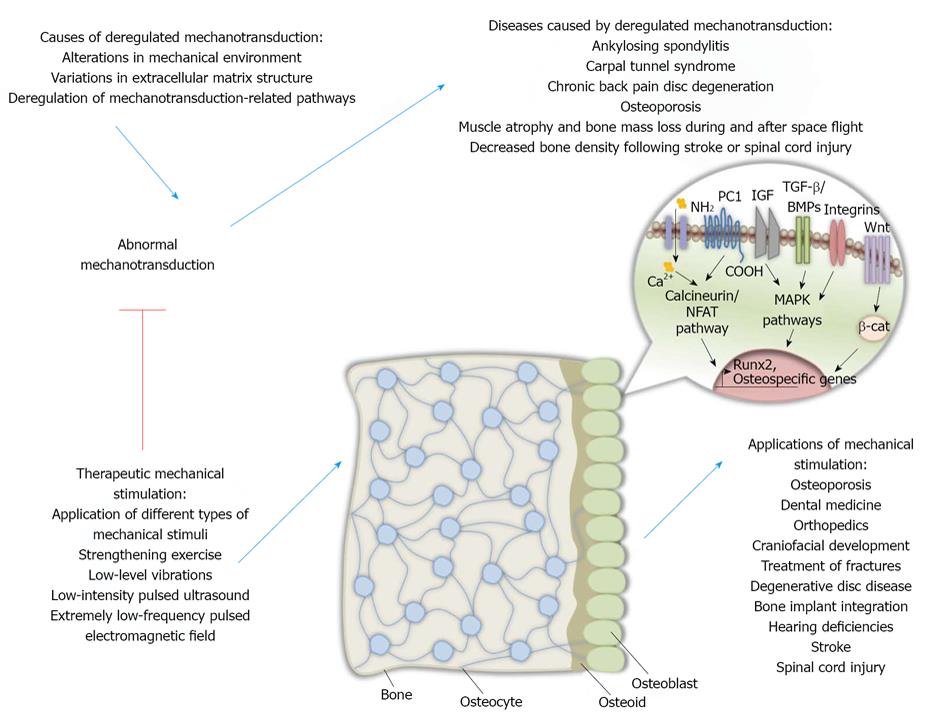
Mechanical stimulation in osteocytes has also been under investigation since it may lead in better mechanotransduction understanding and may represent a potent therapeutic target against bone degenerative diseases. Recent studies have underlined the role of osteocytes in bone remodeling since their absence in mice led in fragile bones, microfractures, deregulated osteoblast functions, bone loss in the trabeculae as well as adipose tissue proliferation in the marrow indicating an aging skeleton. In addition, these mice could not experience bone loss due to unloading, an event that indicates osteocytes’ importance in the procedure of mechanotransduction[81] (Figure 1).
Runx2 which is known to play a significant role in osteoblast differentiation has been shown to be the recipient of mechanical signals in human osteoblast-like cells[82]. As it has been demonstrated, continuous mechanical stimuli of low intensity in human osteoblast-like cells of the periodontal ligament (PDL) result in elevated Runx2 expression and DNA- binding capacity. The mechanical signal, according to the researchers, initiates at the plasma membrane and more specifically from integrins and travels towards the nucleus through MAPK cascades. In the nucleus, the signal targets Runx2 and induces its expression[82]. More specifically, Runx2 demonstrates increased expression at both mRNA and protein levels as well as elevated DNA binding activity. During this process, ERK1 and ERK2 are activated in a parallel manner with the Runx2 DNA- binding capacity elevation. After their activation, ERKs interact, phosphorylate and activate Runx2 in vivo causing osteoblast maturation[7,82].
Runx2 expression depends on an autoregulatory mechanism[83]. More specifically, activated by mechanical stimuli ERKs phosphorylate and activate already existing Runx2 molecules. Those activated Runx2 molecules bind to Runx2 promoter inducing Runx2 expression[82]. In addition, a canonical AP-1 binding site has been found in Runx2 promoter which potentially plays a role in the regulation of Runx2 expression. AP-1 and Runx2 proteins have also been shown to interact and regulate collagenase-3 expression[84].
NF-κB transcription factor in mechanotransduction
NF-κB transcription factor which is implicated in inflammatory response signaling[31] also plays a crucial role in osteoclast formation and thus bone resorption[85]. NF-κB, which is activated either through the RANK-RANKL system or potentially through integrins that transmit signals of mechanical nature to src-kinases[86], besides its role in osteoclast maturation, may be implicated in osteoblast differentiation under mechanical stimulation. This is indicated by the fact that NF-κB is found to be activated and then translocated in the nucleus of osteoblasts that receive mechanical stimuli[26,87] where it has been hypothesized to promote the transcription of osteoblast-specific genes.
The in vitro study of mechanostimulation in osteoblasts, has been made possible with the usage of osteoblast-like cells that are acquired either from healthy tissue (human PDL or mouse MC3T3-E1 calvaria cells) or from osteosarcomas (MG-63, SaOs cells). Different types of mechanical stimulation are applied on the aforementioned cell models, each causing a different response in osteoblast-like cells[3]. Such types of mechanical stimulation include fluid flow, four-point bending and substrate stretch, gravity force, vibration, magnetic bead twisting and atomic force or shockwaves among others[88].
Periodontal ligament (PDL) cell system is a helpful model for the study of mechanotransduction signaling cascades in osteoblasts[89]. More specifically, PDL cells are undifferentiated mesenchymal fibroblasts[90] that bear all the characterized properties of osteoblasts. Furthermore, these cells are adapted to receive mechanical pressure, either because of physiological conditions or orthodontic treatments. Under specific conditions, PDL cells have the ability to differentiate towards more specialized cells capable of taking part in the regeneration and repair of the periodontal ligament as well as its surrounding hard tissue[91].
Furthermore, three dimensional (3-D) constructs, like polydimethylsiloxane microdevices and human trabecular 3-D bone scaffolds, have been used to investigate the effects of mechanical stimulation on osteoblasts[92].
Scientists are trying to develop an effective way to monitor the levels and characteristics of mechanical pressure applied as well as a way to measure the rates of tissue regeneration. In order to achieve the first part, scientists have made either fixation devices with different mechanical pressure characteristics and then monitor their effects in vivo or custom-made devices that accurately control the mechanical stimulation characteristics. With the first type of devices they are able to study bone tissue regeneration under more physiological conditions while with the second they assess the effects to a specific loading signal[93]. In order to study the effect of mechanical signals on healing processes at organs, it is necessary to develop techniques to assess their mechanical environment in vivo. Today, we have found ways to determine loading applied on the affected limb[94], load distribution between implant and bone[95-97], and assess interfragmentary movements[94,98] but the development of techniques to study the intermediate steps and not only the final outcome of loading are imperative.
As mentioned before, deregulated bone remodeling is the main cause of a number of bone diseases. Bone remodeling abnormalities may be due to genetic alterations. For example, a mutant runx2 gene can result in human heritable skeletal disorder CCD[99,100]. A mutation in runx2 gene may also lead in cancer metastasis to bone tissues since Runx2 is responsible for the expression of genes that are implicated in cancer development and more specifically, in cell metastasis in bone. Among those genes regulated by Runx2 are those encoding matrix metalloproteinases (MMPs) MMP-9 and MMP-13 as well as osteopontin and bone sialoprotein[101]. Abnormal mechanotransduction due to lack of mechanical loading or other causes may result in bone remodeling deregulations like ankylosing spondylitis, carpal tunnel syndrome, chronic back pain disc degeneration and osteoporosis.
Recent studies have shown that annulus fibrosus (AF) cells that originate from non degenerative tissue respond to cyclic tensile strain through IL-1 and IL-4 dependent mechanisms, something that does not apply in AF cells coming from degenerative tissue[102]. Furthermore, annulus fibrosus cells from degenerative discs have been found to have little capacity to successfully respond to application of mechanical stimuli and exhibit an intense response to inflammatory stimuli. The above observations may explain the different responses observed in patients with intervertebral disc degeneration after specific therapies[103].
During space flight, astronauts are exposed to microgravity and thus altered mechanical stimuli are applied on their skeletons. As a result, their muscles atrophy and their bones experience bone mass loss. Short exposure to microgravity has been shown to result in increased bone resorption evidenced by the urinary calcium excretion observed[104]. Under long periods of microgravity, the structural alterations occurring in bones have even more crucial effects on bone strength than was previously thought while counteracting measurements like exercise seem to have little or no effects[104]. The mechanism behind bone loss is not yet clarified but probably is a result of decreased hydrostatic pressures and thus decreased intramedullary pressure which may lead in reduced fluid flow shear stresses on osteocytes and thus enhanced bone loss. Since exercise does not seem to prevent bone loss, it has been suggested that the decreased hydrostatic pressure may result in impaired mechanosensitivity in the bone tissue. Furthermore, other physiologic alterations on the body under reduced gravity conditions may contribute to the observed bone loss in co-operation with the reduced hydrostatic pressures like low vitamin D levels, oxidative stress, radiation exposure and acidosis[105-109].
Neurologic injury results in bone loss in the affected paretic limb whereas the other limb is characterized either by reduced or increased bone mass. Those effects are probably due to alterations in muscle mass and strength and load pressure applied. More specifically, strokes result in decreased bone density mostly in the paretic limb and its effects are more intense in the upper extremities. The pattern of bone loss observed in stroke patients is generally limited to the paretic side and is more evident in the upper extremities than in the lower extremities. The pathogenesis of the observed bone loss after stroke probably depends between others on immobilization, duration of paresis, loss of muscle activity, endocrine disorders, nutritional deficiencies as well as medications[110].
Following spinal cord injury, bone loss is observed in pelvis and lower extremities of paraplegics and in the upper and lower extremities of tetraplegics after spinal cord injury[111]. Those effects are predominantly observed in trabecular bone. Recent data indicate the presence of endocortical resorption without periosteal synthesis[112]. Absence of mechanical stimulation, muscle contraction, neuroendocrine alterations as well as neural innervation alteration are probably responsible for the observed bone loss after those types of injuries[113,114] (Figure 1).
Pharmaceutical treatments like anabolic treatments or treatments with anti-resorptive agents have been the norm in order to achieve increased bone density until now[3]. Nowadays, mechanical stimulation is considered to be of great importance in designing new therapies for bone diseases, avoiding this way the unwanted side effects of pharmaceutical products.
A number of studies demonstrate the role of mechanostimulation in acquiring a higher bone mass quantity and thus its role in treatment of bone diseases. For example, it has been shown that low intensity mechanical signals result in bone remodeling activation and increased bone mass and that following a period of time confer regenerative abilities to bone tissues[115]. It has also been observed that mechanical signal application on PDL and osteoblast cell lines leads in enhanced OPG expression[116,117] and therefore in RANK-RANKL signaling interruption which results in decreased osteoclastogenesis. Furthermore, mechanical stimulation has been shown to activate Cox enzymes and prostaglandins which reduce RANKL production and thus block bone resorption in vitro[77,118]. Mechanical stimuli have also been demonstrated to activate the Wnt-b-catenin pathway on osteoblasts resulting in enhanced osteoblast differentiation and bone synthesis[119]. Studies on three dimensional models have showed that osteoblasts receiving dynamic application of mechanical pressure, expressed elevated ALP, Runx2 and osteocalcin levels[120,121]. Additionally, application of mechanical pressure resulted in increased mineralized matrix production in 3-D, partially demineralized bone scaffold- cultured human bone marrow stromal cells[122].
Considering the aforementioned and other results, researchers have turned to mechanical stimulation in order to design treatments against bone diseases which will avoid the undesirable effects of pharmacological treatments[115]. Application of mechanostimulation has already a variety of applications in dentistry, orthopedics, the craniofacial development and treatment of fractures.
More specifically, strengthening exercises in osteoporotic patients has been shown to result in increased bone mineral content[123] and physical exercise has been observed to prevent post-menopausal and age-related ECM bone mineral decrease[124]. Moreover, other types of mechanical stimulation like low-level vibrations at intensity safe for the bone integrity may play a protective role in osteoporosis[125]. A functional mechanical environment seems to be of importance in the treatment of degenerative disc disease as well as other skeletal deregulations[126]. Mechanical signals of specific ratio[127], form[128] and intensity in osteoblasts have also been shown to be beneficial in bone fracture treatment[128]. Additionally, low-intensity pulsed ultrasound has been indicated to promote osteoblast differentiation and bone formation in bone fractures[129]. Extremely low-frequency pulsed electromagnetic field has been demonstrated to result in osteoblast proliferation and maturation[130].
In addition, mechanostimulation was found to have positive effects in bone implant integration by modulating osteoblast differentiation through regulation of Cbfα1 as well as osteocalcin levels. Cbfα1 and osteocalcin levels were shown to be frequency-, magnitude-, and duration of mechanical application- dependent. Furthermore, osteoblast cells under strain in the implant seem to produce factors that have the ability to activate DNA synthesis and thus cell proliferation in a larger scale than non-strained cells[131].
Mechanical stimulation has also its applications in the treatment of hearing problems. For example, SPAHA, which comprises a novel bone conduction hearing device, whose effects are accomplished through elastic bending of the bone and not the application of a point force which results in cochlea vibration as previous devices used to do[132].
Exercise has not been shown to meliorate bone loss in space flights until now[104]. Furthermore, there is no indication that osteoporosis drug therapies would be successful during or following space flight. Exercise seems to be helpful in increasing bone density after stroke or spinal cord injury according to a recent study[133,134]. Bisphosphonates have been shown to be able to prevent bone loss after a stroke[134]. Mechanical stimulation may have some positive effects on preventing bone loss after spinal cord injury, with early application demonstrated to bear better results[135,136]. Furthermore, bisphosphonate early administration after spinal cord injury may be able to prevent bone loss[137].
Researchers have investigated whether sympathetic nervous system inhibition could be beneficial against bone loss in osteopenia induced by absence of mechanical signals. They found that its inhibition led in blockade of neurectomy-induced bone resorption but further studies need to be conducted[138].
Although mechanical loading is thought to be an anabolic beneficial procedure against osteoporosis, abnormal mechanotransduction in conjunction with age seem to counteract its beneficial effects in elderly people. Recently, a research group presented an agent-based model of real-time Ca2+/NFAT signaling in bone cells that successfully described periosteal bone synthesis induced by different types of mechanical stimulation in young and aged animals. The model demonstrated age-related pathway changes being responsible for the decrease in bone synthesis during senescence. This way the group managed to identify important pathway alterations that comprise potent therapeutic targets. In accordance, the researchers applied an in vivo intervention and showed that application of mechanical stimuli along with Cyclosporin A can prohibit the decrease in bone synthesis in the bones of elderly people. This study not only provided a potent inexpensive treatment for osteoporosis in the elderly but also demonstrated the significance of real-time cellular signaling and in silico techniques in studying, intervening and treating bone diseases like osteoporosis[139].
The primary cilium was shown to modulate fluid flow mechanotransduction in human mesenchymal stem cells by maintaining fluid flow-induced osteogenic gene expression elevation and preventing fluid flow-induced increased proliferation[43]. Therefore, fluid flow systems may be effective in designing techniques to develop bone-like tissues for bone regenerative purposes. Furthermore, the role of cilium in developing techniques that imitate loading in order to treat bone loss in bone diseases needs to be investigated. Last but not least, studying the events taking place during acute proliferation of mesenchymal stem cells with not functional cilia receiving mechanical cues could help in understanding the mechanisms behind ciliopathies and cystic diseases[43] (Figure 1).
Bone remodeling is of major importance for the proper structure and metabolic functions of the bone. Deregulations in bone remodeling can result in a variety of bone diseases like osteoporosis, hyperparathyroidism, hyperthyroidism, Paget’s and osteopetrosis among others. Therefore, the investigation of mechanisms and pathways behind bone remodeling and mechanotransduction, which comprises of the most important variables of bone remodeling, is of great significance.
There is a lot that we don’t know about bone biology and bone diseases as well as the implication of mechanical signals in the aforementioned procedures. The better understanding of the underlying mechanisms will potentially result in designing a successful strategy for treating bone diseases, avoiding the unpleasant side effects of conventional treatments like the administration of pharmaceutical substances. Furthermore, it will help us design techniques to successfully predict and prevent bone diseases when possible.
Undeniable is the necessity of innovative new ways to monitor bone density, to identify hormonal or metabolic risk factors for bone loss, to develop effective ways to apply mechanical stimulation with successful results against reduced bone density, to assess the effect of newly developed anabolic drugs against osteoporosis and their effects on bone loss characterizing bone diseases due to absence of mechanical stimuli, as well as to develop trials investigating the improvement of bone health under the afore mentioned conditions. In addition, the study on the effects of mechanostimulation on bone tissue and organ healing is of great significance for future interventions. In order for this to be achieved, we need to develop an effective way to monitor the levels and characteristics of mechanical pressure applied on bone tissue, a way to measure the rates of tissue regeneration as well as techniques to assess mechanical environment of organs in vivo[106].
Currently, researchers have started using mechanostimulation with encouraging results for certain bone conditions but further study is required. Mechanostimulation is considered to comprise the future in treating bone diseases that have their origin in absence of mechanical cues. Further investigation of the molecular players and pathways involved in mechanotransduction and bone remodeling will amplify our knowledge and understanding of these processes and help us build successful prevention, prediction and treatment strategies for a variety of bone diseases.
P- Reviewer: Song GB S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45:863-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 2. | Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 3. | Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3326] [Cited by in F6Publishing: 3163] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 6. | Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3364] [Cited by in F6Publishing: 3249] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 7. | Ziros PG, Basdra EK, Papavassiliou AG. Runx2: of bone and stretch. Int J Biochem Cell Biol. 2008;40:1659-1663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2205] [Cited by in F6Publishing: 2140] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 9. | Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P. High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol. 2002;22:6222-6233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103-25108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 762] [Cited by in F6Publishing: 782] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 12. | Ziros PG, Georgakopoulos T, Habeos I, Basdra EK, Papavassiliou AG. Growth hormone attenuates the transcriptional activity of Runx2 by facilitating its physical association with Stat3beta. J Bone Miner Res. 2004;19:1892-1904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Ikeda T, Kasai M, Utsuyama M, Hirokawa K. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology. 2001;142:1419-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev. 2007;12:113-145. [PubMed] [Cited in This Article: ] |
| 16. | Papachristou DJ, Basdra EK, Papavassiliou AG. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med Res Rev. 2012;32:611-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1044] [Cited by in F6Publishing: 1135] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 18. | Bucaro MA, Fertala J, Adams CS, Steinbeck M, Ayyaswamy P, Mukundakrishnan K, Shapiro IM, Risbud MV. Bone cell survival in microgravity: evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann N Y Acad Sci. 2004;1027:64-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Krane SM. Identifying genes that regulate bone remodeling as potential therapeutic targets. J Exp Med. 2005;201:841-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Hatton JP, Pooran M, Li CF, Luzzio C, Hughes-Fulford M. A short pulse of mechanical force induces gene expression and growth in MC3T3-E1 osteoblasts via an ERK 1/2 pathway. J Bone Miner Res. 2003;18:58-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Raab-Cullen DM, Akhter MP, Kimmel DB, Recker RR. Periosteal bone formation stimulated by externally induced bending strains. J Bone Miner Res. 1994;9:1143-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Rubin CT, Gross TS, McLeod KJ, Bain SD. Morphologic stages in lamellar bone formation stimulated by a potent mechanical stimulus. J Bone Miner Res. 1995;10:488-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 593] [Cited by in F6Publishing: 495] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R. A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17:899-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Yamamoto T, Kita M, Kimura I, Oseko F, Terauchi R, Takahashi K, Kubo T, Kanamura N. Mechanical stress induces expression of cytokines in human periodontal ligament cells. Oral Dis. 2006;12:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007;52:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Tsuji K, Uno K, Zhang GX, Tamura M. Periodontal ligament cells under intermittent tensile stress regulate mRNA expression of osteoprotegerin and tissue inhibitor of matrix metalloprotease-1 and -2. J Bone Miner Metab. 2004;22:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 819] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 32. | Cillo JE, Gassner R, Koepsel RR, Buckley MJ. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Koay EJ, Ofek G, Athanasiou KA. Effects of TGF-beta1 and IGF-I on the compressibility, biomechanics, and strain-dependent recovery behavior of single chondrocytes. J Biomech. 2008;41:1044-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology. 1995;136:528-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Jones DB, Nolte H, Scholübbers JG, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101-110. [PubMed] [Cited in This Article: ] |
| 36. | Weyts FA, Bosmans B, Niesing R, van Leeuwen JP, Weinans H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif Tissue Int. 2003;72:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Liu J, Zhao Z, Li J, Zou L, Shuler C, Zou Y, Huang X, Li M, Wang J. Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 not p38 MAPK signaling involved. J Cell Biochem. 2009;107:224-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Kuipers AJ, Middelbeek J, van Leeuwen FN. Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur J Cell Biol. 2012;91:834-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Papachristou DJ, Papachroni KK, Basdra EK, Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays. 2009;31:794-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 41. | Basdra EK, Papavassiliou AG, Huber LA. Rab and rho GTPases are involved in specific response of periodontal ligament fibroblasts to mechanical stretching. Biochim Biophys Acta. 1995;1268:209-213. [PubMed] [Cited in This Article: ] |
| 42. | Dalagiorgou G, Piperi C, Georgopoulou U, Adamopoulos C, Basdra EK, Papavassiliou AG. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol Life Sci. 2013;70:167-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30:2561-2570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Pommerenke H, Schmidt C, Dürr F, Nebe B, Lüthen F, Muller P, Rychly J. The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J Bone Miner Res. 2002;17:603-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 46. | Huang Z, Cheng SL, Slatopolsky E. Sustained activation of the extracellular signal-regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J Biol Chem. 2001;276:21351-21358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443-14450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 306] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 49. | Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 50. | Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3632] [Cited by in F6Publishing: 3649] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 51. | Brivanlou AH, Darnell JE. Signal transduction and the control of gene expression. Science. 2002;295:813-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 425] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 52. | Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun). Ann Rheum Dis. 2010;69 Suppl 1:i86-i88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Kletsas D, Basdra EK, Papavassiliou AG. Effect of protein kinase inhibitors on the stretch-elicited c-Fos and c-Jun up-regulation in human PDL osteoblast-like cells. J Cell Physiol. 2002;190:313-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Peverali FA, Basdra EK, Papavassiliou AG. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med. 2001;7:68-78. [PubMed] [Cited in This Article: ] |
| 55. | Inaoka T, Lean JM, Bessho T, Chow JW, Mackay A, Kokubo T, Chambers TJ. Sequential analysis of gene expression after an osteogenic stimulus: c-fos expression is induced in osteocytes. Biochem Biophys Res Commun. 1995;217:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26:100-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Papachristou DJ, Papachroni KK, Papavassiliou GA, Pirttiniemi P, Gorgoulis VG, Piperi C, Basdra EK. Functional alterations in mechanical loading of condylar cartilage induces changes in the bony subcondylar region. Arch Oral Biol. 2009;54:1035-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Ozaki S, Kaneko S, Podyma-Inoue KA, Yanagishita M, Soma K. Modulation of extracellular matrix synthesis and alkaline phosphatase activity of periodontal ligament cells by mechanical stress. J Periodontal Res. 2005;40:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Searby ND, Steele CR, Globus RK. Influence of increased mechanical loading by hypergravity on the microtubule cytoskeleton and prostaglandin E2 release in primary osteoblasts. Am J Physiol Cell Physiol. 2005;289:C148-C158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280:31353-31359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 61. | Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674-2682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T, Matsushita M. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci USA. 2002;99:4580-4585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res. 2002;17:668-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 65. | Chatterjee S, Sivakamasundari V, Lee WJ, Chan HY, Lufkin T. Making no bones about it: Transcription factors in vertebrate skeletogenesis and disease. Trends Dev Biol. 2012;6:45-52. [PubMed] [Cited in This Article: ] |
| 66. | Komori T. A fundamental transcription factor for bone and cartilage. Biochem Biophys Res Commun. 2000;276:813-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, Pilz RB. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem. 2009;284:14796-14808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 69. | Reijnders CM, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007;192:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;2004:RE12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 72. | Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Duncan RL. Transduction of mechanical strain in bone. ASGSB Bull. 1995;8:49-62. [PubMed] [Cited in This Article: ] |
| 74. | Mitsui N, Suzuki N, Maeno M, Yanagisawa M, Koyama Y, Otsuka K, Shimizu N. Optimal compressive force induces bone formation via increasing bone morphogenetic proteins production and decreasing their antagonists production by Saos-2 cells. Life Sci. 2006;78:2697-2706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Koyama Y, Mitsui N, Suzuki N, Yanagisawa M, Sanuki R, Isokawa K, Shimizu N, Maeno M. Effect of compressive force on the expression of inflammatory cytokines and their receptors in osteoblastic Saos-2 cells. Arch Oral Biol. 2008;53:488-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Herman S, Krönke G, Schett G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med. 2008;14:245-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Tang L, Lin Z, Li YM. Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun. 2006;344:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Cheng MZ, Rawlinson SC, Pitsillides AA, Zaman G, Mohan S, Baylink DJ, Lanyon LE. Human osteoblasts’ proliferative responses to strain and 17beta-estradiol are mediated by the estrogen receptor and the receptor for insulin-like growth factor I. J Bone Miner Res. 2002;17:593-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Lacouture ME, Schaffer JL, Klickstein LB. A comparison of type I collagen, fibronectin, and vitronectin in supporting adhesion of mechanically strained osteoblasts. J Bone Miner Res. 2002;17:481-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Zhan M, Zhao H, Han ZC. Signalling mechanisms of anoikis. Histol Histopathol. 2004;19:973-983. [PubMed] [Cited in This Article: ] |
| 81. | Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 558] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 82. | Ziros PG, Gil AP, Georgakopoulos T, Habeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002;277:23934-23941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000;219:461-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 84. | Hess J, Porte D, Munz C, Angel P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem. 2001;276:20029-20038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Trouvin AP, Goëb V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Courter DL, Lomas L, Scatena M, Giachelli CM. Src kinase activity is required for integrin alphaVbeta3-mediated activation of nuclear factor-kappaB. J Biol Chem. 2005;280:12145-12151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Liu J, Zou L, Zheng Y, Zhao Z, Li Y, Yang P, Luo S. NF-kappaB responds to mechanical strains in osteoblast-like cells, and lighter strains create an NF-kappaB response more readily. Cell Biol Int. 2007;31:1220-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Scott A, Khan KM, Duronio V, Hart DA. Mechanotransduction in human bone: in vitro cellular physiology that underpins bone changes with exercise. Sports Med. 2008;38:139-160. [PubMed] [Cited in This Article: ] |
| 89. | Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod. 1997;19:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 90. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2371] [Cited by in F6Publishing: 2336] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 91. | Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J Biosci Bioeng. 2007;103:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Wendt D, Jakob M, Martin I. Bioreactor-based engineering of osteochondral grafts: from model systems to tissue manufacturing. J Biosci Bioeng. 2005;100:489-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Epari DR, Duda GN, Thompson MS. Mechanobiology of bone healing and regeneration: in vivo models. Proc Inst Mech Eng H. 2010;224:1543-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Klein P, Schell H, Streitparth F, Heller M, Kassi JP, Kandziora F, Bragulla H, Haas NP, Duda GN. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res. 2003;21:662-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 95. | Cruz M, Lourenço AF, Toledo EM, da Silva Barra LP, de Castro Lemonge AC, Wassall T. Finite element stress analysis of cuneiform and cylindrical threaded implant geometries. Technol Health Care. 2006;14:421-438. [PubMed] [Cited in This Article: ] |
| 96. | Kinoshita H, Nakahara K, Matsunaga S, Usami A, Yoshinari M, Takano N, Ide Y, Abe S. Association between the peri-implant bone structure and stress distribution around the mandibular canal: a three-dimensional finite element analysis. Dent Mater J. 2013;32:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Mesnard M, Ramos A, Simoes JA. Influences of implant condyle geometry on bone and screw strains in a temporomandibular implant. J Craniomaxillofac Surg. 2013;May 29; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Augat P, Penzkofer R, Nolte A, Maier M, Panzer S, v Oldenburg G, Pueschl K, Simon U, Bühren V. Interfragmentary movement in diaphyseal tibia fractures fixed with locked intramedullary nails. J Orthop Trauma. 2008;22:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Otto F, Kanegane H, Mundlos S. Mutations in the RUNX2 gene in patients with cleidocranial dysplasia. Hum Mutat. 2002;19:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Winslow MM, Pan M, Starbuck M, Gallo EM, Deng L, Karsenty G, Crabtree GR. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 101. | Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, Stein GS. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 102. | Gilbert HT, Hoyland JA, Freemont AJ, Millward-Sadler SJ. The involvement of interleukin-1 and interleukin-4 in the response of human annulus fibrosus cells to cyclic tensile strain: an altered mechanotransduction pathway with degeneration. Arthritis Res Ther. 2011;13:R8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Sowa GA, Coelho JP, Vo NV, Pacek C, Westrick E, Kang JD. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli: a pilot study. Am J Phys Med Rehabil. 2012;91:846-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7:33-47. [PubMed] [Cited in This Article: ] |
| 105. | Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285-27297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 500] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 106. | Amin S. Mechanical factors and bone health: effects of weightlessness and neurologic injury. Curr Rheumatol Rep. 2010;12:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Hamilton SA, Pecaut MJ, Gridley DS, Travis ND, Bandstra ER, Willey JS, Nelson GA, Bateman TA. A murine model for bone loss from therapeutic and space-relevant sources of radiation. J Appl Physiol (1985). 2006;101:789-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45-52. [PubMed] [Cited in This Article: ] |
| 109. | Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J Nutr. 2005;135:437-443. [PubMed] [Cited in This Article: ] |
| 110. | Carda S, Cisari C, Invernizzi M, Bevilacqua M. Osteoporosis after stroke: a review of the causes and potential treatments. Cerebrovasc Dis. 2009;28:191-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 112. | Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 113. | Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol (Oxf). 2006;65:555-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 114. | Marenzana M, Chenu C. Sympathetic nervous system and bone adaptive response to its mechanical environment. J Musculoskelet Neuronal Interact. 2008;8:111-120. [PubMed] [Cited in This Article: ] |
| 115. | Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 426] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 116. | Kusumi A, Sakaki H, Kusumi T, Oda M, Narita K, Nakagawa H, Kubota K, Satoh H, Kimura H. Regulation of synthesis of osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand in normal human osteoblasts via the p38 mitogen-activated protein kinase pathway by the application of cyclic tensile strain. J Bone Miner Metab. 2005;23:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Tang LL, Xian CY, Wang YL. The MGF expression of osteoblasts in response to mechanical overload. Arch Oral Biol. 2006;51:1080-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 119. | Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715-20727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 120. | Cartmell SH, Porter BD, García AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 121. | Leclerc E, David B, Griscom L, Lepioufle B, Fujii T, Layrolle P, Legallaisa C. Study of osteoblastic cells in a microfluidic environment. Biomaterials. 2006;27:586-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 122. | Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 123. | Iwamoto J, Takeda T, Ichimura S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci. 2001;6:128-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 124. | Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch Intern Med. 2004;164:1084-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 125. | Patel MJ, Chang KH, Sykes MC, Talish R, Rubin C, Jo H. Low magnitude and high frequency mechanical loading prevents decreased bone formation responses of 2T3 preosteoblasts. J Cell Biochem. 2009;106:306-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Sengupta DK. Dynamic stabilization devices in the treatment of low back pain. Orthop Clin North Am. 2004;35:43-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 127. | Wolf S, Augat P, Eckert-Hübner K, Laule A, Krischak GD, Claes LE. Effects of high-frequency, low-magnitude mechanical stimulus on bone healing. Clin Orthop Relat Res. 2001;192-198. [PubMed] [Cited in This Article: ] |
| 128. | Augat P, Simon U, Liedert A, Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005;16 Suppl 2:S36-S43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 129. | Li L, Zhu Z, Huang C, Chen W. Ultrasound: a potential technique to improve osseointegration of dental implants. Med Hypotheses. 2008;71:568-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Wei Y, Xiaolin H, Tao S. Effects of extremely low-frequency-pulsed electromagnetic field on different-derived osteoblast-like cells. Electromagn Biol Med. 2008;27:298-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Kokkinos PA, Zarkadis IK, Kletsas D, Deligianni DD. Effects of physiological mechanical strains on the release of growth factors and the expression of differentiation marker genes in human osteoblasts growing on Ti-6Al-4V. J Biomed Mater Res A. 2009;90:387-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 132. | Adamson RB, Bance M, Brown JA. A piezoelectric bone-conduction bending hearing actuator. J Acoust Soc Am. 2010;128:2003-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Eng JJ, Pang MY, Ashe MC. Balance, falls, and bone health: role of exercise in reducing fracture risk after stroke. J Rehabil Res Dev. 2008;45:297-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Marsden J, Gibson LM, Lightbody CE, Sharma AK, Siddiqi M, Watkins C. Can early onset bone loss be effectively managed in post-stroke patients? An integrative review of the evidence. Age Ageing. 2008;37:142-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Biering-Sørensen F, Hansen B, Lee BS. Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord. 2009;47:508-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489-500. [PubMed] [Cited in This Article: ] |
| 137. | Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:1385-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 138. | Huang TH, Lin HS, Chen HI, Yang RS. The effects of systemic chemical sympathectomy on local bone loss induced by sciatic neurectomy. J Orthop Sci. 2011;16:629-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 139. | Srinivasan S, Ausk BJ, Prasad J, Threet D, Bain SD, Richardson TS, Gross TS. Rescuing loading induced bone formation at senescence. PLoS Comput Biol. 2010;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |