Published online Nov 9, 2022. doi: 10.5492/wjccm.v11.i6.364
Peer-review started: April 26, 2022
First decision: June 8, 2022
Revised: June 12, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: November 9, 2022
Scoring systems have not been evaluated in oncology patients. We aimed to assess the performance of Acute Physiology and Chronic Health Evaluation (APACHE) II, APACHE III, APACHE IV, Simplified Acute Physiology Score (SAPS) II, SAPS III, Mortality Probability Model (MPM) II0 and Sequential Organ Failure Ass
To compare the efficacy of seven commonly employed scoring systems to predict outcomes of critically ill cancer patients.
We conducted a retrospective analysis of 400 consecutive cancer patients admitted in the medical intensive care unit over a two-year period. Primary outcome was hospital mortality and the secondary outcome measure was comparison of var
In our study, the overall intensive care unit and hospital mortality was 43.5% and 57.8%, respectively. All of the seven tested scores underestimated mortality. The mortality as predicted by MPM II0 predicted death rate (PDR) was nearest to the actual mortality followed by that predicted by APACHE II, with a standardized mortality rate (SMR) of 1.305 and 1.547, respectively. The best calibration was shown by the APACHE III score (χ2 = 4.704, P = 0.788). On the other hand, SOFA score (χ2 = 15.966, P = 0.025) had the worst calibration, although the difference was not statistically significant. All of the seven scores had acceptable discrimination with good efficacy however, SAPS III PDR and MPM II0 PDR (AUROC = 0.762), had a better performance as compared to others. The correlation between the different scoring sys
All the severity scores were tested under-predicted mortality in the present study. As the diff
Core Tip: Scoring systems are important for patient triaging, benchmarking intensive care unit (ICU) performance, comparing different ICUs and may also help in patient prognostication, selecting treatment options and resource utilization. However, validity and utility of these scores may be questionable in the patient population apart from where they were developed. Hence, these scores need to be tested and validated in different patient populations, in different geographical areas and over different time periods. There is a lack of an ideal score for prognostication of critically ill cancer patients. In our retrospective study, analyzing data from 400 patients and comparing seven commonly employed critical illness scores, we observed that all the scores had similar efficacy and under-predicted mortality. Therefore, the selection of severity of illness score should depend on the ease of use and local preferences.
- Citation: Beniwal A, Juneja D, Singh O, Goel A, Singh A, Beniwal HK. Scoring systems in critically ill: Which one to use in cancer patients? World J Crit Care Med 2022; 11(6): 364-374
- URL: https://www.wjgnet.com/2220-3141/full/v11/i6/364.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i6.364
The application of prognosticating scoring systems is considered as an important phase in intensive care units (ICUs) since these severity scoring systems estimate the probability of mortality for patients. These scores help the physicians to facilitate resource utilization or continuous quality improvement and to stratify the patients for clinical research[1,2]. ICU scoring systems can help both patients as well as their attendants to select from further treatment options. Further, the scores calculated by these scoring systems help in evaluating the impact of newer treatment modalities and organizational changes which in turn contributes towards the development of treatment standards. In addition to the above, the scoring systems’ outcomes also help in benchmarking ICU performance and comparing the scores secured by different ICU patient populations so as to find out the differences in mortality. However, these systems are unreliable in predicting the clinical outcomes of an individual though it has proven efficacy in predicting mortality for a particular patient cohort[3].
Acute Physiology and Chronic Health Evaluation (APACHE) II and Simplified Acute Physiology Score (SAPS) II are arguably the two most-commonly used and validated tools used in the prediction of ICU patient outcomes[4,5]. These scoring systems were developed in the 1980s and have become outdated due to technological and clinical advancements in critical care management of patients in recent years. Hence, there is a need to develop new scoring systems that include APACHE IV, SAPS III and Mortality Probability Model (MPM) II0[6-9]. Such newly-created systems encompass a large number of variables and are highly complicated to compute.
In addition, both validity and utility of the existing scoring systems may be questionable in terms of current patient population compared to the patient population during which they were developed. These scores are widely used and the scoring systems have been validated for a notable time to predict the outcome in general medical or surgical procedures conducted upon critically ill patients. However, whether these systems can predict the mortality accurately among cancer patients remains unknown[10]. There is a dearth of studies that compare different generations of scoring systems and especially the ones used upon cancer patients admitted in medical oncology ICUs. Only a few studies have ass
A retrospective observational cohort study was carried out at a multi-disciplinary onco-medical ICU of a tertiary care center in India. We have an advanced ICU setup and 24-h intensivist coverage with state-of-the-art facilities. Approval for the study and a consent waiver from the institutional ethics committee was obtained.
The data from the records of adult patients who were admitted between January 2018 and February 2020, i.e., 2 years, was collected and analyzed. If the patient was readmitted to the ICU more than once during his/her hospital stay, only the first admission was included in the study. Patients who had ICU stays of less than 12 h, post-operative patients and those admitted from or discharged to another ICU were excluded from the study. Patients fulfilling inclusion criteria were serially recruited. The re
The collected data was then transformed into variables, coded and entered in Microsoft Excel. Then, it was statistically analyzed using SPSS software (version. PC-25). Quantitative data was expressed in mean ± SD or median with an interquartile range. Normality distribution difference between two comparable groups was measured using student’s t-test or Mann Whitney ‘U’ test. Qualitative data was expressed in percentage whereas the statistical differences between the proportions were tested using chi square or Fisher’s exact test, as appropriate.
Standardized Mortality Ratio (SMR) was computed by dividing the observed 28 d’ mortality by predicted hospital mortality based on different scores. Further, 95% confidence interval (CI) was calculated for SMR by considering the observed mortality as a Poisson variable and then dividing its 95%CI by predicted mortality.
The calibration of the scores was executed using Hosmer-Lemeshow goodness-of-fit statistics which divides the subjects into deciles based on the predicted probabilities of death. Afterwards, it computes a Chi-square value from the observed and expected frequencies. Low Chi-square values and high P values (P > 0.5) correspond to a better fit. The ability of the scores to predict ICU mortality was explored and discrimination was tested using Area Under Receiver Operating Characteristic (AUROC) curves. If the AUROC curves are more than 0.8, it denotes excellent outcome while 0.6-0.8 are considered to be acceptable. The cut-off values were calculated for different scores using Youden’s index based on which sensitivity and specificity of the scores were calculated.
Clinically-relevant variables that produced P < 0.05 during univariate analyses and are easily accessible on admission were also entered into multiple logistic regression models as the outcome variable of interest. Odds ratio (OR) was calculated along with 95%CI. A P value < 0.05 was considered to be statistically significant.
The sample size calculation was done for the estimation of the AUROC curve for APACHE 2 score, using the following formula:
n ≥ Z2α/2 V (AUC) ÷ d2
Where, V(AUC) = 0.0099 × e-a2/2 × (6a2 + 16), a = ϕ-1 (AUC) × 1.414 and ϕ-1 is the inverse of standard cumulative normal distribution for AUC.
For a 95% level of confidence Zα/2 = 1.96; d = 0.05 which is the margin of error in estimation and AUC was obtained from a similar study conducted by Schellongowski et al[12] who reported an AUC of 0.776 for the APACHE II score.
Substituting these values in the above formula gives n ≥ 196. As our study was retrospective in nature, we included 400 patients.
During the study period, the data from 400 patients who fulfilled the inclusion criteria were included in the final analysis. Thirty-eight patients were excluded because 31 were admitted from or discharged to another ICU, five were post-operative patients and two had ICU stays less than 12 h. Their baseline characteristics are given in Table 1 and the comparison between various scores is given in Table 2.
| Parameters | Survivors, n = 169 | Non-survivors, n = 231 | Total, n = 400 | P value |
| Age in yr | 62.85 ± 12.49 | 61.45 ± 14.82 | 62.04 ± 13.88 | 0.527 |
| Male | 98 (58.0%) | 142 (61.5%) | 240 (60.0%) | 0.48 |
| Female | 71 (42.0%) | 89 (38.5%) | 160 (40.0%) | |
| DM | 56 (33.1%) | 62 (26.8%) | 118 (29.5%) | 0.17 |
| Hypertension | 61 (36.1%) | 63 (27.3%) | 124 (31.0%) | 0.06 |
| Reason for ICU admission | ||||
| Sepsis | 42 (24.9%) | 68 (29.4%) | 110 (27.5%) | 0.31 |
| Respiratory distress/failure | 76 (45.0%) | 93 (40.3%) | 169 (42.2%) | 0.34 |
| Cardiac arrest | 1 (0.6%) | 8 (3.5%) | 9 (2.2%) | 0.08 |
| Gastrointestinal bleed | 15 (8.9%) | 14 (6.1%) | 29 (7.2%) | 0.33 |
| Altered sensorium | 33 (19.5%) | 45 (19.5%) | 78 (19.5%) | 1 |
| Acute kidney injury | 2 (1.2%) | 3 (1.3%) | 5 (1.2%) | 1 |
| Type of malignancy | ||||
| Solid organ | 135 (79.9%) | 187 (81.0%) | 322 (80.5%) | 0.78 |
| Hematological | 34 (20.1%) | 44 (19.0%) | 78 (19.5%) | |
| Metastasis | 80 (59.3%) | 145 (77.5%) | 225 (69.9%) | 0.001 |
| Previous history of surgery for CA | ||||
| Yes | 72 (42.6%) | 74 (32.0%) | 146 (36.5%) | 0.03 |
| No | 97 (57.4%) | 157 (68.0%) | 254 (63.5%) | |
| ICU stay | 5 (3-8) | 4 (2-10) | 5 (3-9) | 0.58 |
| Hospital stay | 14 (8-21) | 11 (5-22) | 12 (7-21) | 0.006 |
| Use of MV | 24 (14.2%) | 130 (56.3%) | 154 (38.5%) | < 0.001 |
| Days of MV | 5 (3-7.75) | 3 (2-6) | 3 (2-7) | 0.002 |
| Use of renal support | 7 (4.1%) | 29 (12.6%) | 36 (9.0%) | 0.004 |
| Days of renal support | 2.14 ± 0.90 | 2.48 ± 2.06 | 2.42 ± 1.88 | 0.786 |
| Use of vasopressor support | 26 (15.4%) | 174 (75.3%) | 200 (50.0%) | < 0.001 |
| Days of vasopressor support | 3 (2-4) | 2 (1.75-4.0) | 2 (2-4) | 0.276 |
| Scoring system | Survivors, n = 169 | Non-survivors, n = 231 | Total, n = 400 | P value |
| APACHE II | 17.66 ± 4.96 | 22.82 ± 8.34 | 20.64 ± 7.55 | < 0.001 |
| APACHE II PDR | 28.10 ± 17.74 | 44.04 ± 25.88 | 37.30 ± 24.10 | < 0.001 |
| APACHE III | 59.01 ± 16.95 | 81.36 ± 31.37 | 71.92 ± 28.46 | < 0.001 |
| APACHE III PDR | 17.59 ± 15.80 | 37.59 ± 28.51 | 29.14 ± 25.91 | < 0.001 |
| APACHE IV | 58.80 ± 16.98 | 80.45 ± 31.70 | 71.30 ± 28.55 | < 0.001 |
| APACHE IV PDR | 20.45 ± 14.99 | 40.45 ± 27.91 | 32.00 ± 25.33 | < 0.001 |
| SAPS II | 34.67 ± 11.83 | 49.20 ± 19.87 | 43.06 ± 18.39 | < 0.001 |
| SAPS II PDR | 19.81 ± 16.97 | 42.83 ± 30.51 | 33.10 ± 28.06 | < 0.001 |
| SAPS III PDR | 18.12 ± 16.95 | 34.66 ± 24.12 | 27.67 ± 22.88 | < 0.001 |
| SOFA Score | 5.76 ± 2.80 | 9.02 ± 4.58 | 7.64 ± 4.24 | < 0.001 |
| MPM II0 PDR | 33.39 ± 15.08 | 52.16 ± 26.63 | 44.23 ± 24.31 | < 0.001 |
All of the scoring systems tested in the current study underestimated the mortality (Table 3). The mortality, predicted by MPM II0 PDR, was nearest to the actual mortality with an SMR of 1.305, followed by APACHE II (1.547) and SAPS II (1.74).
| Scoring system | Actual mortality | Predicted mortality | SMR | 95%CI |
| APACHE II | 0.577 | 0.373 | 1.547 | 1.423-1.678 |
| APACHE III | 0.577 | 0.291 | 1.982 | 1.824-2.151 |
| APACHE IV | 0.577 | 0.320 | 1.803 | 1.659-1.956 |
| SAPS II | 0.577 | 0.331 | 1.743 | 1.604-1.891 |
| SAPS III | 0.577 | 0.277 | 2.083 | 1.917-2.26 |
| MPM II0 PDR | 0.577 | 0.442 | 1.305 | 1.201-1.416 |
Using the Lemeshow-Hosmer goodness-of fit test, APACHE III (4.704) achieved the best calibration with P = 0.788 whereas SOFA score (15.966) was the worst with P = 0.025 (Table 4). The least statistically significant discrepancy between the predicted and observed mortality was shown by the APACHE III score.
| Scoring system | Chi square value | P value |
| APACHE II | 9.366 | 0.312 |
| APACHE II PDR | 12.159 | 0.144 |
| APACHE III | 4.707 | 0.788 |
| APACHE III PDR | 6.471 | 0.595 |
| APACHE IV | 9.331 | 0.315 |
| APACHE IV PDR | 10.763 | 0.216 |
| SAPS II | 9.479 | 0.304 |
| SAPS II PDR | 10.410 | 0.237 |
| SAPS III PDR | 10.787 | 0.214 |
| SOFA Score | 15.966 | 0.025 |
| MPM II0 PDR | 11.265 | 0.187 |
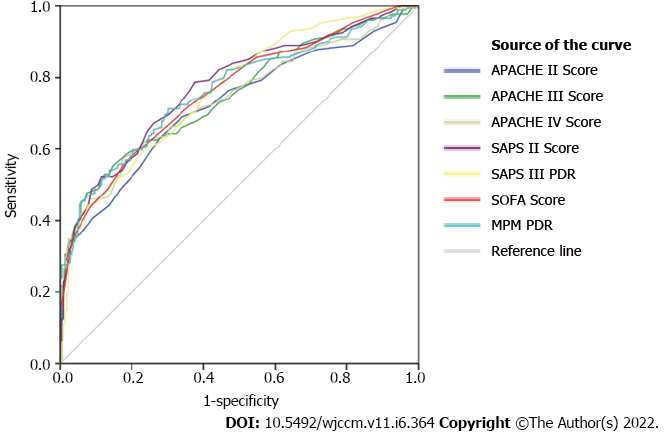
The efficacy of various scores is given in Figure 1. All the scores tested in the current study exhibited good efficacy, even though there was no statistically significant difference between AUROCs and SAPS III PDR. On the other hand, MPM II0 PDR (AUROC = 0.762) yielded the best performance (Table 5).
| Scoring system | AUC | P value | 95%CI | Cut off | Sensitivity | Specificity |
| APACHE II | 0.688 | < 0.001 | 0.637-0.739 | > 18.5 | 67.5% | 62.7% |
| APACHE III | 0.720 | < 0.001 | 0.672-0.769 | > 78.5 | 46.8% | 87.6% |
| APACHE IV | 0.708 | < 0.001 | 0.659-0.758 | > 72.5 | 53.7% | 79.3% |
| SAPS II | 0.734 | < 0.001 | 0.685-0.782 | > 34.5 | 76.2% | 60.4% |
| SAPS III PDR | 0.762 | < 0.001 | 0.715-0.808 | 39.0 | 44.3% | 92.0% |
| SOFA Score | 0.715 | < 0.001 | 0.665-0.764 | > 7.5 | 58.0% | 79.3% |
| MPM II0 PDR | 0.762 | < 0.001 | 0.714-0.810 | 36.45 | 71.3% | 69.9% |
As shown in Table 6, there was a significant correlation found among various scoring systems (P < 0.001) as assessed by linear regression analysis.
| Scoring system | APACHE II Score | A2 PDR | APACHE III Score | A3 PDR | APACHE IV Score | A4 PDR | SAPS II Score | SAPS2 PDR | SAPS 3 PDR | SOFA score | |
| APACHE II Score | r value | 0.898 | 0.892 | 0.836 | 0.883 | 0.826 | 0.820 | 0.812 | 0.748 | 0.679 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| A2 PDR | r value | 0.898 | 0.824 | 0.832 | 0.814 | 0.805 | 0.751 | 0.752 | 0.716 | 0.635 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| APACHE III Score | r value | 0.892 | 0.824 | 0.929 | 0.966 | 0.895 | 0.910 | 0.902 | 0.820 | 0.753 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| A3 PDR | r value | 0.836 | 0.832 | 0.929 | 0.897 | 0.895 | 0.851 | 0.852 | 0.763 | 0.711 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| APACHE IV Score | r value | 0.883 | 0.814 | 0.966 | 0.897 | 0.915 | 0.890 | 0.877 | 0.821 | 0.762 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| A4 PDR | r value | 0.826 | 0.805 | 0.895 | 0.895 | 0.915 | 0.836 | 0.839 | 0.782 | 0.727 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| SAPS II Score | r value | 0.820 | 0.751 | 0.910 | 0.851 | 0.890 | 0.836 | 0.972 | 0.814 | 0.756 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| SAPS 2 PDR | r value | 0.812 | 0.752 | 0.902 | 0.852 | 0.877 | 0.839 | 0.972 | 0.813 | 0.773 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| SAPS 3 PDR | r value | 0.748 | 0.716 | 0.820 | 0.763 | 0.821 | 0.782 | 0.814 | 0.813 | 0.684 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| SOFA score | r value | 0.679 | 0.635 | 0.753 | 0.711 | 0.762 | 0.727 | 0.756 | 0.773 | 0.684 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| MPM II0 PDR | r value | 0.704 | 0.653 | 0.777 | 0.729 | 0.759 | 0.734 | 0.790 | 0.805 | 0.714 | 0.700 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
Five factors that showed significance in univariate analysis such as hypertension, surgery for cancer, use of MV, vasopressors and renal support were used in multivariate analysis as well. Out of the five factors, two factors, i.e. need for MV (OR 2.437, 95%CI = 1.315-4.515, P = 0.005) and vasopressor support (OR 10.465, 95%CI = 5.901-18.557, P = 0.000) were statistically associated with hospital mortality.
The current study compared various mortality prediction scoring systems and found that all the scores under-predicted the mortality in critically-ill cancer patients. Amongst the scoring systems considered, mortality predicted by MPM PDR was the closest to that of the actual mortality with an SMR of 1.305. AUROC values showed that all of the seven scoring systems had good efficacy and acceptable discrimination. MPM PDR and SAPS III PDR achieved the best discrimination. We found the best sensitivity in SAPS II score (76.2%) and best specificity in SAPS III PDR score (92%). The Lemeshow-Hosmer goodness-of fit tests showed that the APACHE III score had the best calibration although there was no statistically significant difference.
In the current study, all of the scores were significantly higher among non-survivors (P value < 0.001) as reported in the literature[13-18]. However, all the scores tested in this study underestimated the mortality (SMR > 1), like previous studies[14,15,19,20].
Discrimination is the ability to determine the patients who may die and who will survive. Measures of discrimination include sensitivity, specificity and AUROC curve. But no single scoring system excelled in all of the three areas. SAPS III PDR and MPM II0 PDR (AUROC = 0.762) had the best AUROC values whereas sensitivity was at its best for SAPS II and specificity was at its best for SAPS III PDR. However, these differences were not statistically significant. In the current study, AUROC outcomes showed that discrimination is acceptable in all the scoring systems tested as reported in the literature[14-16,20-22]. All the severity illness scores showed good efficacy with no statistically significant difference in AUROCs.
Calibration evaluates the accuracy of the degree of correspondence between the estimated probability of mortality and the observed actual mortality. Calibration is good if the predicted mortality is close to the observed mortality. APACHE III (4.704) had the best calibration with P = 0.788. This infers that it had the least statistically significant discrepancy between the predicted and observed mortality. Good calibration of these scores have also been reported by other authors[14-16,20].
A significant correlation was found among various scoring systems (P < 0.001) as per linear regression analysis. This correlation may be attributed to the overlap of multiple variables, considered for calculating the scores. Sculier et al[21] also reported an excellent correlation between APACHE II and SAPS II in their study on oncology patients. ICU mortality rate among cancer patients was reportedly high and in the range of 30% to 77%[23-26]. The overall ICU mortality rate in the current study was 43.5%. Even though it is higher, the ICU mortality of the current cohort does not differ from the mortality reported in similar studies conducted earlier[23,24]. The hospital mortality rate in the current study was 57.8% which is again similar as reported earlier[27,28].
Use of MV and vasopressor support have a direct association with hospital mortality. Similar studies conducted earlier have also reported the need for organ support in the form of MV. At times, vaso
The accuracy of scoring systems may differ over a period of time and may produce varied results in different countries due to differences in ethnicity, patient population, healthcare systems, ICU structure and organization. So, its accuracy cannot be generalized and all such models need external validation in independent patient populations to prove its reproducibility. Therefore, it becomes imperative to compare and test the validity of scoring systems under different geographical areas and upon different patient populations. The current study is one of the few studies conducted on the Indian subcontinent and the researchers have compared a huge number of scoring systems developed for cancer patients in a significantly large cohort of patients.
The current study has a limitation to address, i.e. being a single center retrospective study where concerns may arise in terms of generalizing the conclusions arrived in this study. The missing data may have also led to information bias. Nonetheless, the study has several salient features such as the com
The current study concludes that all of the scoring systems considered for this study cohort under-predicted the mortality. However, the APACHE III score had the least discrepancy between the predicted and observed mortality. There was no statistically significant difference in efficacy and all the scores tested had good calibration and acceptable discrimination. Hence, the choice of scoring system in critically-ill oncology patients should not only be based on the performance of the score, but also on other factors such as ease of use and local preferences.
The application of prognosticating scoring systems is considered as an important phase in intensive care units (ICUs) since these severity scoring systems estimate the probability of mortality for patients. These scores help the physicians to facilitate resource utilization or continuous quality improvement and to stratify the patients for clinical research. ICU scoring systems can help both patients as well as their attendants to select from further treatment options. Further, the scores calculated by these scoring systems help in evaluating the impact of newer treatment modalities and organizational changes which in turn contributes towards the development of treatment standards. In addition to the above, the scoring systems’ outcomes also help in benchmarking ICU performance and comparing the scores secured by different ICU patient populations so as to find out the differences in mortality.
There is a dearth of studies that compare different generations of scoring systems especially the ones used upon cancer patients admitted in medical oncology ICUs. Only a few studies have assessed their usefulness in cancer patients with conflicting results.
To compare the efficacy of seven commonly employed scoring systems to predict outcomes of critically ill cancer patients.
We conducted a retrospective analysis of 400 consecutive cancer patients admitted in the medical intensive care unit over a 2-year period. The primary outcome was hospital mortality and the secondary outcome measure was comparison of various scoring systems in predicting hospital mortality.
Overall ICU mortality in our study was 43.5% whereas hospital mortality was 57.8%. All scoring systems tested underestimated the mortality. Mortality predicted by MPM II0 predicted death rate (PDR), was closest to that of the actual mortality followed by that of APACHE II, with a standardized mortality rate (SMR) of 1.305 and 1.547, respectively. APACHE III (χ2 = 4.704, P = 0.788) had the best calibration and SOFA score (χ2 = 15.966, P = 0.025) had the worst calibration, but the difference was not statistically significant. All the scores tested had good efficacy and acceptable discrimination, however SAPS III PDR and MPM II0 PDR (AUROC = 0.762), performed better than others. There was a significant correlation between the various scoring systems (P < 0.001).
Overall, all the scores in our study cohort under-predicted the mortality. The difference in efficacy was not statistically significant in all scores. The choice of scoring system should depend on the ease of use and local preferences as all the scores tested had similar performance.
There is a lack of an ideal score for prognostication of critically ill cancer patients. In our retrospective study, analyzing data from 400 patients and comparing seven commonly employed critical illness scores, we observed that all the scores had similar efficacy but under-predicted mortality. Therefore, the choice of scoring system should depend on the ease of use and local preferences.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Surani S, United States; Zhang ZH, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Sakr Y, Krauss C, Amaral AC, Réa-Neto A, Specht M, Reinhart K, Marx G. Comparison of the performance of SAPS II, SAPS 3, APACHE II, and their customized prognostic models in a surgical intensive care unit. Br J Anaesth. 2008;101:798-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Moreno R, Matos R. New issues in severity scoring: interfacing the ICU and evaluating it. Curr Opin Crit Care. 2001;7:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Aygencel G, Turkoglu M, Turkoz Sucak G, Benekli M. Prognostic factors in critically ill cancer patients admitted to the intensive care unit. J Crit Care. 2014;29:618-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10902] [Cited by in F6Publishing: 10643] [Article Influence: 272.9] [Reference Citation Analysis (0)] |
| 5. | Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 2135] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 6. | Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1044] [Cited by in F6Publishing: 1075] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 7. | Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR; SAPS 3 Investigators. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005;31:1336-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 427] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 8. | Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR; SAPS 3 Investigators. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 864] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 9. | Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III). Crit Care Med. 2007;35:827-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Juneja D, Singh O, Nasa P, Dang R. Comparison of newer scoring systems with the conventional scoring systems in general intensive care population. Minerva Anestesiol. 2012;78:194-200. [PubMed] [Cited in This Article: ] |
| 11. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2004] [Cited by in F6Publishing: 2263] [Article Influence: 251.4] [Reference Citation Analysis (0)] |
| 12. | Schellongowski P, Benesch M, Lang T, Traunmüller F, Zauner C, Laczika K, Locker GJ, Frass M, Staudinger T. Comparison of three severity scores for critically ill cancer patients. Intensive Care Med. 2004;30:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J. 2008;31:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kopterides P, Liberopoulos P, Ilias I, Anthi A, Pragkastis D, Tsangaris I, Tsaknis G, Armaganidis A, Dimopoulou I. General prognostic scores in outcome prediction for cancer patients admitted to the intensive care unit. Am J Crit Care. 2011;20:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Soares M, Silva UV, Teles JM, Silva E, Caruso P, Lobo SM, Dal Pizzol F, Azevedo LP, de Carvalho FB, Salluh JI. Validation of four prognostic scores in patients with cancer admitted to Brazilian intensive care units: results from a prospective multicenter study. Intensive Care Med. 2010;36:1188-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Berghmans T, Paesmans M, Sculier JP. Is a specific oncological scoring system better at predicting the prognosis of cancer patients admitted for an acute medical complication in an intensive care unit than general gravity scores? Support Care Cancer. 2004;12:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Xia R, Wang D. Intensive care unit prognostic factors in critically ill patients with advanced solid tumors: a 3-year retrospective study. BMC Cancer. 2016;16:188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. |
Vincent F, Soares M, Mokart D, Lemiale V, Bruneel F, Boubaya M, Gonzalez F, Cohen Y, Azoulay E, Darmon M; GrrrOH: Groupe de recherche respiratoire en réanimation en Onco-Hématologie (Group for respiratory research in intensive care in Onco-Hematology, |
| 19. | Soares M, Fontes F, Dantas J, Gadelha D, Cariello P, Nardes F, Amorim C, Toscano L, Rocco JR. Performance of six severity-of-illness scores in cancer patients requiring admission to the intensive care unit: a prospective observational study. Crit Care. 2004;8:R194-R203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Xing X, Gao Y, Wang H, Huang C, Qu S, Zhang H, Sun K. Performance of three prognostic models in patients with cancer in need of intensive care in a medical center in China. PLoS One. 2015;10:e0131329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sculier JP, Paesmans M, Markiewicz E, Berghmans T. Scoring systems in cancer patients admitted for an acute complication in a medical intensive care unit. Crit Care Med. 2000;28:2786-2792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Staudinger T, Stoiser B, Müllner M, Locker GJ, Laczika K, Knapp S, Burgmann H, Wilfing A, Kofler J, Thalhammer F, Frass M. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med. 2000;28:1322-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Anisoglou S, Asteriou C, Barbetakis N, Kakolyris S, Anastasiadou G, Pnevmatikos I. Outcome of lung cancer patients admitted to the intensive care unit with acute respiratory failure. Hippokratia. 2013;17:60-63. [PubMed] [Cited in This Article: ] |
| 24. | Müller AM, Gazzana MB, Silva DR. Outcomes for patients with lung cancer admitted to intensive care units. Rev Bras Ter Intensiva. 2013;25:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ostermann M, Raimundo M, Williams A, Whiteley C, Beale R. Retrospective analysis of outcome of women with breast or gynaecological cancer in the intensive care unit. JRSM Short Rep. 2013;4:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Yoo H, Suh GY, Jeong BH, Lim SY, Chung MP, Kwon OJ, Jeon K. Etiologies, diagnostic strategies, and outcomes of diffuse pulmonary infiltrates causing acute respiratory failure in cancer patients: a retrospective observational study. Crit Care. 2013;17:R150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wang YG, Zhou JC, Wu KS. High 28-day mortality in critically ill patients with sepsis and concomitant active cancer. J Int Med Res. 2018;46:5030-5039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Shin SH, Lee H, Kang HK, Park JH. Twenty-eight-day mortality in lung cancer patients with metastasis who initiated mechanical ventilation in the emergency department. Sci Rep. 2019;9:4941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kostakou E, Rovina N, Kyriakopoulou M, Koulouris NG, Koutsoukou A. Critically ill cancer patient in intensive care unit: issues that arise. J Crit Care. 2014;29:817-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |