Peer-review started: October 20, 2014
First decision: December 17, 2014
Revised: December 28, 2014
Accepted: January 9, 2015
Article in press: January 12, 2015
Published online: February 20, 2015
Angina bullosa hemorrhagica (ABH) is an enigmatic oral disorder described for the first time by Badham in 1967 to define blisters with a hematic content in the oral cavity and oropharynx unrelated to any hematological, dermatological or systemic disease. The ABH is an uncommon disease of the oral cavity distinctively affecting adults, with the highest incidence over the 5th decade of life. This process is considered nowadays to have a multifactorial etiopathogenesis, where mild oral traumatisms can trigger the blisters in susceptible individuals. Certain association on the onset of the lesion with the chronic use of inhaled steroids and, more controversially, with triggering systemic disorders, such as, diabetes or hypertension has been described. Characteristically, the ABH blisters are acute and are located on the lining mucosa, more frequently on the soft palate. Usually, the lesions are solitary and rupture easily, resulting in a superficial ulceration that heals quickly without scarring. The histopathological analysis shows a subepithelial blister containing blood and direct immunofluorescence on the epithelium is negative. The differential diagnosis should consider all oral vesiculo-bullous disorders with hematic content, including mucocutaneos, hematological or cystic pathology. The diagnosis of ABH is clearly clinical, although the biopsy might be helpful on atypical or abnormally recurrent cases. The general prognosis of ABH is good and the treatment is symptomatic.
Core tip: Although it is an uncommon disease, the angina bullosa hemorrhagica should be considered in the differential diagnosis of oral vesiculo-bullous processes. Acknowledging this entity will help in differentiating it from important mucocutaneous and hematological diseases such as pemphigus vulgaris, mucous membrane pemphigoid or coagulation disorders. In this review we analyze the main etiopathogenic, clinicopathological, diagnostic and therapeutic aspects of this enigmatic oral condition.
- Citation: Alberdi-Navarro J, Gainza-Cirauqui ML, Prieto-Elías M, Aguirre-Urizar JM. Angina bullosa hemorrhagica an enigmatic oral disease. World J Stomatol 2015; 4(1): 1-7
- URL: https://www.wjgnet.com/2218-6263/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.5321/wjs.v4.i1.1
Angina bullosa haemorrhagica (ABH) is an uncommon and benign subepithelial disorder appearing as hematic blisters on the oral and oropharyngeal mucosa and no relation with any dermatological, haemostatic or systemic condition[1]. Badham[1] in 1967 defined these lesions with this term, although according to Stephenson et al[2] in 1987 and Grinspan et al[3] in 1999, similar lesions had been previously described by other authors such as Haryng[4] in 1890 referred to this condition as “Traumatic Oral Hemophlyctenosis” or Baliña[5] in 1933 as “Angina Ulcerosa Benigna” 1933. This entity has received multiple names, such as Benign Hemorrhagic Bullous Stomatitis[6] or Localized Oral Purpura[7]. In 1994 Kirtschig and Happle[8] named it “Stomatopompholyx hemorrhagica”, as “angina” was an inadequate term for this disease. However, despite all the attempts in changing its name, ABH continues as the most commonly used term in the literature.
The ABH is an uncommon oral pathology, although its real prevalence is unknown. The study performed by Mehrotra et al[9] in 2010 is the most accurate as they analyze the prevalence of oral pathologies of the soft tissue in a sample of 3030 Indian adults reporting a prevalence of ABH of only 0.03%. Retrospective studies show a prevalence of 0.5% on patients diagnosed with ABH in Oral Medicine and Oral Pathology clinics[3,10]. However, many authors[1,10-13] estimate a higher prevalence of this disease, justifying its rare diagnosis to its frequent asymptomatic character and the fast resolution of the lesions, which would lead the patient to seek less attention, thus to be undiagnosed.
This disease distinctively affects adult patients from the 3rd decade of life, with a peak incidence over the 5th decade[2,3,10,14-17].
Regarding the gender distribution, in his first description, Badham[1] observed a higher prevalence of ABH in women, although later published series of cases[2,3,10] have shown that the differences between genders are non-significant and, some authors[17], even describe a higher prevalence in males.
The etiopathogenesis of this lesion is yet unknown thus being considered nowadays as a multifactorial disease with local trauma on the oral mucosa as the trigger on susceptible individuals[16]. Several authors[1,3], have considered ABH an acquired disease without a recognized genetic component; however, some[2,18] have described certain familial predisposition in developing ABH.
Classically, it has been suggested that a loss of cohesion between the epithelium and the chorion can cause the rupture of the subepithelial capillaries after trauma and condition the emergence of a blood-containing blister[15].
An important percentage of the cases (35%-100%) report a known triggering traumatic event, with the intake of hard or crunchy foods as the most cited[2,10,13,15-17,19]. Nevertheless, it is worth mentioning that, in a study[3], only 24% of the patients could identify the traumatic factor. We believe that this datum is lower due to the retrospective character of many ABH studies that force the patient to remember the existence of a previous traumatic event[2].
Different foods are associated with ABH, including toasts, chips and hot meals[1]. Together with hard and crunchy foods (75%), a previous intake of acidic and citrus fruits has also been reported[17,18]. As an anecdote, other hard foods, such as a fish bone or a chicken bone, have been linked[19]. Along with food, beverage consumption has been associated with the onset of ABH, although the type and its characteristics are yet to be described[16].
Several clinical cases are associated to trauma from dental procedures, including impressions[2], dental preparations[20], a crown as a traumatic factor[21], certain conservative treatments[15], the injection of local anesthesia[22-24] or a periodontal treatment[25]. Isolated cases of ABH from other traumatisms have been described, including intubations or endoscopies[1,26], or even after coughing or sneezing roughly[11,15].
In 1987, Stephenson et al[2] suggested the suction habit as the main cause for the formation of these lesions; although, incidentally, none of the 30 patients from their study described this circumstance. Subsequently, de las Heras et al[27] described that the suction habit could lead to multiple ABH lesions.
Together with local traumatic factors, certain inhaled drugs, mainly the chronic use of topical corticosteroids, have been associated with the onset of ABH[28,29]. High and Main[28] performed a study in 1988 in two groups of patients with asthma undergoing treatment with aerosols, one with and one without steroids. When comparing the incidence of ABH, lesions were present only in the group using steroids (35.7%). In these cases, the prolonged contact of the steroid with the oral mucosa may cause epithelial atrophy and may alter the distribution of the chorionic elastic fibers, which would weaken the epithelium-connective tissue junction, and would favor the onset of a subepithelial blister in a local traumatic event[19,28,29].
Another inhaled drug linked to the onset of ABH is Ipratropium Bromide, an antimuscarinic bronchodilator[30].
Badham[1] described in his study certain association between ABH and systemic conditions, including menstruation in some of his patients.
Subsequently, ABH has been linked to different systemic processes, although this is still unfounded as its etiopathogenic base is yet to be described. The main systemic conditions associated with ABH are diabetes mellitus and hypertension (Table 1).
| Ref. | n | Diabetes | Hypertension |
| Grinspan et al[3] | 54 | 24 (44%)1 | 0 (0%) |
| Giuliani et al[16] | 8 | 1 (12.5%) | 0 (0%) |
| Yamamoto et al[13] | 11 | 4 (36.4%) | 3 (27.3%) |
| Horie et al[17] | 16 | 1 (6.25%) | 6 (37.5%) |
| Deblauwe and van der Waal[11] | 9 | 1 (11.1%) | 0 (0%) |
| Serra et al[31] | 4 | 0 (0%) | 2 (50%) |
| Martini et al[19] | 4 | 0 (0%) | 2 (50%) |
| Rosa et al[10] | 47 | 4 (8.5%) | 17 (32.2%) |
The high prevalence of diabetes, described only by Grinspan et al[3] in 1999 is worth mentioning as 44% of the ABH patients showed altered serological levels of glucose or family history of diabetes mellitus. It is possible that considering that both entities share the same age range and that diabetes has a high incidence among adults, it could be a coincidental relation and not a direct pathological association.
Regarding hypertension, several authors[10,17] outline circumstances similar to diabetes mellitus. Moreover, several cases of patients with chronic kidney failure are described in the literature[13,32,33].
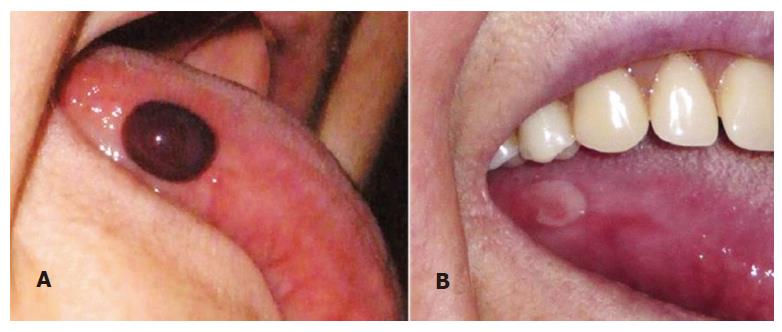
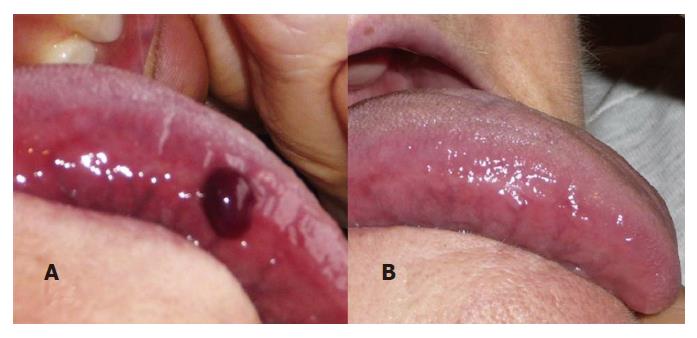
The characteristic lesion of ABH is a dark red-violet blister with a hematic content[1] (Figure 1A and Figure 2A).
Two types of patients have been distinguished according to its clinical presentation[15]. Some, the most frequent, have a large solitary lesion located in the soft palate and recurring spaced in time; others, less frequent, have a greater number of lesions in different locations and with a higher recurrence rate. Subsequent studies avoid separating into these subtypes as they distinguish the solitary lesion as the most frequent clinical presentation[2,3,10,18,16]. Nonetheless, in 30% of the patients multiple lesions are present, with up to 4 simultaneous lesions being described[15,34].
The formation of the blisters is characteristically acute as the lesions may appear abruptly within seconds[1,3,15,35]. The lesions have a diameter of 0.3 to 4 cm, but generally over 1 cm[2,3,15,17,25,33]. Despite most authors, Rosa et al[10], in their study on 47 patients, observed that most lesions measured less than 1 cm in diameter.
The lesions might cause mild unspecific discomfort for which it may be diagnosed casually on a dental revision[3,13,16,19,35]. However, Rosa et al[10], described pain, mainly of a mild intensity, in 36.1% of their patients.
In some cases, and previous to the blister formation, a burning or itching sensation, or even a stabbing pain has been described[15,36].
Regarding the location of the lesions, there is agreement in suggesting that the most affected site is the soft palate, followed by the borders of the tongue and the buccal mucosa[2,3,10,13-15,17,19]. Nonetheless, in addition to the above mentioned locations, cases have been described in the ventral surface of the tongue[2], the lip[15] and the floor of the mouth[3,15,16]. It is important to point out that all of these locations are part of the “lining mucosa” of the oral cavity which is non-keratinized. Some authors[2,15,27] have defended that the keratinized masticatory mucosa (hard palate, gingiva and lingual dorsum) remains unaffected in this pathology. Even so, several cases are described in these locations[25,32,33]. In addition to the intraoral involvement, Badham included lesions in the pharynx and esophagus[1].
The time the blister stays complete in the oral cavity is variable, from a few minutes to hours[2,8,14-16,35] or even days[32,34], and depends on the location and the size. When ruptured, generally spontaneously or while eating, its hematic content is emptied giving rise to an ulcerated area with minor symptomatology[2,3,16,19]. Martini et al[19], described the formation of petechiae in the periphery of the blister immediately after its rupture, which they suggest to be caused by a venous obstruction in the area, although it is unclear if it is a cause or a consequence of the blister. A similar event, although surrounding intact blisters, was described by Hopkins and Walter in 1985[15], defining it as an “ecchymotic halo”. Furthermore, Grinspan et al[3], described that the blood in the blister may occasionally be coagulated.
Although the blister is the defining lesion of ABH, it is frail and the patient might seek attention for an unspecific ulcer instead (Figure 1B)[10,16,19]. These ulcers heal within 7-14 d without leaving scars (Figure 2B)[2,3,16].
The recurrence of ABH lesions is frequent, between 25% and 100% of the cases[2,3,10,15,16,19,31], with the lesions appearing in the same location or on another area of the oral mucosa[3]. It is interesting that, despite most authors, Horie et al[17] show no recurrence in a series of 16 cases.
The frequency of recurrence of ABH is variable, with patients reporting lesions only once or twice per year while others show them continuously[2,3,15,16]. Recurrences for more than 24 years have been described[2,33].
The cases where the ABH lesions have been biopsied before its rupture show a subepithelial blister with a hematic content and an atrophic squamous epithelium surrounding the lesion[2]. A mild perilesional inflammatory infiltrate, generally chronic, is also observed[2,3]. In certain cases, an abundant acute subepithelial inflammatory infiltrate with a certain perivascular disposition has been described[34]. The biopsy of the ulcer formed after the rupture of the blister shows an unspecific ulcer with chronic inflammatory infiltrate, mainly lymphocytic[16].
Silver special staining has shown a decrease in elastic fibers in the chorion[29]. In addition, a capillary vascular hypertrophy, similar to that of patients with diabetes or porphyria, has been described[3].
Studies with direct immunofluorescence may be useful to rule out other oral vesiculo-bullous diseases of an immunological basis and with a poorer prognosis, such as Pempighus Vulgaris or Mucous Membrane Pemphigoid[14]. Unlike these diseases, direct immunofluorescence of ABH lesions is negative for IgA, IgG, IgM, fibrinogen and the C3 complement fraction. However, Stephenson et al[2] described certain basal positivity for IgG and C3 in some cases.
The differential diagnosis of ABH should be made with all vesiculo-bullous diseases of the oral cavity, including hematological disorders, mucocutaneous immunological pathology and cystic pathology.
Some hematological pathologies, such as thrombocytopenia or the von Willebrand Disease, may present lesions similar to ABH[14,37] Therefore, a complete blood test should always be performed, including coagulation tests which in these cases are altered, while in ABH are normal[3,14,16].
In addition to these pathologies, Serra et al[31] mention other hematological entities, including leukemia and vasculitis that should be considered in the differential diagnosis. In these cases, the lesions are usually multiple and widespread appearing in other locations of the body and generally producing systemic symptoms.
The mucocutaneous immunological diseases are the most important differential diagnosis of ABH and should include pemphigus vulgaris, mucous membrane pemphigoid, lineal IgA disease, epidermolysis bullosa acquisita and bullous amyloidosis[16]. All of these pathologies have a characteristic immunological basis and sometimes have clinical or even histological characteristics similar to ABH. The main clinical characteristics that differentiate these entities are shown in Table 2.
| Disease | Type of lesion | Content of the blister | Location | Cutaneous involvement | Involvement of other mucosal membranes |
| Angina bullosa hemorrhagica | Subepithelial blister | Hematic | LM (soft palate) | No | Oropharynx and esophageal |
| Mucous membrane pemphigoid[38] | Subepithelial blisters and vesicles | Serous and serohematic | MM and LM (gingiva) | Yes | Ocular, genital, oropharynx, nasal and esophageal |
| Pemphigus vulgaris[39] | Intraepithelial blisters and vesicles | Serous | MM and LM (areas of friction) | Yes | Nasal, ocular, esophageal, genital, pharyngeal |
| Linear IgA disease[40] | Subepithelial blisters and vesicles | Serous and serohematic | MM and LM | Yes | Ocular, nasal, genital |
| Epidermolysis bullosa acquisita[41] | Subepithelial blister | Serous, serohematic or hematic | MM and LM | Yes | Ocular, anal, vaginal, esophageal (depending on the subtype) |
| Bullous amyloidosis[42] | Subepithelial blister | Hematic | MM and LM | Yes | Not described |
To perform a correct differential diagnosis on these entities, a good medical history is essential, focusing on the presence of lesions in skin or other mucosal membranes[14]. The most important differential diagnosis for patients with an ABH ulcer is, without a doubt, pemphigus vulgaris.
In cases of solitary lesions showing the typical characteristics of ABH (acute onset and associated to a traumatic event) a biopsy is often unnecessary[17]. The histopathological analysis should be performed only in cases with multiple or recurrent lesions or on atypical lesions. In these cases, together with the conventional hematoxylin and eosin histopathological analysis, it is convenient to perform direct immunofluorescence for IgA, IgG, IgM and C3 in order to exclude other mucocutaneous processes[2,14,16].
The differential diagnosis with oral cystic pathologies includes superficial mucocele. This lesion often shows acute clinical features generating a subepithelial blister that initially contains mucus but, after traumatic events, may contain blood and be mistaken with ABH[43].
Finally, some genetic syndromes with blisters containing blood in the oral cavity and oropharynx, such as the Kindler syndrome[44] or the vascular type of the Ehler-Danlos syndrome, should be excluded[45]. It is important to consider that the lesions of ABH are only present in adults, while on these processes, they appear in young people.
Given the clinical characteristics of this disease, a specific treatment is unnecessary in most cases, recommending a symptomatic treatment of the lesions[2,3,15-17].
A complete blood test is necessary to rule out a possible systemic compromise while a histopathological analysis would be helpful in those cases with a complicated differential diagnosis.
The benign nature of the process should always be explained to the patients[2]. Given the possible traumatic etiology, this should be avoided by establishing general measures and eliminating all possible irritants[3,17]. Serra et al[31] recommend patients undergoing treatment with inhaled topical steroids to rinse with water after each use as a prevention measure of ABH.
In ABH patients with discomfort or pain, the treatment of the symptoms includes different drugs such as a mouthwash of benzydamine hydrochloride[2], several anti-inflammatory drugs[28], or even topical beclomethasone[32].
To avoid the superinfection of the ulcer resulting from the rupture of the blister, Hopkins and Walker[15] recommended rinsing with chlortetracycline. However, most authors[14,16,28], support the use of chlorhexidine gluconate mouthwashes in concentrations between 0.12%-0.25%.
To avoid possible recurrences, ascorbic acid and citroflavonoids have been suggested to be administered to the patients[3], without effective results reported.
The general prognosis for ABH is good; however, large lesions and on the soft palate and oropharynx may cause a feeling of suffocation due to a compromise of the upper airway, which leads the patient to seek urgent attention and even compromises his or her life[2,15,26,32,46]. Therefore, large blisters are recommended to be ruptured, mainly those located in the soft palate and oropharynx, as to decrease the possibility of causing obstruction of the upper airway and avoiding an unpleasant choking sensation on the patient[2,15-17,36].
The ABH is an uncommon disease of the oral cavity and oropharynx that should be considered when a blister with a hematic content is observed. It is important for the dentist to acknowledge this condition as to differentiate it from other oral vesicular processes with a poorer prognosis such as Pemphigus Vulgaris, Mucous Membrane Pemphigoid or certain hematological diseases.
P- Reviewer: Said SAM S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Badham NJ. Blood blisters and the oesophageal cast. J Laryngol Otol. 1967;81:791-803. [PubMed] [Cited in This Article: ] |
| 2. | Stephenson P, Lamey PJ, Scully C, Prime SS. Angina bullosa haemorrhagica: clinical and laboratory features in 30 patients. Oral Surg Oral Med Oral Pathol. 1987;63:560-565. [PubMed] [Cited in This Article: ] |
| 3. | Grinspan D, Abulafia J, Lanfranchi H. Angina bullosa hemorrhagica. Int J Dermatol. 1999;38:525-528. [PubMed] [Cited in This Article: ] |
| 4. | Haryng T. Verhandelungen X Section IV. 1890;. [Cited in This Article: ] |
| 5. | Baliña PL. Hemoflictenosis bucal traumatica. Rev Arg Dermatol. 1933;17:194-196. [Cited in This Article: ] |
| 6. | Antoni-Bach N, Couilliet D, Garnier J, Tortel MC, Grange F, Guillaume JC. [Case for diagnosis. Benign hemorrhagic bullous stomatitis]. Ann Dermatol Venereol. 1999;126:525-526. [PubMed] [Cited in This Article: ] |
| 7. | Scully C. The oral cavity. In: Rook AJ, Wilkinson DS, Ebling FJG, editors. Textbook of Dermatology. 5th ed. Oxford Scientific Publications 1992; 2732-2733. [Cited in This Article: ] |
| 8. | Kirtschig G, Happle R. Stomatopompholyx hemorrhagica. J Am Acad Dermatol. 1994;31:804-805. [PubMed] [Cited in This Article: ] |
| 9. | Mehrotra R, Thomas S, Nair P, Pandya S, Singh M, Nigam NS, Shukla P. Prevalence of oral soft tissue lesions in Vidisha. BMC Res Notes. 2010;3:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Rosa A, Geraldo Pappen F, Neutzing Gomes AP. Angina bullosahemorrhagica: a rare condition? RSBO. 2012;9:190-192. [Cited in This Article: ] |
| 11. | Deblauwe BM, van der Waal I. Blood blisters of the oral mucosa (angina bullosa haemorrhagica). J Am Acad Dermatol. 1994;31:341-344. [PubMed] [Cited in This Article: ] |
| 12. | Slezák R. Traumatic haemorragic bullae of the oral mucosa (angina bullosa haemorrhagica). Folia Gastroenterol Hepatol. 2005;3:122-127. [Cited in This Article: ] |
| 13. | Yamamoto K, Fujimoto M, Inoue M, Maeda M, Yamakawa N, Kirita T. Angina bullosa hemorrhagica of the soft palate: report of 11 cases and literature review. J Oral Maxillofac Surg. 2006;64:1433-1436. [PubMed] [Cited in This Article: ] |
| 14. | Stephenson P, Scully C, Prime SS, Daly HM. Angina bullosa haemorrhagica: lesional immunostaining and haematological findings. Br J Oral Maxillofac Surg. 1987;25:488-491. [PubMed] [Cited in This Article: ] |
| 15. | Hopkins R, Walker DM. Oral blood blisters: angina bullosa haemorrhagica. Br J Oral Maxillofac Surg. 1985;23:9-16. [PubMed] [Cited in This Article: ] |
| 16. | Giuliani M, Favia GF, Lajolo C, Miani CM. Angina bullosa haemorrhagica: presentation of eight new cases and a review of the literature. Oral Dis. 2002;8:54-58. [PubMed] [Cited in This Article: ] |
| 17. | Horie N, Kawano R, Inaba J, Numa T, Kato T, Nasu D, Kaneko T, Kudo I, Shimoyama T. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36. [PubMed] [Cited in This Article: ] |
| 18. | Edwards S, Wilkinson JD, Wojnarowska F. Angina bullosa haemorrhagica--a report of three cases and review of the literature. Clin Exp Dermatol. 1990;15:422-424. [PubMed] [Cited in This Article: ] |
| 19. | Martini MZ, Lemos CA, Shinohara EH. Angina bullosa hemorrhagica: report of 4 cases. Minerva Stomatol. 2010;59:139-142. [PubMed] [Cited in This Article: ] |
| 20. | Corson MA, Sloan P. Angina bullosa haemorrhagica: an unusual complication following crown preparation. Br Dent J. 1996;180:24-25. [PubMed] [Cited in This Article: ] |
| 21. | Singh D, Misra N, Agrawal S, Misra P. Angina bullosa haemorrhagica. BMJ Case Rep. 2013;2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Garlick JA, Calderon S. Oral blood blisters in angina bullosa haemorrhagica secondary to trauma of eating and dental injection. Br Dent J. 1988;165:286-287. [PubMed] [Cited in This Article: ] |
| 23. | O’Riordan BC. ‘Oral blood blisters in angina bullosa haemorrhagica secondary to trauma of eating and dental injection’. Br Dent J. 1989;166:7. [PubMed] [Cited in This Article: ] |
| 24. | Seoane Leston J, Gómez-Duaso J, Romero Méndez A, Ortíz Reina S, Aguado Santos A, Iglesias P. Angina bullosa hemorrágica: caso clínico y revisión de la literatura. Stoma (Lisb). 1991;19:43-48. [Cited in This Article: ] |
| 25. | Curran AE, Rives RW. Angina bullosa hemorrhagica: an unusual problem following periodontal therapy. J Periodontol. 2000;71:1770-1773. [PubMed] [Cited in This Article: ] |
| 26. | Hosain SI, Bounds G, Stanford J. Angina haemorrhagica bullosa causing respiratory obstruction postoperatively. Anaesthesia. 1991;46:422. [PubMed] [Cited in This Article: ] |
| 27. | de las Heras ME, Moreno R, Núñez M, Gómez MI, Ledo A. Angina bullosa hemorrhagica. J Dermatol. 1996;23:507-509. [PubMed] [Cited in This Article: ] |
| 28. | High AS, Main DM. Angina bullosa haemorrhagica: a complication of long-term steroid inhaler use. Br Dent J. 1988;165:176-179. [PubMed] [Cited in This Article: ] |
| 29. | Higgins EM, du Vivier AW. Angina bullosa haemorrhagica--a possible relation to steroid inhalers. Clin Exp Dermatol. 1991;16:244-246. [PubMed] [Cited in This Article: ] |
| 30. | Saravanan V, Bankar RN, Kumar S, Williams JG. Hemorrhagic bullae with nebulised ipratropium bromide. J Postgrad Med. 2006;52:235-236. [PubMed] [Cited in This Article: ] |
| 31. | Serra D, De Oliveira HS, Reis JP, Vieira R, Figueiredo A. Angina bullosa haemorrhagica: a disorder to keep in mind. Eur J Dermatol. 2010;20:509-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Pahl C, Yarrow S, Steventon N, Saeed NR, Dyar O. Angina bullosa haemorrhagica presenting as acute upper airway obstruction. Br J Anaesth. 2004;92:283-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Shashikumar B, Reddy RR, Harish M. Oral hemorrhagic blister: an enigma. Indian J Dermatol. 2013;58:407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Kurban M, Kibbi AG, Ghosn S. Expanding the histologic spectrum of angina bullosa hemorrhagica: report of one case. Am J Dermatopathol. 2007;29:477-479. [PubMed] [Cited in This Article: ] |
| 35. | Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of two cases. Indian J Dermatol. 2012;57:503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Yayli S, Yayli AY. Angina bullosa haemorrhagica. J Dtsch Dermatol Ges. 2012;10:436-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Abhinav C, Mahajan VK, Mehta KS, Chauhan PS. Angina bullosa hemorrhagica-like lesions: a rare presentation of drug-induced thrombocytopenia. Int J Dermatol. 2013;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Bagan J, Lo Muzio L, Scully C. Mucosal disease series. Number III. Mucous membrane pemphigoid. Oral Dis. 2005;11:197-218. [PubMed] [Cited in This Article: ] |
| 39. | Black M, Mignogna MD, Scully C. Number II. Pemphigus vulgaris. Oral Dis. 2005;11:119-130. [PubMed] [Cited in This Article: ] |
| 40. | Eguia del Valle A, Aguirre Urízar JM, Martínez Sahuquillo A. Oral manifestations caused by the linear IgA disease. Med Oral. 2004;9:39-44. [PubMed] [Cited in This Article: ] |
| 41. | Gupta R, Woodley DT, Chen M. Epidermolysis bullosa acquisita. Clin Dermatol. 2012;30:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Stoopler ET, Alawi F, Laudenbach JM, Sollecito TP. Bullous amyloidosis of the oral cavity: a rare clinical presentation and review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:734-740. [PubMed] [Cited in This Article: ] |
| 43. | Bermejo A, Aguirre JM, López P, Saez MR. Superficial mucocele: report of 4 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:469-472. [PubMed] [Cited in This Article: ] |
| 44. | Solanki SL, Jain A, Bhukal I, Samanta S. Anesthetic management in a patient with Kindler’s syndrome. Saudi J Anaesth. 2011;5:430-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 45. | Colebatch AN, Shaw EC, Foulds NC, Davidson BK. Hemorrhagic bullae of the oral mucosa as an early manifestation of vascular-type ehlers-danlos syndrome. J Clin Rheumatol. 2011;17:383-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Prabhakar Shashikala R. Angina bullosa haemorrhagica rare cause of upper airway obstruction. Emerg Med J. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |