INTRODUCTION
The synovium is the soft tissue lining diarthrodial joints, tendon sheaths and bursae. The normal synovium has intimal and subintimal layers and performs the critical homeostatic functions of non-adherence, control of synovial fluid volume and composition and contributes to chondrocyte nutrition. The synovial membrane is involved in several rheumatic diseases including rheumatoid arthritis (RA), osteoarthritis (OA), the spondyloarthritides and the crystal induced arthropathies; and is the primary target of inflammation in RA. Analyses of the synovial membrane have been central to our current understanding of the pathogenetic mechanisms in the inflammatory arthritides in addition to having provided insight into mechanisms of action of targeted therapies.
METHODS OF SYNOVIAL BIOPSY
The synovium is amenable to biopsy by arthroscopy or by using blind needle or ultrasound directed techniques[1]. Blind needle techniques have been established for decades and have a good safety and feasibility record[2]. They can be undertaken in an office setting, are relatively low-cost, and do not require special facilities. The major concerns of blind biopsy techniques lie in failure to obtain satisfactory samples, especially if the joint is clinically quiescent because of failure to visualize involved areas; in addition the joints that are amenable to biopsy by this technique are limited[2]. In one series with more than 800 Parker-Pearson biopsy procedures, sufficient tissue was obtained in about 85% of patients for histological examination; the authors found that the procedure failed especially in joints that were not swollen. There were no haemarthroses or infections reported in this series[3]. This failure rate, however would not be acceptable within the context of “proof of concept” phase IB or II RCTs in which arthroscopic synovial biopsy would be more acceptable[2].
Arthroscopic synovial biopsy (usually done with a small-bore arthroscope) is the “gold standard”[2]; it has the advantage of macroscopic examination, visually directed biopsies with better sampling from areas of interest, its major disadvantages being the need for a “learning curve” and the requirement of a sterile area and operation theatre facilities[2]. In addition arthroscopic biopsy techniques allow biopsies from sites adjacent to cartilage (the “cartilage-pannus junction”), an area that differs quantitatively and qualitatively from the synovium as opposed to needle biopsies in which this area is difficult if not impossible to access[1]. Complications with arthroscopies performed by rheumatologists are similar to those reported in the orthopaedic literature: in a study evaluating 16532 arthroscopies in which 50.5% and 49.5% of the arthroscopies had a clinical and research indication respectively revealed a complication rate of joint infection in 0.1%, wound infection in 0.1%, haemarthrosis in 0.9%, deep venous thrombosis in 0.2% and neurological damage, thrombophlebitis and other complications in 0.02%, 0.08% and 0.06% respectively. Irrigation volume correlated with wound infection rate and centres that performed cartilage biopsy had a higher rate of haemarthrosis[4]. Further information on details of arthroscopic synovial biopsy performed as a day procedure has been reviewed elsewhere[5].
Ultrasound guided synovial biopsy is a relatively recent technique which combines the advantage of being minimally invasive, enables sampling under guidance, and may be of particular utility in synovial biopsy of small joints[6,7].
MACROSCOPIC APPEARANCE OF THE SYNOVIUM
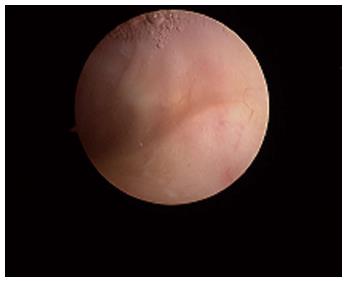
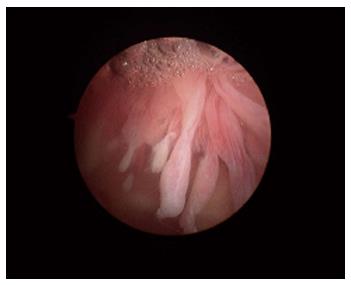
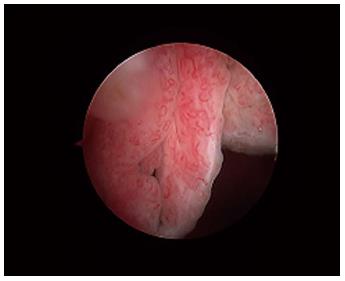
Macroscopically, the normal synovium (Figure 1) looks bland and devoid of villi, granularity or increased vascularity. In contrast, synovium in RA (Figure 2) has a distinct vascularity pattern with straight vessels as opposed to tortuous vessels or a mixed pattern seen in spondyloarthritides (SpA), reactive arthritis and psoriatic arthritis (Figure 3). The macroscopic appearance may predict histological changes (albeit with only a very moderate correlation) and clinical parameters with the straight pattern portending a worse outcome. However there is no widely accepted scoring system or well-validated method of description of macroscopic changes, and no reliable method to predict microscopic features, especially in an individual patient[1,6,8].
Figure 1 The normal synovium.
Figure 2 The synovium in rheumatoid arthritis.
Note the villous appearance and straight vessels.
Figure 3 The synovium in psoriatic arthritis.
Note the hypervascular villous hypertrophy and tortuous vessels.
THE UNDERLYING DISEASE PROCESS AND REPRESENTATIVENESS OF SYNOVIAL TISSUE
A single study[9] has examined arthroscopic synovial biopsies from the knee joint and wrists or metacarpophalangeal joints in a small number of patients and found that the numerous biopsy specimens correlated well with regards to qualitative and quantitative markers of cellular infiltrate.
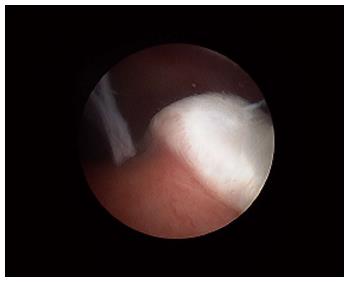
Various studies have also looked at whether there was significant variability of synovial tissue within regions of the same joint; this distinction is particularly relevant in the context of inflammatory arthritis such as RA where the cartilage-pannus (which is the inflamed tongue of abnormal synovium at the junction of the cartilage and bone and is thought to be central in causing bone destruction known as erosions; see Figure 4) junction may have a different cellular and cytokine profile as compared to synovium elsewhere. While some differences in certain cellular populations can be recognised, in general the features of inflammation and expression of mediators of inflammation and destruction are thought to be largely similar, though cellular markers responsible for bone destruction may be one notable exception[2,10,11].
Figure 4 The cartilage-pannus junction.
To minimise intra-joint variability and to maximise reliability it has been demonstrated that a minimum of six synovial biopsy specimens are necessary[2].
HANDLING OF SYNOVIAL TISSUE
The handling and processing of synovial tissue is largely dictated by the indication for the synovial biopsy. For routine histopathology by light microscopy, synovial biopsy samples should be fixed in 4% formalin and then embedded in paraffin. If infection is suspected, the tissue should be transported in suitable culture media. For detection of bacterial DNA using the PCR, it is critical to avoid contamination of the samples by foreign DNA and the samples should be snap frozen in liquid nitrogen[6]. The latter method is also preferred for synovial sample processing and storage in the research setting, and enables immunohistochemical staining to be performed without the need for antigen retrieval. For detection of crystals, synovial tissue needs to be sent in absolute alcohol (as crystals dissolve in most other fixatives)[6].
METHODS OF QUANTIFICATION OF CELLULAR, CYTOKINE, CHEMOKINE AND RECEPTOR EXPRESSION IN SYNOVIAL BIOPSIES
The histological assessment of synovium in RA includes intimal thickness and density and composition of the cellular infiltrate[1]. The preferred method to analyse cellular infiltrate, cell phenotype, cell surface receptor expression and cellular adhesion molecule expression is by immunohistochemical techniques. Quantification can be performed using either manual counting (reliable but time consuming), semiquantitative scoring (the fastest of all techniques with proven intra- and inter-observer reliability) or digital image analysis (expensive and time consuming but has been validated with regards to inter- and intra-observer reliability for the widest range of biological variables including cytokines, metalloproteinases, vascular markers, adhesion molecules and chemokines)[2]. The synovium can also be subject to analyses, by real time quantitative polymerase chain reaction (PCR) for gene expression profiling, and to potentially analyse all genes, expression microarrays can be used; the latter method has revealed considerable heterogeneity and identified molecularly distinct forms of RA[12,13].
NORMAL SYNOVIUM
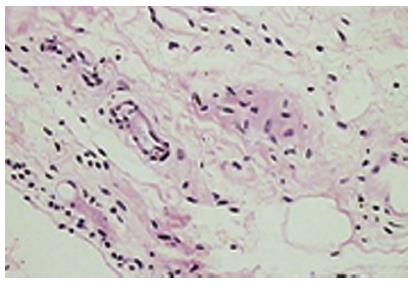
The normal synovium has an intimal layer, which is 20 to 40 μm thick in cross section, and an areolar subintima, which can be up to 5 mm in thickness; considerable variability in this pattern exists and three main types of synovia have been described on the basis of their subintima: fibrous, areolar and adipose[14]. Of these, areolar synovium (Figure 5) is the most specialized form. Often crimped into folds, the lining consists of 2-3 layers of cells. Beneath this layer are capillaries and further down a network of arterioles, venules and lymphatic vessels. Nerve fibres are present in association with blood vessels. The supporting tissue matrix is rich in type I collagen which forms the physical membrane. Still deeper is a loose layer that enables the synovial membrane to move freely[15-18]. The adipose synovium occurs as fat pads and within villi; the deeper tissue is fat, there is a superficial net of capillaries and a complete intimal layer. Fibrous synovium can be difficult to accurately define and consists of fibrous tissue such as ligament or tendon on which lies an intimal layer of cells.
Figure 5 The normal (areolar) synovium.
The synovium is devoid of a true basement membrane. Most of the soluble components and proteins in synovial fluid exit the synovial microcirculation through pores or fenestration in the vascular endothelium, traverse the interstitium and then enter the joint space. A rich microvascular network lies beneath the synovial surface with the larger venules, arterioles and lymphatics forming an anastomosing quadrilateral array[15,17,18]. The subintima in health, is generally devoid of inflammatory cells apart from a few macrophages and mast cells.
Recent evidence[19] from an animal model suggests that cadherin-11 (cadherins are cell-cell adhesion molecules controlling animal morphogenesis) on synovial fibroblasts may be critical in the development of a recognisable synovial lining by facilitation of cellular organization, compaction, and matrix development and may be critical for normal synovial tissue development.
Synovial intimal cells are of two types: type A cells (macrophages) and type B cells (fibroblasts). Evidence suggests that synovial macrophages are true macrophages derived from bone marrow derived precursors in contrast to synovial fibroblasts which are locally derived. In health, synovial fibroblasts are the dominant population (in disease states, macrophages may constitute as much as 80% of the intimal cells)[20-24]. Synovial fibroblasts are adapted to hyaluronan production and demonstrate high activity of the enzyme uridine diphosphoglucose dehydrogenase which is essential for synthesis of hyaluronan[25]. Synovial intimal fibroblasts express CD55; this feature distinguishes them from CD68 positive (but CD55 negative) intimal macrophages.
SYNOVIUM IN DISEASE STATES
RA
Microvascular changes occur very early in the course of the disease. As noted above, synovial blood vessels in RA are relatively straight in contrast to tortuous and bushy appearance in early psoriatic arthritis and SpA[26].
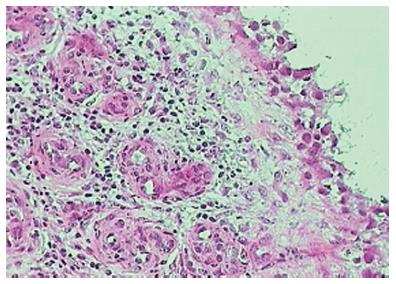
As early as four to six weeks following the onset of symptoms of RA, synovial lining layer thickening of up to 10 cells is noted along with vascular proliferation and diffuse subintimal inflammatory infiltrate consisting of macrophages, lymphocytes, neutrophils, mast cells and dendritic cells (DCs)[27]. Synovial fibroblast proliferation and macrophage recruitment are both thought to be contributors to the lining layer thickening[28] and are of particular importance as they are both implicated in engendering joint damage[29-31]. Importantly, cellular changes in joints are well evident and established before the clinical onset of disease[32] and asymptomatic joints also show cellular changes mainly in the form of lining layer hyperplasia, though to a lesser degree than in clinically involved joints[33]. While most of the cellular subtypes (including monocytes, macrophages, lymphocytes and plasma cells thought to be more typical of established disease) are similar in early and late disease (including the degree of lymphocytic, plasma cell and neutrophil infiltration), lymphoid follicles (Figure 6) are generally a feature of established RA as compared to early disease[26,34,35]. Of note, “early” arthritis is a clinical and not a histological definition and changes at the synovial level precede clinical symptoms by an undetermined period of time[36].
Figure 6 Lymphoid follicles in rheumatoid arthritis.
In general, there is no definite evidence to suggest a distinct histopathological pattern in early as opposed to late disease; the differences observed may relate not to disease duration but how active the disease was at the time of sampling: this was illustrated in a well-designed study[37] that included 16 patients with early RA (and similar numbers with late RA, early SpA and OA). In this study except for maximal synovial lining thickness, no difference was found between early and late RA, though differences were found between RA and SpA/OA.
Spondyloarthritides including psoriatic arthritis
In the study alluded to above[37], patients with SpA showed similar degree of synovial villous hypertrophy in RA vs SpA, with a clear difference in vascular pattern. The mean synovial intimal layer thickness was similar. On microscopy vascularity was increased and few had lymphoid follicles. Another study[38] confirmed the increased vascularity of the psoriatic arthritic synovium as compared to RA and found less intimal layer hyperplasia, fewer macrophages but similar numbers of B cells, T cells and T cell subsets. There has been increasing interest recently in the Th-17 T cell pathway and related cytokines in the spondyloarthritides; despite therapeutic success of drugs targeting this pathway[39] and demonstration of increased frequency of IL-23 positive cells in facet joints of ankylosing spondylitis[40], this pathway probably has diverse roles in the pathogenesis of psoriatic SpA, with little or no expression of IL-22 in the psoriatic synovium[41].
Synovium as an immunologic target; synovial response to therapy in the inflammatory arthritides and targeted therapy
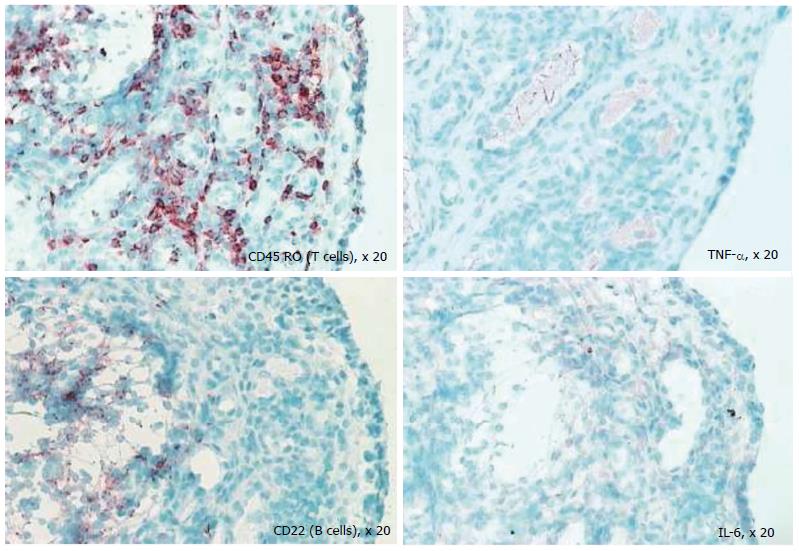
Despite the fact that the synovium is the primary target of many inflammatory arthritides, synovial tissue specific antigens that may elicit an immune response per se are virtually unknown; when the rheumatic diseases are associated with autoantibodies, the antigens involved are ubiquitous. One of the most important reasons in undertaking a study of the synovium in health and disease is to gain insight into immunopathologic processes that may suggest a therapeutic target in the inflammatory arthritides and identify biomarkers to predict outcomes in individual patients. Several studies have used serial synovial biopsies to attempt to identify biomarkers; these studies have evaluated responses to corticosteroids, gold, methotrexate, leflunomide, tumour necrosis factor inhibitors and rituximab, among others[42-46]. It is now well established that the inflammatory arthritides are very heterogeneous; indeed RA is now considered to be an umbrella term encompassing multiple disease subtypes with different phenotypes correlating to different genotypes. This heterogeneity[13,47] perhaps explains the variable response to therapy. It remains unexplained why some patients with RA fail to respond to standard and biologic disease modifying therapy; in addition, a proportion of patients initially respond only to lose response later. The exact reasons for this remain elusive. Studies in our laboratory (Figure 7) show that at the level of the synovial cellular infiltrate, the dominance of infiltrate is markedly heterogeneous and therapeutic selections that are not individualised may be one explanation for primary or secondary non-response to therapy.
Figure 7 Differential staining of synovial biopsy for T cells, tumour necrosis factor, B cells (CD22), and interleukin-6 in a patient with active rheumatoid arthritis prior to disease-modifying therapy.
TNF: Tumour necrosis factor; IL: Interleukin.
Synovial proliferative conditions
Pigmented villonodular synovitis (PVNS) is a nodular and villous thickening of the synovial membrane that affects males and females equally with the knee being the most commonly involved joint[48]. Microscopy reveals synovial intimal cells overlying a lobular and sheet-like arrangement of mononuclear rounded (containing large hemosiderin granules) and epithelioid cells, multinucleated osteoclast-like giant cells, and lipid rich cells; an extra-articular analogue of PVNS is the giant cell tumour of the tendon sheath. Haemosiderotic synovitis (which usually happens as a complication of chronic intra-articular haemorrhage in haemophiliacs) differs from PVNS; the synovium lacks the thickened fronds and nodules of PVNS and becomes thickened and opaque due to repeated scarring[48].
Synovial chondromatosis is characterised by multiple nodules of metaplastic hyaline cartilage that are present in the synovial sublining and often have foci of calcification or endochondral ossification[48].
Crystal induced synovial disease: Gout and Calcium pyrophosphate dehydrate deposition disease (pseudogout)
Monosodium urate crystal deposits in the synovium appear as creamy white tophi (which demonstrate needle shaped crystals that are strongly negatively birefringent on polarised light microscopy) and lead to membrane opacification and papillary thickening; histological examination of the synovial membrane in acute gout shows lining layer hyperplasia and intense infiltration with neutrophils, mononuclear cells and lymphocytes[48-50]. Synovial sections can also be stained with the DeGolantal stain for urate to identify gouty synovitis[6]. Calcium pyrophosphate dehydrate (CPPD) disease, in contrast also involves cartilage; CPPD crystals are rhomboid shaped and positively birefringent on polarised light microscopy. Synovial deposits are usually smaller than gout, are associated with foci of metaplastic cartilage and usually elicit a histiocytic reaction[48].
Infectious and granulomatous, infiltrating and other deposition diseases
Synovial biopsies can assist in diagnosing bacterial species when blood and synovial cultures are negative especially when antibiotic treatment has been initiated[51] before culturing synovial fluid and with fastidious and difficult to grow bacteria[52]. Synovial biopsies may also help in diagnosis of granulomatous, infiltrating and deposition diseases including amyloidosis, sarcoidosis, arthritis caused by foreign body material and Erdheim-Chester disease[6].
CONCLUSION
Synovial biopsy is a safe and generally well-tolerated procedure; of the several methods used to obtain synovial biopsies, arthroscopic techniques, though more complicated, allow macroscopic examination and have a consistent and higher yield especially after effective therapy when synovial tissue volume has reduced.
Analyses of the synovial membrane provides useful insight into the etiopathogenesis of and direct evidence about events in the synovial tissue in various arthritides; in particular, evaluation of serial biopsy specimens has been very useful in understanding the effects of targeted therapies. In addition, synovial biopsies have a role in the diagnostic evaluation of neoplastic or granulomatous disease or infection when synovial fluid analysis is non-contributory. Finally, synovial biopsies are invaluable as a research tool for proof of concept studies to assess efficacy and mechanisms of new therapies, provide tissue for in-vitro studies, proteomics and microarrays and allow evaluation for biomarkers that may help predict response to therapy and identify new targets for drug development.
P- Reviewer: Fenichel I, Malik MHA S- Editor: Song XX L- Editor: A E- Editor: Wu HL