Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.316
- This article has been retracted.
- Retraction in: World J Orthop. Mar 18, 2018; 9(3): 58-59 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Revised: July 7, 2013
Accepted: July 12, 2013
Published online: October 18, 2013
AIM: To examine the effects of treatment with risedronate for 1 year on speed of sound (SOS) of the calcaneus and bone turnover markers in postmenopausal women with osteoporosis.
METHODS: Thirty-eight postmenopausal women with osteoporosis who had been treated with risedronate for > 1 year were enrolled in the study. The SOS and bone turnover markers were monitored during treatment with risedronate for 1 year.
RESULTS: The urinary levels of cross-linked N-terminal telopeptides of type I collagen and serum levels of alkaline phosphatase were significantly decreased at 3 mo (-34.7%) and 12 mo (-21.2%), respectively, compared with the baseline values. The SOS increased modestly, but significantly by 0.65% at 12 mo compared with the baseline value. Treatment with risedronate elicited an increase in the SOS of the calcaneus exceeding the coefficient of variation in vivo (0.27%).
CONCLUSION: The present study confirmed that risedronate suppressed bone turnover and elicited a clinically significant increase in the SOS of the calcaneus in postmenopausal women with osteoporosis.
Core tip: The effects of risedronate treatment on quantitative ultrasound parameters of the calcaneus remain to be established in patients with osteoporosis. The aim of the present clinical practice-based observational study was to examine the effects of treatment with risedronate for 1 year on speed of sound (SOS) of the calcaneus and bone turnover markers in postmenopausal women with osteoporosis. The present study confirmed that risedronate suppressed bone turnover and elicited a clinically significant increase in the SOS of the calcaneus in postmenopausal women with osteoporosis.
- Citation: Iwamoto J, Takada T, Sato Y, Matsumoto H. Effect of risedronate on speed of sound in postmenopausal women with osteoporosis. World J Orthop 2013; 4(4): 316-322
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/316.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.316
Osteoporosis mostly affects postmenopausal women and substantially increases their risk of fracture. Risedronate is widely used as the first-line drug for treating postmenopausal osteoporosis because of its efficacy, demonstrated in the Vertebral Efficacy with Risedronate Therapy Study, the Hip Intervention Program Study, and a systematic review of 11 randomized controlled trials (RCTs), in which it reduced the incidence of vertebral, non-vertebral, and hip fractures[1-4].
Because risedronate increases the bone mineral density (BMD) of the lumbar spine, femoral neck, and total hip in postmenopausal women with osteoporosis[1,2], the BMD measured by dual-energy X-ray absorptiometry (DXA) remains the optimal method for monitoring the response to risedronate treatment. Quantitative ultrasound (QUS) is a more recently developed non-invasive method to determine bone density and structure in vivo. QUS parameters, including speed of sound (SOS), broadband ultrasound attenuation, and stiffness index, can predict the risk of hip, wrist, and total non-vertebral fractures up to 10 years later[5]. QUS may also provide a better assessment of the structural changes of bone compared with DXA[6].
The SOS of the calcaneus can be measured using a QUS device (CM-200; Elk Corp., Osaka, Japan). Recently, we reported the effects of 1 year of treatment with alendronate treatment on the SOS as well as bone turnover markers in Japanese postmenopausal women with osteoporosis[7]. In that study, alendronate reduced the urinary levels of cross-linked N-terminal telopeptides of type Icollagen (NTX) and serum levels of alkaline phosphatase (ALP), and modestly increased the SOS. To date, however, very few studies have examined the effects of risedronate on QUS parameters in postmenopausal women with osteoporosis. We hypothesized that risedronate, similar to alendronate, would increase the SOS at the calcaneus in postmenopausal women with osteoporosis. Therefore, the aim of the present clinical practice-based observational study was to examine the effects of 1 year of treatment with risedronate on the SOS and bone turnover markers in Japanese postmenopausal women with osteoporosis. We also discuss the differential effects of risedronate and alendronate on the SOS and bone turnover markers in Japanese postmenopausal women with osteoporosis.
Thirty-eight Japanese postmenopausal women with osteoporosis who had been treated with risedronate (17.5 mg weekly) for more than 1 year were recruited at the outpatient clinic of Hiyoshi Medical Clinic (Kanagawa, Japan) during the 6-month period between July 1 and December 31, 2012. This dose of risedronate is the dose used in Japan to treat osteoporosis in postmenopausal women, and has shown safety and efficacy[8-11]. Patients were eligible if they had postmenopausal osteoporosis defined according to the Japanese diagnostic criteria[12,13] as: (1) BMD < 70% of the young adult mean (YAM) or the “presence” of osteopenia on X-ray images of the spine; and (2) BMD of 70%-80% of the YAM or “possible” osteopenia on X-ray images of the spine together with a history of osteoporotic fractures. Because DXA of the spine is useful for monitoring osteoporosis in Japanese women, and QUS appears to be less useful[14], the diagnosis of osteoporosis was made using both the SOS (< 70% of the YAM or 70%-80% of the YAM together with a history of osteoporotic fractures) and X-ray findings of the spine (i.e., presence of osteopenia or possible osteopenia along with a history of osteoporotic fractures). Patients were excluded if they had a history of reflux esophagitis, gastric or duodenal ulcer, gastrectomy, renal failure, and bone diseases, including cancer-induced bone loss because of aromatase inhibitors, primary hyperparathyroidism, hyperthyroidism, Cushing’s syndrome, multiple myeloma, Paget’s disease of the bone, rheumatoid arthritis, or osteogenesis imperfecta.
The assessment performed before starting risedronate treatment included a medical history, physical examination, plain radiography of the thoracic and lumbar spine, measurement of the SOS of the calcaneus, and blood (e.g., serum calcium, phosphorus, and ALP) and urinary (e.g., NTX) biochemical tests. The urinary NTX levels were measured at 3 mo after starting treatment. The serum levels of calcium, phosphorus and ALP, and the SOS of the calcaneus were measured every 6 mo after starting treatment. Plain X-rays of the thoracic and lumbar spine were taken after 1 year of treatment. We evaluated the outcome of risedronate treatment for 1 year.
Plain lateral X-ray films of the thoracic and lumbar spine were obtained at the start of treatment to detect evidence of morphometric vertebral fractures. According to the Japanese criteria, a vertebral fracture is defined according to the vertebral height on lateral X-ray films[12,13]. Briefly, the vertebral height is measured at the anterior (A), central (C), and posterior (P) parts of the vertebral body. The presence of a vertebral fracture was defined as: (1) a reduction in the vertebral height of > 20% (A, C, and P) as compared with the height of the adjacent vertebrae; (2) the C/A or C/P ratio is < 0.8; or (3) the A/P ratio is < 0.75. Vertebral fractures were assessed at the T4-L4 level.
Low-traumatic osteoporotic clinical fractures were assessed. Clinical vertebral fractures were determined based on the clinical symptoms and findings of radiographic or magnetic resonance images of the lumbar and thoracic spine. Non-vertebral fractures, including major osteoporotic fractures of the distal radius, proximal humerus, and hip, were determined based on the clinical symptoms and radiographic images of the wrist, shoulder and hip joints, respectively.
Serum and urine samples were sent to Kotobiken Medical Laboratories, Inc. (Yokohama, Kanagawa, Japan) for the following biochemical analyses. Serum calcium and phosphorus levels were measured using standard laboratory techniques. Serum ALP levels were measured using the JSCC reference methods. The coefficient of variation (CV = 100 × standard deviation/mean) of two consecutive measurements made within 1 d was < 1.15% for 20 people. The CV of two measurements at the same time on two consecutive days was < 4.08% for 6 people. Urinary NTX levels were measured using an enzyme-linked immunosorbent assay. The CV of two consecutive measurements made within 1 d was < 7.4% for 10 people. The CV of two measurements made at the same time on two consecutive days was < 15.0% for 24 people.
The SOS of the left calcaneus was measured using a QUS device (CM-200; Elk Corp., Osaka, Japan). The reliability and reproducibility of this QUS device have already been reported, and the CV was 0.15% using the phantom technique and 0.27% in vivo[15].
Data are expressed as mean ± SD. One-way analysis of variance (ANOVA) with repeated measurements was used to determine the significance of the longitudinal changes in the SOS and biochemical markers. Univariate regression analysis was used to determine associations between the change in urinary NTX at 3 mo and the changes in the SOS at 6 and 12 mo. All statistical analyses were performed using StatView-J5.0 software (SAS Institute, Cary, NC, United States) on a Windows computer. A significance level of P < 0.05 was used in all comparisons.
Table 1 shows the anthropometry, SOS, and biochemical markers of the study subjects at the start of treatment. The mean age of the subjects was 71.1 years (range: 49-88 years). The mean SOS was 1473 m/s, which corresponds to 68.9% of the YAM. The mean serum calcium, phosphorus, and ALP levels were 9.2 mg/dL, 3.5 mg/dL, and 229 IU/L, respectively, which were within the normal ranges (8.4-10.2 mg/dL, 2.5-4.5 mg/dL, and 100-340 IU/L, respectively). However, the mean urinary NTX level was 56.2 nmol bone collagen equivalent (BCE)/mmol Cr, which was higher than the normal range for Japanese women (9.3-54.3 nmol BCE/mmol Cr)[16], indicating a high bone turnover in these women, a characteristic of osteoporosis.
| mean ± SD | Range | |
| Age (yr) | 71.1 ± 9.7 | 49-88 |
| Height (m) | 1.54 ± 0.06 | 1.40-1.70 |
| Body weight (kg) | 53.2 ± 7.6 | 40-80 |
| Body mass index (kg/m2) | 22.4 ± 3.1 | 18.7-35.0 |
| SOS (m/s) | 1473 ± 13 | 1442-1500 |
| SOS as % of YAM | 68.9 ± 5.9 | 53-79 |
| Calcium (mg/dL) | 9.2 ± 0.4 | 8.6-10.2 |
| Phosphorus (mg/dL) | 3.5 ± 0.3 | 2.9-4.5 |
| ALP (IU/L) | 229 ± 63 | 142-365 |
| Urinary NTX (nmol BCE /mmol Cr) | 56.2 ± 17.8 | 35.2-99.9 |
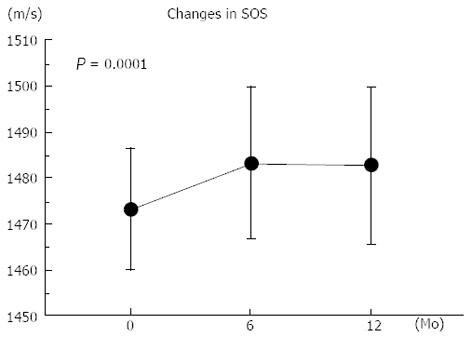
Figure 1 shows the changes in the SOS of the calcaneus. One-way ANOVA with repeated measurements showed a significant longitudinal increase in the SOS at 1 year (P = 0.0001). The mean percent changes in the SOS from the baseline after 6 and 12 mo of treatment were +0.68% and +0.65%, respectively (Table 2), which were beyond the coefficient of variation in vivo (0.27%)[15].
| Baseline | 3 mo | 6 mo | 12 mo | |
| SOS (m/s) | 1473 ± 13 | 1483 ± 16 | 1483 ± 17 | |
| Percent changes from baseline | 0.68% ± 1.10% | 0.65% ± 1.24% | ||
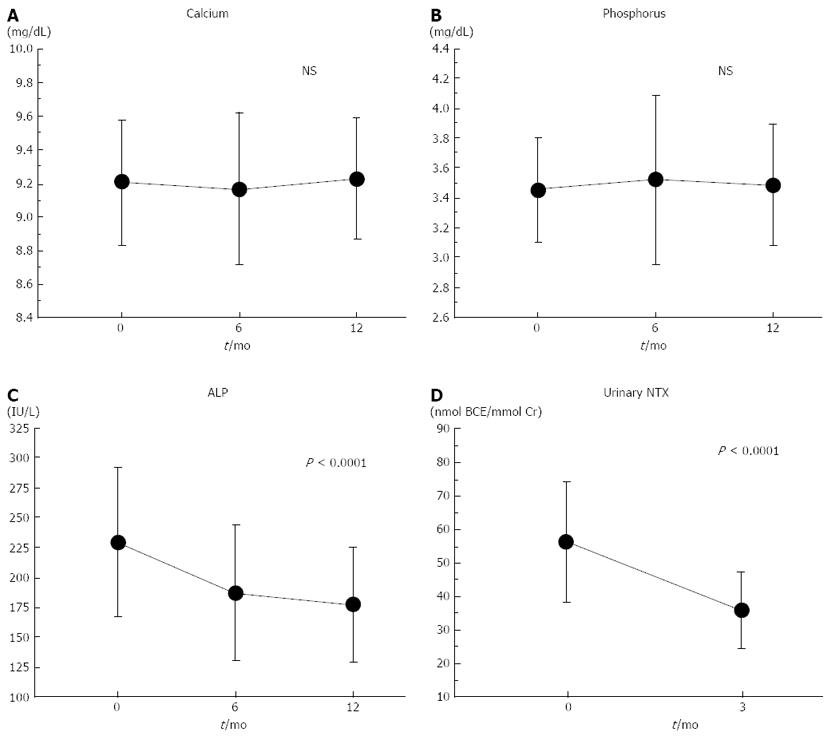
| Calcium (mg/dL) | 9.2 ± 0.4 | 9.2 ± 0.5 | 9.2 ± 0.4 | |
| Percent changes from baseline | −0.43% ± 3.36% | 0.30% ± 3.68% | ||
| Phosphorus (mg/dL) | 3.5 ± 0.3 | 3.5 ± 0.6 | 3.5 ± 0.4 | |
| Percent changes from baseline | 2.48% ± 17.6% | 1.17% ± 9.85% | ||
| ALP (IU/L) | 229 ± 63 | 187 ± 57 | 177 ± 48 | |
| Percent changes from baseline | −17.2% ± 16.7% | − 21.2% ± 16.7% | ||
| Urinary NTX (nmol BCE/mmol Cr) | 56.2 ± 17.8 | 35.9 ± 11.3 | ||
| Percent changes from baseline | −34.7% ± 15.0% |
Figure 2 shows the changes in the biochemical markers. The mean urinary NTX levels decreased to the normal range for Japanese women (9.3-54.3 nmol BCE/mmol Cr)[16] after 3 mo of treatment. The mean serum ALP levels also decreased and remained within the normal range (135-340 IU/L) during the 1-year treatment period. One-way ANOVA with repeated measurements showed significant longitudinal decreases in the serum ALP and urinary NTX levels (both, P < 0.0001). There were no significant longitudinal changes in the serum calcium or phosphorus levels. The mean percent change in the urinary NTX level from the baseline after 3 mo of treatment was -34.7% (Table 2), while those for serum ALP levels after 6 and 12 mo of treatment were -17.2% and -21.2%, respectively (Table 2).
Univariate regression analysis showed no significant associations between the percent decrease in urinary NTX at 3 mo and the percent increase in the SOS at either 6 or 12 mo.
During the 1-year treatment period, one patient experienced a rib fracture and one patient experienced a morphometric vertebral fracture.
The present study confirmed that treatment with risedronate decreased the urinary NTX and serum ALP levels (by -34.7% at 3 mo and -21.2% at 12 mo, respectively), and elicited a modest increase in the SOS of the calcaneus (by +0.68% at 6 mo and +0.65% at 12 mo) in Japanese postmenopausal women with osteoporosis. The objectives of this study were to determine: (1) whether decreases in bone turnover markers would be similar to those reported in our previous studies; and (2) whether the increase in the SOS of the calcaneus would be significant and greater than the range of reproducibility. We also compared the effects of risedronate and alendronate on the changes in these parameters.
Urinary NTX levels were measured at 3 mo after starting treatment, because measurement of urinary NTX levels at this time helps to assess whether the antiresorptive effects of risedronate (2.5 mg daily and 17.5 mg weekly) are sufficient or clinically significant[8,11]. Previous RCTs showed that risedronate together with calcium supplementation decreased urinary NTX (by about -38% to -40% at 3 mo) and serum ALP (by about -28% to -30% at 1 year) in Japanese postmenopausal women with osteoporosis[8,9,11]. The decreases in urinary NTX and serum ALP levels in the present study were slightly smaller than those in the previous RCTs. One reason for this discrepancy is that calcium supplementation was not used in the present study unlike in the previous RCTs. Nevertheless, this clinical practice-based observational study confirmed that treatment with risedronate for 1 year suppressed bone turnover in postmenopausal women with osteoporosis. Optimal vitamin D repletion is thought to be necessary to maximize the response to antiresorbers in terms of BMD changes and reducing the risk of fracture in postmenopausal women with osteoporosis[20]. Thus, improvements in vitamin D status may be necessary for greater response of the SOS and bone turnover markers to risedronate.
The mean age of the study subjects at the start of treatment was 71.1 years. The reference values of the SOS of the calcaneus in healthy Japanese women aged 65-69, 70-74, and 75-79 years are 1487, 1481, and 1475 m/s, respectively[21]. Treatment with risedronate for 1 year increased the SOS of the calcaneus from 1473 m/s at the start of treatment to 1483 m/s at 12 mo. Therefore, it seems that risedronate might help to increase the SOS of the calcaneus in postmenopausal women with osteoporosis. The percent increase in SOS from the baseline was +0.65% at 12 mo. Although this increase appeared to be modest, it was likely to exceed the CV of the SOS of the calcaneus in vivo (0.27%)[15].
We previously reported the effects of 1 year of treatment with alendronate (35 mg weekly) on the SOS and bone turnover markers in postmenopausal women with osteoporosis (mean age: 69.0 years)[7], In that study, alendronate reduced urinary NTX levels (by -44.9% at 3 mo) and increased the SOS (by +0.6% at 12 mo). In the present study, 1 year of treatment with risedronate (17.5 mg weekly) decreased the urinary NTX levels (by -34.7% at 3 mo) and increased the SOS of the calcaneus (by +0.65% at 12 mo) in postmenopausal women with osteoporosis (mean age: 71.1 years). In prior Japanese RCTs in postmenopausal women with osteoporosis, 1 year of treatment with alendronate (5 mg daily and 35 mg weekly) increased BMD of the lumbar spine by 5.8%-6.4% from baseline[22-24], while risedronate (2.5 mg daily and 17.5 mg weekly) increased it by 4.9%-5.9%[8-11]. The reductions in levels of bone turnover markers were also greater with alendronate than with risedronate[8-11,22-24]. Thus, alendronate may be elicit greater reductions in bone turnover and greater increases in BMD of the lumbar spine in postmenopausal Japanese women with osteoporosis compared with risedronate. However, the increase in the SOS was very similar with both treatments, suggesting that risedronate and alendronate have similar effects on bone structure and quality.
Dufresne et al[25] investigated the effect of 1 year of treatment with risedronate on bone structure by analyzing iliac crest bone biopsy specimens from women enrolled in a double-blind, placebo-controlled study of risedronate for the prevention of early postmenopausal bone loss using three-dimensional microcomputed tomography. The placebo group experienced decreases in bone volume (placebo: -5.1%, risedronate: +3.5%), trabecular thickness (placebo: -20 μm, risedronate: +23 μm), and trabecular number (placebo: -0.223 mm-1, risedronate: +0.099 mm-1), and increases in percent plate (placebo: +2.79%, risedronate: -3.23%), trabecular separation (placebo: +79 μm, risedronate: -46 μm) and marrow star volume (placebo: +2.80 mm3, risedronate: -2.08 mm3) as compared with the risedronate group. These changes in the trabecular structure appeared to partly reflect changes in the SOS.
There was no further increase in the SOS after 6 mo of treatment with risedronate, although serum ALP levels continued to decrease. However, the reduction in the serum ALP levels were blunted after 6 mo (229 IU/L at the baseline, 187 IU/L at 6 mo, and 177 IU/L at 12 mo). It has been reported that the anti-fracture effect of risedronate against clinical vertebral fractures is recognized as early as 6 mo after the start of treatment in postmenopausal women with osteoporosis[26], suggesting the rapid skeletal effects of risedronate. It was likely that risedronate rapidly improved the trabecular architecture by suppressing bone turnover, thereby increased the SOS from the baseline, and maintained the trabecular structure thereafter.
There are several limitations of the present study. In particular, the statistical quality of the present analyses may be relatively poor because of the small sample size, the absence of statistical power for the fracture incidence, and the retrospective nature of the analyses. Further studies are needed to confirm our results.
In conclusion, the present study confirmed that risedronate suppresses bone turnover, producing a modest but significant increase in the SOS of the calcaneus in Japanese postmenopausal women with osteoporosis. The results of this study and our previous studies suggest risedronate and alendronate have similar beneficial effects on the SOS of the calcaneus.
The effects of risedronate treatment on quantitative ultrasound (QUS) parameters of the calcaneus remain to be established in patients with osteoporosis.
The aim of the present clinical practice-based observational study was to examine the effects of treatment with risedronate for 1 year on speed of sound (SOS) of the calcaneus and bone turnover markers in postmenopausal women with osteoporosis.
The urinary levels of cross-linked N-terminal telopeptides of type I collagen (NTX) and serum levels of alkaline phosphatase were significantly decreased at 3 mo (-34.7%) and 12 mo (-21.2%), respectively, compared with the baseline values. The SOS increased modestly, but significantly by 0.65% at 12 mo compared with the baseline value. Treatment with risedronate elicited an increase in the SOS of the calcaneus exceeding the coefficient of variation in vivo (0.27%).
The present study confirmed that risedronate suppressed bone turnover and elicited a clinically significant increase in the SOS of the calcaneus in postmenopausal women with osteoporosis.
This study convincingly demonstrates that risedronate slightly increases the speed of sound in the calcaneous, as well as reduce the rate of bone turnover based on lower concentrations of circulating collagen and alkaline phosphatase. The methodology is sound and sufficiently detailed, the results are clearly presented, and the conclusions are supported by the results.
P- Reviewers Elder SH, Lee KH S- Editor Wen LL L- Editor A E- Editor Wang CH
| 1. | Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, Brown J, Eriksen EF, Hoseyni MS. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352. [PubMed] [Cited in This Article: ] |
| 2. | Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91. [PubMed] [Cited in This Article: ] |
| 3. | McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333-340. [PubMed] [Cited in This Article: ] |
| 4. | Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;23:CD004523. [Cited in This Article: ] |
| 5. | Fujiwara S, Sone T, Yamazaki K, Yoshimura N, Nakatsuka K, Masunari N, Fujita S, Kushida K, Fukunaga M. Heel bone ultrasound predicts non-spine fracture in Japanese men and women. Osteoporos Int. 2005;16:2107-2112. [PubMed] [Cited in This Article: ] |
| 6. | Wüster C, Heilmann P, Pereira-Lima J, Schlegel J, Anstätt K, Soballa T. Quantitative ultrasonometry (QUS) for the evaluation of osteoporosis risk: reference data for various measurement sites, limitations and application possibilities. Exp Clin Endocrinol Diabetes. 1998;106:277-288. [PubMed] [Cited in This Article: ] |
| 7. | Iwamoto J, Takada T, Sato Y, Matsumoto H. Influence of treatment with alendronate on the speed of sound, an ultrasound parameter, of the calcaneus in postmenopausal Japanese women with osteoporosis: a clinical practice-based observational study. Ther Clin Risk Manag. 2012;8:287-293. [PubMed] [Cited in This Article: ] |
| 8. | Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K. A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporos Int. 2002;13:971-979. [PubMed] [Cited in This Article: ] |
| 9. | Shiraki M, Fukunaga M, Kushida K, Kishimoto H, Taketani Y, Minaguchi H, Inoue T, Morita R, Morii H, Yamamoto K. A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group). Osteoporos Int. 2003;14:225-234. [PubMed] [Cited in This Article: ] |
| 10. | Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, Kaneda K, Morii H, Nawata H, Yamamoto K. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab. 2004;22:469-478. [PubMed] [Cited in This Article: ] |
| 11. | Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y. Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab. 2006;24:405-413. [PubMed] [Cited in This Article: ] |
| 12. | Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Tanaka H, Ikai T. Diagnostic criteria of primary osteoporosis. J Bone Miner Metab. 1998;16:139-150. [DOI] [Cited in This Article: ] |
| 13. | Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331-337. [PubMed] [Cited in This Article: ] |
| 14. | Ito M, Nishida A, Kono J, Kono M, Uetani M, Hayashi K. Which bone densitometry and which skeletal site are clinically useful for monitoring bone mass? Osteoporos Int. 2003;14:959-964. [PubMed] [Cited in This Article: ] |
| 15. | Yamaguchi T, Yamamoto M, Kanazawa I, Yamauchi M, Yano S, Tanaka N, Nitta E, Fukuma A, Uno S, Sho-no T. Quantitative ultrasound and vertebral fractures in patients with type 2 diabetes. J Bone Miner Metab. 2011;29:626-632. [PubMed] [Cited in This Article: ] |
| 16. | Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Chaki O. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005;23:97-104. [PubMed] [Cited in This Article: ] |
| 17. | Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491. [PubMed] [Cited in This Article: ] |
| 18. | Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267-2294. [PubMed] [Cited in This Article: ] |
| 19. | Pazianas M, Compston J, Huang CL. Atrial fibrillation and bisphosphonate therapy. J Bone Miner Res. 2010;25:2-10. [PubMed] [Cited in This Article: ] |
| 20. | Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE, Minisola S, Rossini M. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20:239-244. [PubMed] [Cited in This Article: ] |
| 21. | Kishimoto H, Yoh K, Ohta H, Gorai I, Hashimoto J, Nakatsuka Y, Yoshimoto Y, Makita K. Reference and cut-off values of QUS parameters measured using CM-100. Osteoporosis Japan. 2003;11:307-310 (in Japanese). [Cited in This Article: ] |
| 22. | Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T, Morii H. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int. 1999;10:183-192. [PubMed] [Cited in This Article: ] |
| 23. | Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, Kaneda K, Fukunaga M, Inoue T, Nakashima M. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab. 2004;22:462-468. [PubMed] [Cited in This Article: ] |
| 24. | Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab. 2005;23:382-388. [PubMed] [Cited in This Article: ] |
| 25. | Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423-432. [PubMed] [Cited in This Article: ] |
| 26. | Roux C, Seeman E, Eastell R, Adachi J, Jackson RD, Felsenberg D, Songcharoen S, Rizzoli R, Di Munno O, Horlait S. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004;20:433-439. [PubMed] [Cited in This Article: ] |