Published online Aug 24, 2021. doi: 10.5306/wjco.v12.i8.675
Peer-review started: December 30, 2020
First decision: May 4, 2021
Revised: May 5, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: August 24, 2021
Axillary sentinel lymph node biopsy (SLNB) is standard treatment for patients with clinically and pathological negative lymph nodes. However, the role of completion axillary lymph node dissection (cALND) following positive sentinel lymph node biopsy (SLNB) is debated.
To identify a subgroup of women with high axillary tumor burden undergoing SLNB in whom cALND can be safely omitted in order to reduce the risk of long-term complications and create a Preoperative Clinical Risk Index (PCRI) that helps us in our clinical practice to optimize the selection of these patients.
Patients with positive SLNB who underwent a cALND were included in this study. Univariate and multivariate analysis of prognostic and predictive factors were used to create a PCRI for safely omitting cALND.
From May 2007 to April 2014, we performed 1140 SLN biopsies, of which 125 were positive for tumor and justified to practice a posterior cALND. Pathologic findings at SLNB were micrometastases (mic) in 29 cases (23.4%) and macrometastasis (MAC) in 95 cases (76.6%). On univariate analysis of the 95 patients with MAC, statistically significant factors included: age, grade, phenotype, histology, lymphovascular invasion, lymph-node tumor size, and number of positive SLN. On multivariate analysis, only lymph-node tumor size (≤ 20 mm) and number of positive SLN (> 1) retained significance. A numerical tool was created giving each of the parameters a value to predict preoperatively which patients would not benefit from cALND. Patients with a PCRI ≤ 15 has low probability (< 10%) of having additional lymph node involvement, a PRCI between 15-17.6 has a probability of 43%, and the probability increases to 69% in patients with a PCRI > 17.6.
The PCRI seems to be a useful tool to prospectively estimate the risk of nodal involvement after positive SLN and to identify those patients who could omit cALND. Further prospective studies are necessary to validate PCRI clinical generalization.
Core Tip: The role of completion axillary lymph node dissection (cALND) following a positive sentinel lymph node biopsy (SLNB) is being actively debated. Patients with a positive SLNB performed at our institution who also underwent a cALND were analyzed. Univariate and multivariate analysis of prognostic and predictive factors were used to create a Preoperative Clinical Risk Index (PCRI). The PCRI could help estimate the risk in removing extra positive nodes beyond the SLN in order to identify which patients could safely avoid cALND. Further prospective studies are necessary to validate clinical generalization for suggested tool.
- Citation: Herrero M, Ciérvide R, Calle-Purón ME, Valero J, Buelga P, Rodriguez-Bertos I, Benassi L, Montero A. Macrometastasis at selective lymph node biopsy: A practical going-for-the-one clinical scoring system to personalize decision making. World J Clin Oncol 2021; 12(8): 675-687
- URL: https://www.wjgnet.com/2218-4333/full/v12/i8/675.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i8.675
The presence of axillary nodal involvement continues to be a major breast cancer prognostic factor. Complete axillary lymph node dissection (cALND) has a dual role both to ensure local control of the disease and to add staging information to adapt adjuvant treatment. The extent of cALND has evolved over time. In 1990, consensus from National Institute of Health concluded that the local treatment of axillary disease in operable breast cancer included at least the removal of axillary levels I and II[1]. In many European countries, cALND included all three Berg levels[2].
Axillary recurrence after cALND is infrequent[3-6], although treatment-related morbidity could be relevant both in the short- and long-term, including lymphedema, which occurs in 23 to 25% of women[7-11], pain, and decreased mobility of the arm[10,12]. During the 1980s, the possibility to identify a regional lymph node that was the first lymph node drainage station for breast cancer began to be considered[13]. Years later, different studies established the utility of selective sentinel lymph node biopsy (SLNB) as an alternative to axillary dissection in patients with clinically negative lymph nodes (cN0)[14-16], with the advantage of decreasing the risk of lymphedema associated with cALND[9,10,17].
The presence of sentinel node involvement was associated with the need to complete the study and treatment of the axilla with cALND. In recent years, emphasis has been placed on the possibility of reducing the need of cALND in patients with positive SLNB without worsening the prognosis. The results of the ACOSOG Z0011[18] and IBCSG 23-01[19] trials suggest that cALND could be avoided when sentinel node is minimally involved, eliminating complications of axillary surgery without negatively affecting survival. In addition, the AMAROS[7] and OTOASOR[20] trials concluded that axillary radiation therapy was not inferior to cALND in those patients with clinically node-negative breast cancer who had a positive SLN. However, doubts remain regarding macroscopic involvement after SLNB. Aforementioned studies show wide variability regarding tumor burden in the sentinel node, including patients with isolated tumor cells (ITC), micrometastasis and macrometastasis making it impossible to definitively establish the effect of omitting cALND in patients with a higher tumor burden in the SLNB.
Our objective was to identify a subgroup of women with high axillary tumor burden in SLNB in whom cALND can be safely omitted in order to reduce the risk of long-term complications. The second endpoint is to create a Preoperative Clinical Risk Index (PCRI) that helps us in our clinical practice to optimize the selection of these patients.
We retrospectively reviewed the experience of our Breast Cancer Unit with SLNB and cALND procedures from 2007 to 2014. This study received ethical approval from the Inhouse Local Ethics and Clinical Committee (Code: 16.04.0940-GHM) the April 27, 2016.
All patients included in this analysis had breast cancer diagnosed by core needle biopsy. Axillar presurgical ultrasound with fine needle biopsy was done if required. Magnetic resonance imaging (MRI) of the breast was performed in all patients where it was technically feasible. Before 2012, SLNB was only done in patients with tumors less than 3 cm. However, since then, tumors smaller than 5 cm were also considered for SLNB. The sentinel lymph node assessment was performed intraoperatively, with hematoxylin-eosin and immunohistochemistry analyses deferred. Data from all patients with positive SLNB who underwent cALND were collected. From 2012, cALND was not carried out in the presence of micrometastasis.
Database information included the patients’ age, menopausal status, and laterality, as well as tumor characteristics at diagnosis (tumor size, number of foci, IHC, Her-2 status, grade, presence of intraductal carcinoma), surgical treatment data (surgery type, date, margins status, postoperative tumor size), and data of lymph nodes analysis (SLN, nodes from the accessory axillary fat and nodes from axillary dissection). In each of the sections, isolated tumor cells (ITC), micrometastasis (mic) and macrometastasis (MAC) were differentiated.
All patients with pathologic involvement of the axilla received adjuvant whole breast/chest wall radiotherapy together with comprehensive regional nodal irradiation (RNI) according to published international guidelines[21,22].
Patients´ information was obtained from the patients´ records, and collected data were subsequently analyzed with the SPSS software, version 17.0 (Released 2008. IBM SPSS Statistics for Windows. Version 17.0 Armonk, NY: IBM Corp).
Once the data had been validated and refined, the statistical analysis was carried out following this way: (1) Exploratory analysis of all selected variables; (2) Descriptive analysis of the sample: distribution of all variables; (3) Description of the clinical data: patients, tumor and type of surgery; (4) Description of sentinel node biopsy procedures (detailed pathological results of SLNs, axillary fat and axillary dissections); (5) Descriptive statistics of the patients with macrometastasis in the sentinel node; (6) Univariate analysis with the Chi-square test was performed to compare every clinical parameter with the variable “presence” or “absence” of positive nodes in the lymphadenectomy”. The parameters considered in the univariate analysis of the 95 cases of MAC were: age, menopausal status, laterality, tumor size, uni- or multicentricity, grade, phenotype, histology, association to in situ carcinoma, type of surgery, number of removed nodes, number of MAC, and the sum of MAC and mic; (7) For those significant variables in the univariate analysis, the Odds Ratio were calculated by using a logistic regression model using the Stepwise method to know which of them had the greatest impact on final results. The Pearson correlation coefficient was used. The level of significance used was α = 0.05 (95% confidence interval), therefore those differences whose P value < 0.05 were considered significant; and (8) Finally, a numeric value was assigned to selected variables by calculating the logarithms of the results to develop a numerical tool, the PCRI. Different cut-off points of this tool, according to the cut-off point recommended by Krag et al[14] in the NSABP 32 trial, were simulated in order to find the one that left the least number of false negatives (low risk, FN must be < 10%, specificity of 90%).
From May 2007 to April 2014 a total of 1140 women underwent an SLNB procedure. One-hundred and twenty-five (11%) were positive for tumor and, hence, underwent posterior cALND. The average number of sentinel nodes dissected was 2.12 (1-5). Pathologic findings after SLNB were micrometastasis (mic) in 29 cases (23.4%) and macrometastasis (MAC) in 95 cases (76,6%).
We focused our analysis on patients with MAC. In our patients, the probability of harboring additional lymph node disease in the cALND in patients with MAC in SLNB was 36%.
Patients’ characteristic of the 95 patients with SLN macrometastasis are summarized in Table 1.
| Characteristics | n = 95 | % |
| Age (range), yr | 51.9 (23-82) | - |
| Average tumor size, mm | 17.4 | - |
| Tumor size | ||
| 0-10 | 14 | 14.7 |
| 11-15 | 25 | 26.3 |
| 16-20 | 26 | 27.4 |
| 21-30 | 18 | 18.9 |
| 31-40 | 6 | 6.3 |
| > 50 | 1 | 1.1 |
| In situ | 5 | 5.3 |
| Menopausal status | ||
| Pre | 50 | 52.6 |
| Post | 45 | 47.4 |
| Histology type | ||
| Ductal | 76 | 80 |
| Lobular | 15 | 15.8 |
| Others | 4 | 4.2 |
| Phenotype | ||
| Luminal | 83 | 87.4 |
| Basal | 9 | 9.5 |
| Her-2 | 3 | 3.2 |
| Grade | ||
| I | 16 | 16.8 |
| II | 54 | 56.8 |
| I + II | 70 | 73.7 |
| III | 24 | 25.3 |
| Unknown | 1 | 1.1 |
| Surgery | ||
| Conservative | 63 | 66.3 |
| Mastectomy | 32 | 33.7 |
| Number of SN removed | ||
| 1 | 35 | 36.8 |
| 2 | 28 | 29.5 |
| 3 | 20 | 21.1 |
| ≥ 4 | 12 | 12.6 |
| Nº of positive SN (Mic + MAC) | ||
| 0 | 4 | 4.2 |
| 1 | 65 | 68.4 |
| 2 | 20 | 21.1 |
| 3 | 4 | 4.2 |
| ≥ 4 | 2 | 2.1 |
| Size of the SLN metastasis | ||
| ITC (i+) | 0 | 0 |
| Micrometastasis (Mic) | 0 | 0 |
| Macrometastasis (MAC) | 95 | 100 |
| Number of positive SLN | ||
| 0 | 55 | 57.9 |
| 1 | 21 | 22.1 |
| 2-3 | 11 | 11.6 |
| 1-3 | 32 | 33.7 |
| ≥ 4 | 8 | 8.4 |
| Adjuvant treatments | ||
| Radiotherapy | 75/90 | 82.4 |
| Chemotherapy | 72/90 | 80 |
| Hormonotherapy | 79/90 | 87.7 |
| Immunotherapy | 4/92 | 4.3 |
For the 95 patients with MAC, the parameters that showed statistical significance in the univariate analysis were: Age (cut point 40 years-old), grade, phenotype, histology, lymphovascular invasion, tumoral size, and number of positive sentinel nodes (P < 0.05 for all comparisons) (Table 2).
| No additional metastatic lymph nodes | Additional metastatic lymph nodes | P value | |
| Age | |||
| < 40 | 66% | 44% | 0.04 |
| ≥ 40 | 32.6% | 67.4% | |
| Hormonal status | |||
| Premenopausal | 70% | 30% | NS |
| Postmenopausal | 60% | 40% | |
| T stage | |||
| T1 | 75.8% | 24.2% | < 0.05 |
| T2 | 47% | 53% | |
| T3 | 0 | 100% | |
| Tumor size | |||
| ≤ 15 mm | 85% | 15% | < 0.05 |
| > 15 mm | 49% | 51% | |
| Histologic type | |||
| Ductal carcinoma | 67.1% | 32.9% | NS |
| Lobular carcinoma | 47% | 53% | |
| Grade | |||
| I + II | 68.6% | 31.4% | NS |
| III | 54% | 46% | |
| Molecular subtype | |||
| Luminal A/B | 65.6% | 34.4% | < 0.05 |
| Basal | 22.2% | 77.8% | |
| HER2 | 100% | 0 | |
| LVI | |||
| Present | 66.7% | 33.3% | NS |
| Absent | 61.5% | 38.5% | |
| Number of positive SLN | |||
| 1 | 69.6% | 31.4% | < 0.05 |
| ≥ 2 | 43.7% | 66.3% | |
| ACOSOG Z0011 criteria | |||
| ≤ 2 SLN+ | 68.5% | 31.5% | < 0.05 |
| > 2 SLN+ | 16% | 84% |
When analyzing all the parameters that had been significant in the univariate analysis, only the tumor size less or equal to 15 mm and the presence of 1 affected SLN remained significant. The variable of up to 2 sentinel lymph nodes affected by MAC or mic as suggested by ACOSOG Z0011 was also studied. In our population, the presence of one MAC is the variable with the greatest statistical significance for predicting disease in the axillary dissection (Table 3). Of the 95 patients collected with MAC, 79 cases had only 1 affected SLN. For this reason, this data has been used as a cut-off point for the population analyzed.
| P value | Odds ratio | ||
| Tumor size | ≤ 15 mm vs > 15 mm | 0.002 | 2.004 |
| Number of metastatic SLN | 1 vs > 1 | 0.021 | 2.573 |
| ACOSOG Z0011 criteria | ≤ 2 SLN+ vs > 2 SLN+ | 0.045 | 10.195 |
To avoid bias in the results due to the simultaneous presence of mic, a subgroup of 79 patients with exclusively one sentinel node with MAC in SLNB was selected from our original population, which served as the basis for developing the PCRI tool. Although having such a group of cases with little probability of involvement of other nodes in the cALND, these patients form a heterogeneous group. Thus, in the 79 women with only 1 affected SLN, we still found an additional 30.4% of disease in cALND, which is not an acceptable result to justify stopping cALND in all patients.
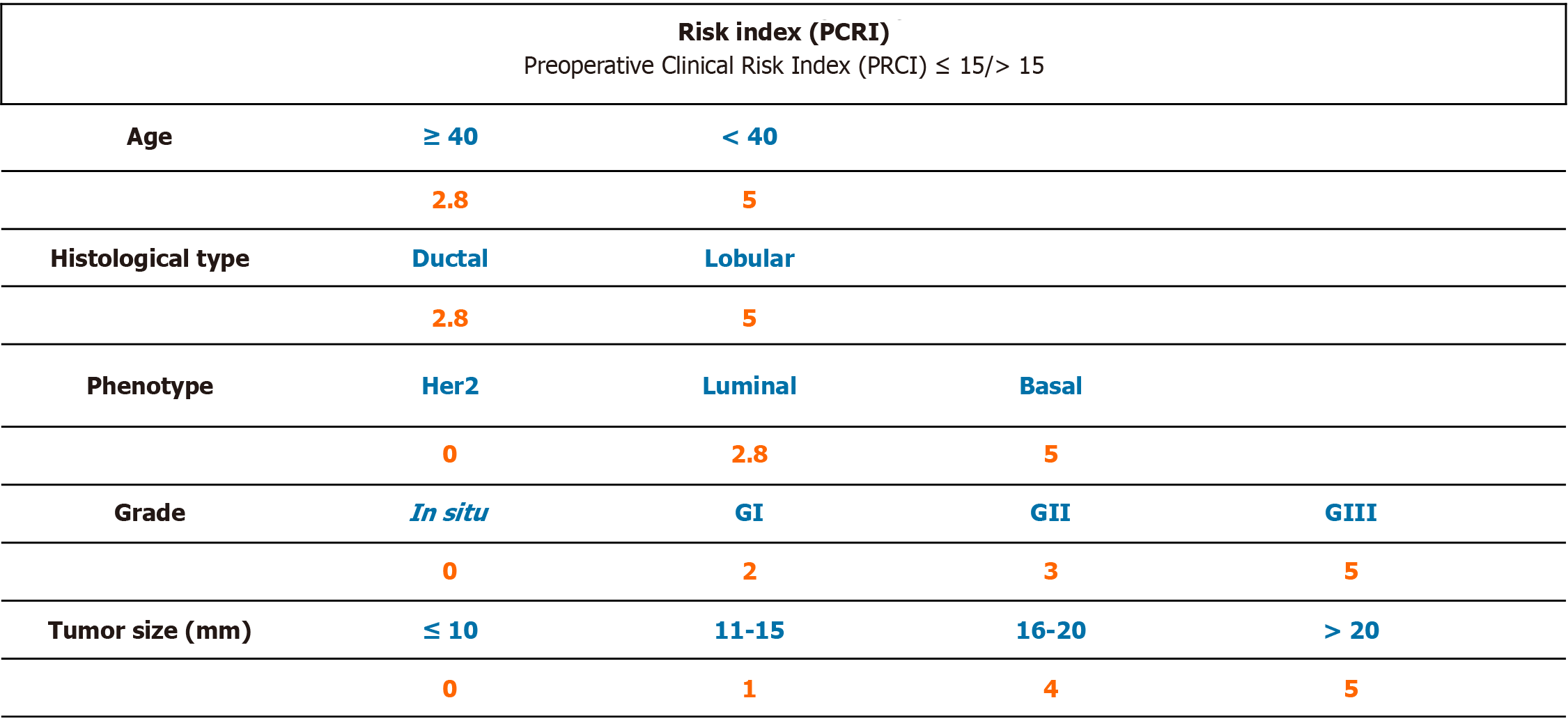
Based on the results of the univariate logistic regression of those variables that are related to the presence of additional disease on cALND (size, age, histology, grade and phenotype) in the group of 79 cases with presence of only one macrometastatic SLN, a numerical tool was created by giving each of the parameters a numeric value in order to be able to predict preoperatively which patients would not benefit from a cALND (Table 4). To facilitate the usability of the prognostic index, we decided to include only those factors that could be known before breast surgery. Appropriate numeric value was calculated by the resulting rate of involvement when considering this value in isolation and then taking these results to base 10 (Figure 1).
| Additional metastatic lymph nodes, n = 79 | |
| Age | |
| < 40 | 50% |
| ≥ 40 | 28% |
| ≤ 15 mm | 85% |
| > 15 mm | 49% |
| Histologic type | |
| Ductal carcinoma | 28% |
| Lobular carcinoma | 50% |
| Mucinous | 0 |
| Tumor size | |
| ≤ 10 mm | 7% |
| 11-15 mm | 10% |
| 16-20 mm | 42% |
| > 20 mm | 47% |
| Grade | |
| DCIS | 0 |
| I | 18% |
| II | 31% |
| III | 45% |
| Molecular subtype | |
| Luminal A/B | 28% |
| Basal | 55% |
| HER2 | 0 |
Numeric values assigned to age, grade, size, histological type, and phenotype were included. The resulting value of the PCRI was related to the risk of having other lymph nodes involved in the axillary dissection. According to the PCRI, patients could be divided into two groups based upon a cut-off point of 15.
Patients in the low-risk group (index score ≤ 15, 42 patients) were at risk of having other affected nodes less than 10% and in whom the cALND would have added to morbidity with minimal extra information. Only 5% changed from the pN1a state after axillary surgery. Ninety-five percent of patients had 1 or 2 positive nodes in total.
Patients in the high risk group (index score > 15, 37 patients) had a 54% of risk of having nodal involvement in the cALND. The pN1a stage changed in 13.5% of patients after lymphadenectomy. Seventy-three percent of the patients had 1 or 2 positive nodes in total, and 13.5% had 3 affected lymph nodes. For these patients, axillary dissection would be indicated, not only as information for adjuvant treatment, but also as a therapeutic approach of the axillary disease itself.
Of the total of 79 cases with only one MAC detected by SLNB, using the cut-off point at 15 for the PCRI, less than 10% of the cases would have more affected nodes, and only 5% of the cases would change from the pN1a stage.
The American Society for Medical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) initially proposed in their clinical guidelines to always perform cALND in patients with positive SLNs[23,24]. However, studies had shown that 53% of patients with positive SLN did not have disease in the non-sentinel nodes after cALND[25], and that positive SLN was the only metastasis in 40% to 60% of cases[14,26-29]. In about half of the women undergoing cALND, surgery would not provide more prognostic information, although it would add a greater risk of complications derived from the procedure. Therefore, the current recommendations of international Guidelines and Consensus Statements on the locoregional treatment of breast cancer state the possibility of omitting cALND in the case of a positive SLNB is considered low risk, especially in those patients who are going to receive radiotherapy after surgery[24,30]. Omission of cALND is more frequently offered to patients who present lymph node involvement in the form of micrometastasis exclusively than in those patients with macrometastasis in the SLN[30-32].
When this study was conceived and designed, 2 large randomized trials had demonstrated the efficacy and safety of omitting cALND in select groups of patients[7,33]. Compared with what was observed by ACOSOG Z0011[18] and AMAROS[7] trials, our study presents very similar characteristics, both in the mean age and in the rate of ductal or lobular tumors, although in our series we found 8% more grade I/II tumors and half the cases of lymphovascular invasion (LVI) than reported in ACOSOG Z0011, which is considered a marker of aggressiveness and worse prognosis[34]. Finally, it should be noted that the tumor phenotype is known in all of our patients including hormone receptors, overexpression of the HER2 protein and the proliferation factor Ki-67. These data were not collected in the AMAROS, however, the ACOSOG Z0011 study collected data on hormone receptors, but not on Her2 or Ki 67, making a direct comparison difficult. With respect to the surgery performed, in ACOSOG Z0011, all patients received conservative surgery. In AMAROS, the number of conservative surgeries drop to 82%. In our study, 70% of the patients had conservative surgery while 30% received mastectomies. This could allow us to apply the study's conclusions regardless of the type of surgery. The results of the SLNB and cALND are very similar in our study to those reported by AMAROS and ACOSOG Z0011. In our study, 69% of patients did not have additional metastatic involvement in cALND, similar to 72% and 73% of AMAROS and ACOSOG Z0011, respectively.
Our study seeks to identify which patients will not have more axillary disease, even though the SLNB is positive for macrometastasis. The presence of macrometastasis in SLNB has been related to a higher probability of finding additional metastatic lymph nodes after cALND. According to other groups’ experience, the incidence of involvement in the cALND beyond the SLN was from 40 to 58%[23] when in the presence of MAC, while for mic it fell to 20% (23), and for ITC (isolated tumor cells), it was roughly 12% (23). In our series, the probability of harboring additional lymph node disease in the cALND in patients with MAC in SLNB was 36%.
Currently, there are 3 ongoing trials to provide evidence in this regard. The SINODAR ONE trial[35] randomized patients with 1 or 2 macrometastasis in SLNB and treated with conservative surgery or mastectomy to receive either cALND remain under observation. Both groups received adjuvant therapy. The INSEMA trial[36] included patients with tumors T1-2 and conservative surgery or mastectomy and were randomized to undergo either SLNB remain under observation. Patients with a positive SLNB with 1 or 2 affected nodes are then re-randomized to exclusive SLNB or cALND. Finally, in the POSNOC trial[37], T1-2 breast carcinomas with 1 or 2 macrometastasis in SLNB are randomized after conservative surgery or mastectomy to either undergo axillary treatment with cALND or RNI or remain under observation. In both arms the indicated systemic treatment is added. The results of these 3 studies might elucidate. the feasibility and safety of omitting cALND in patients with macrometastasis in SLNB.
Attempts have been made to develop tools to estimate the risk of finding additional positive lymph nodes beyond the SLN based on the presence of different factors: multifocality, presence of LVI, or the hormone receptors status. To date, there are currently seven published nomograms in use: the Memorial Sloan Kettering Cancer Center nomogram[38], the Cambridge nomogram[39], the Turkish Federation nomogram[40], the Stanford nomogram[41], the MD Anderson Cancer Center nomogram[42], the Tenon nomogram[43], and the MOU nomogram[44]. However, these nomograms do not seem to have good reproducibility in populations other than their own[45]. For a nomogram to be successful and clinically applicable, it must identify the majority of patients who might have non-sentinel node involvement, and the false negative rate should be ≤ 10%[45]. We have analyzed prognostic factors in our treated patients in order to design a personalized tool that, preoperatively and based on clinical and tumoral data, would help us to select patients with macrometastasis determined by SLNB with a risk of less than 10% of additional positive nodes in cALND, which would potentially allow them to omit this procedure regardless of the type of surgery and adjuvant treatments, similar to the initial approach of Sentinel Lymph Node Biopsy in NSABP. Our clinical index differs from other widely distributed nomograms, such as the Memorial Sloan Kettering Cancer Center nomogram (MSKCCn) and the MD Anderson Cancer Center nomogram (MDACCn) designed to infer involvement beyond the SLNB. One factor that is different in our clinical index is that our index takes into account the age of the patient, which is not included in either the MSKCCn or the MDACCn. However, tumor size is assessed identically in both the MSKCCn and the MDACCn, as well as in our PCRI. Histology and grade are assessed jointly in the MSKCCn, while MDACCn does not assess tumor grade. Both nomograms assess the total number of nodes removed: MSKCCn as negative sentinel nodes, and MDACCn as total nodes removed. The presence of multifocality was associated with a higher risk in the MSKCCn, but not in our index, and it was not assessed in the MDACCn. LVI is recorded in both nomograms, since it implies a worse prognosis. Our data support this result, but as it is not a parameter that we can obtain preoperatively, and therefore was not included in our index. Likewise, the extranodal extension included in the MDACCn is not a parameter that we can have preoperatively, so it has also not been included in our index. Finally, the tumor phenotype is not collected in the MDACCn and is only partially collected in the MSKCCn, which assesses the absence or presence of estrogen receptor in the tumor.
The most important strength is that our PCRI is that the information can be obtained preoperatively, allowing a personalized decision-making process that involves the patient before surgery. The biggest weakness of our study is the retrospective nature of the study, which limits the findings of the study. The population we assessed does not correspond to a specific geographic area, nor has participated in a standard screening program, making the findings less biased for a specific population. Our patients come both from our own gynecology consultations, where opportunistic screening is performed following the American guidelines[23], as well as from other centers. However, the patients have private health insurance policies that could cause selection bias from the population. Additionally, comparing the characteristics of our population with the multicenter studies, American Z0011 and European AMAROS, we did not find any significant differences between the three patient populations. In addition, the phenotypes of basal and Her-2 were not as represented as other phenotypes in the total of patients, suggesting those patients should be specifically discussed in the Tumor Board.
More than half of our patients with the presence of MAC in the SLNB had no involvement of the non-sentinel nodes, and only 1 in 8 changed from the pN1a stage, which means that we have performed too many cALND on our patients.
The PCRI we outlined is based on a detailed analysis of the group of patients with MAC in the SLN, avoiding the biases of mic common in other trials, and is a useful tool to prospectively estimate the risk of nodal involvement beyond the positive SLN and to identify those patients who would benefit from a cALND afterwards.
Further studies are necessary to validate feasibility and accuracy of this PCRI.
The role of completion axillary lymph node dissection (cALND) following positive sentinel lymph node biopsy (SLNB) is being actively debated.
Patients with positive SLNB performed at our institution who underwent a cALND were analyzed.
This study aims to create a Preoperative Clinical Risk Index (PCRI).
Univariate and multivariate analysis of prognostic and predictive factors were used to create a PCRI.
PCRI could help estimate the risk of having extra positive nodes beyond the SLN in order to identify in which patients cALND could be safely omitted.
Further prospective studies are necessary to validate clinical generalization for the suggested PCRI.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fonseca-Alves CE S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Early stage breast cancer: consensus statement. NIH consensus development conference, June 18-21, 1990. Cancer Treat Res. 1992;60:383-393. [PubMed] [Cited in This Article: ] |
| 2. | Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V, Veronesi P, Intra M, Maisonneuve P, Zucca F, Gatti G, Mazzarol G, De Cicco C, Vezzoli D. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J, Port E, Sacchini V, Sclafani L, VanZee K, Wagman R, Borgen PI, Cody HS 3rd. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462-468; discussion 468-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Veronesi U, Galimberti V, Paganelli G, Maisonneuve P, Viale G, Orecchia R, Luini A, Intra M, Veronesi P, Caldarella P, Renne G, Rotmensz N, Sangalli C, De Brito Lima L, Tullii M, Zurrida S. Axillary metastases in breast cancer patients with negative sentinel nodes: a follow-up of 3548 cases. Eur J Cancer. 2009;45:1381-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Smidt ML, Janssen CM, Kuster DM, Bruggink ED, Strobbe LJ. Axillary recurrence after a negative sentinel node biopsy for breast cancer: incidence and clinical significance. Ann Surg Oncol. 2005;12:29-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Siponen ET, Vaalavirta LA, Joensuu H, Leidenius MH. Axillary and supraclavicular recurrences are rare after axillary lymph node dissection in breast cancer. World J Surg. 2012;36:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1095] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 8. | Giuliano AE, Hawes D, Ballman KV, Whitworth PW, Blumencranz PW, Reintgen DS, Morrow M, Leitch AM, Hunt KK, McCall LM, Abati A, Cote R. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213-5219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 10. | Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE; American College of Surgeons Oncology Group. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657-3663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 610] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 11. | Latosinsky S, Dabbs K, Moffat F; Evidence-Based Reviews in Surgery Group. Canadian Association of General Surgeons and American College of Surgeons Evidence-Based Reviews in Surgery. 27. Quality-of-life outcomes with sentinel node biopsy versus standard axillary treatment in patients with operable breast cancer. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. Can J Surg. 2008;51:483-485. [PubMed] [Cited in This Article: ] |
| 12. | Olson JA Jr, McCall LM, Beitsch P, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE; American College of Surgeons Oncology Group Trials Z0010 and Z0011. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530-3535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Osborne MP, Payne JH, Richardson VJ, McCready VR, Ryman BE. The preoperative detection of axillary lymph node metastases in breast cancer by isotope imaging. Br J Surg. 1983;70:141-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1104] [Cited by in F6Publishing: 1172] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 15. | Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, Costa A, de Cicco C, Geraghty JG, Luini A, Sacchini V, Veronesi P. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864-1867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1519] [Cited by in F6Publishing: 1392] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 16. | Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391-398; discussion 398-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2130] [Cited by in F6Publishing: 1995] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 17. | Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, Fehr MK, Hess T, Oertli D, Bronz L, Schnarwyler B, Wight E, Uehlinger U, Infanger E, Burger D, Zuber M. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264:413-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 19. | Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U; International Breast Cancer Study Group Trial 23-01 investigators. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 782] [Cited by in F6Publishing: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 20. | Sávolt Á, Péley G, Polgár C, Udvarhelyi N, Rubovszky G, Kovács E, Győrffy B, Kásler M, Mátrai Z. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017;43:672-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Duma MN. An Update on Regional Nodal Irradiation: Indication, Target Volume Delineation, and Radiotherapy Techniques. Breast Care (Basel). 2020;15:128-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Torres MA, Horst KC, Freedman GM. Postmastectomy and Regional Nodal Radiation for Breast Cancer. J Clin Oncol. 2020;38:2299-2309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE, Heerdt AS, Helvie M, Huff JG, Khakpour N, Khan SA, Krontiras H, Lyman G, Rafferty E, Shaw S, Smith ML, Tsangaris TN, Williams C, Yankeelov T; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, Cochran AJ, Cody HS 3rd, Edge SB, Galper S, Hayman JA, Kim TY, Perkins CL, Podoloff DA, Sivasubramaniam VH, Turner RR, Wahl R, Weaver DL, Wolff AC, Winer EP; American Society of Clinical Oncology. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703-7720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1360] [Cited by in F6Publishing: 1247] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 25. | Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106:4-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 669] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 26. | Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345-2350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 788] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 27. | Grube BJ, Giuliano AE. Observation of the breast cancer patient with a tumor-positive sentinel node: implications of the ACOSOG Z0011 trial. Semin Surg Oncol. 2001;20:230-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Veronesi U, Paganelli G, Viale G, Galimberti V, Luini A, Zurrida S, Robertson C, Sacchini V, Veronesi P, Orvieto E, De Cicco C, Intra M, Tosi G, Scarpa D. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 547] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 29. | Noguchi M, Motomura K, Imoto S, Miyauchi M, Sato K, Iwata H, Ohta M, Kurosumi M, Tsugawa K. A multicenter validation study of sentinel lymph node biopsy by the Japanese Breast Cancer Society. Breast Cancer Res Treat. 2000;63:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Yi M, Giordano SH, Meric-Bernstam F, Mittendorf EA, Kuerer HM, Hwang RF, Bedrosian I, Rourke L, Hunt KK. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17 Suppl 3:343-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1088] [Cited by in F6Publishing: 1041] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 32. | Kaufmann M, Morrow M, von Minckwitz G, Harris JR; Biedenkopf Expert Panel Members. Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer. 2010;116:1184-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Beaton L, Bergman A, Nichol A, Aparicio M, Wong G, Gondara L, Speers C, Weir L, Davis M, Tyldesley S. Cardiac death after breast radiotherapy and the QUANTEC cardiac guidelines. Clin Transl Radiat Oncol. 2019;19:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Fidalgo F, Rodrigues TC, Pinilla M, Silva AG, Maciel Mdo S, Rosenberg C, de Andrade VP, Carraro DM, Krepischi AC. Lymphovascular invasion and histologic grade are associated with specific genomic profiles in invasive carcinomas of the breast. Tumour Biol. 2015;36:1835-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Tinterri C, Canavese G, Bruzzi P, Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast. 2016;30:197-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Reimer T, Hartmann S, Stachs A, Gerber B. Local treatment of the axilla in early breast cancer: concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel). 2014;9:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Goyal A, Dodwell D. POSNOC: A Randomised Trial Looking at Axillary Treatment in Women with One or Two Sentinel Nodes with Macrometastases. Clin Oncol (R Coll Radiol). 2015;27:692-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, Borgen PI, Cody HS 3rd, Kattan MW. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 580] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 39. | Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95:302-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Gur AS, Unal B, Ozbek U, Ozmen V, Aydogan F, Gokgoz S, Gulluoglu BM, Aksaz E, Ozbas S, Baskan S, Koyuncu A, Soran A; Turkish Federation of Breast Disease Associations Protocol MF08-01 investigators. Validation of breast cancer nomograms for predicting the non-sentinel lymph node metastases after a positive sentinel lymph node biopsy in a multi-center study. Eur J Surg Oncol. 2010;36:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, Henry S, Rouse RV, Bailey L, Philben VJ, Dirbas FM, Dunn JJ, Johnson DL, Wapnir IL, Carlson RW, Stockdale FE, Hansen NM, Jeffrey SS; Bay Area SLN Study. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Lambert LA, Ayers GD, Hwang RF, Hunt KK, Ross MI, Kuerer HM, Singletary SE, Babiera GV, Ames FC, Feig B, Lucci A, Krishnamurthy S, Meric-Bernstam F. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2006;13:310-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Coufal O, Pavlík T, Fabian P, Bori R, Boross G, Sejben I, Maráz R, Koca J, Krejcí E, Horáková I, Foltinová V, Vrtelová P, Chrenko V, Eliza Tekle W, Rajtár M, Svébis M, Fait V, Cserni G. Predicting non-sentinel lymph node status after positive sentinel biopsy in breast cancer: what model performs the best in a Czech population? Pathol Oncol Res. 2009;15:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Nadeem RM, Gudur LD, Saidan ZA. An independent assessment of the 7 nomograms for predicting the probability of additional axillary nodal metastases after positive sentinel lymph node biopsy in a cohort of British patients with breast cancer. Clin Breast Cancer. 2014;14:272-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |