Introduction
Nearly 70% of the human body is composed of water. Diffusion-weighted imaging (DWI) is an MRI sequence that facilitates evaluation of tissue in its microscopic form by dephasing and rephasing water molecules. It is based on free, constant, and random movement of intra- and extracellular water molecules (Brownian motion). In tissue, the motion of water molecules can be decreased (restricted diffusion) or increased (free diffusion) depending on tissue type, intra- and extracellular interactions, or certain pathological causes [1].
DWI is a T2-weighted fast spin echo sequence after applying 2 equal and opposite sensitizing gradients before and after a 180° radiofrequency pulse. If the tissue has restricted diffusion, the second gradient cancels the first, resulting in a hyperintensity appearance on DWI. Conversely, if the tissue has low cellularity and/or defective cell membranes, a relatively high extracellular space allows water molecules to move more freely and randomly, leading to a lower signal on DWI. The respective gradients are acquired with different strengths, characterized by the sensitizing field amplitude, duration, and temporal spacing, which display different b-values.
DWI is sensitive for the detection of diffusivity of water molecules within the brain tissue, thereby revealing information on their microarchitecture. The fundamental principle of DWI is Brownian motion, i.e. constant random motion of free water molecules due to thermal kinetic energy.
This free motion of water molecules within the cell is impeded due to interaction of water molecules with intracellular organelles and the cell wall. Therefore, restriction in the diffusivity of water molecules largely depends on the degree of cellularity of the tissue. High-grade tumours due to increased tumoral cellularity, higher nuclear-to-cytoplasmic ratio, and reduced extracellular volume often demonstrate restricted diffusion. However, rarely, benign central nervous system (CNS) neoplasms also show restricted diffusion because of relative increased cellularity, extracellular matrix composition, and due to the alignment pattern of tumour cells.
After considering the location and tumour characterization on basic sequences (T1, T2, FLAIR), the lesion is further analysed on diffusion and apparent diffusion coefficient (ADC) maps. While interpreting DWI, it is important for the radiologist to be aware of various histological attributes like matrix composition, which can have a significant influence on diffusion characteristics. Some pathological entities, despite being benign, can have inherently increased cellularity and hence can mimic malignant lesions on DWI [1]. Anticipation of such atypicality in benign CNS lesions can reduce the possibility of potential misdiagnosis, thereby improving the diagnostic accuracy [1].
In this article we review 12 benign CNS neoplasms that show non-haemorrhagic restricted diffusion, and we attempt to support the hypothesis with histopathological correlation.
Material and methods
Patient selection
Reports of MRI of all the brain studies done between January 2014 to January 2020 were reviewed from picture archiving and communication systems at our hospital to identify brain tumours that revealed restricted diffusion. Only the reports that showed restriction within a tumour were selected and reviewed. Lesions that were graded as malignant on histopathological records were excluded from the study. Only benign brain tumours that showed restricted diffusion were identified.
MRI and diffusion analysis
A selected cohort of benign brain tumours with restricted diffusion were assessed by radiologists. Medical records and histopathological diagnoses were viewed in all the cases. The presence of diffusion restriction was confirmed on correlation with ADC maps in all the cases. All the cases that demonstrated high signal on DWI with corresponding signal drop on the ADC map and no hemosiderin staining on susceptibility weighted images (SWI) were selected. Only tumours that revealed approximately equal or more than 50% of the entire tumour area were included in the study. Furthermore, individual MR images were evaluated for the location, signal characteristics on T1-, T2-, FLAIR-, and susceptibility-weighted sequences. Contrast enhancement in the areas corresponding to diffusion restriction was recorded as homogenous or heterogenous in all the cases. Cases where there was no corresponding contrast enhancement in the involved areas of restricted diffusion and those that showed corresponding susceptibility changes/haemorrhage were excluded from the study.
MRI technique
All patients were scanned on a 1.5 T MRI using a head coil. The following acquisition parameters were used: T1 (TR 610 ms, TE 9.7 ms), T2 (TE 4500 ms, TR 102 ms), FLAIR (TE 86 ms, TR 8500 ms), susceptibility (TR 49 ms, TE 40 ms).
Diffusion-weighted 3-scan trace in the transverse plane was performed by using echo-planar imaging with b-values of 0, 500, and 1000. Apparent diffusion coefficient (ADC) maps were obtained automatically using commercially available software. A total of 5- ml of intravenous gadolinium (1 ml per 10 kg of body weight) contrast agent was used for the contrast-enhanced study.
Results
There were 274 patients with benign brain tumours that showed restricted diffusion. All the tumours (n = 274) showed about 50% or more than 50% restriction within the tumour area. Additionally, all the tumours (n = 274) demonstrated homogenous or heterogenous type of enhancement corresponding to the areas of restricted diffusion. Males (n = 125) and females (n = 149), ranging in age from 1 to 65 years with varying clinical presentation ranging from: headache, loss of consciousness, seizures, and symptoms of raised intracranial pressure. Of these 274 cases, there were meningiomas (148), pituitary macroadenoma (71), schwannoma (33), central neurocytoma (7), pleomorphic xanthoastrocytoma (5), solitary fibrous tumour (3), pineocytomas (2), desmoplastic infantile gangliogliomas (1), gangliocytoma (1), choroid plexus papilloma (1), pilomyxoid astrocytoma (1), and inflammatory pseudotumor (1). We intend to discuss these individual tumours with histopathological correlation and a review of the literature. We also present a table presenting the demographic distribution and a possible hypothesis for restricted diffusion based on histopathological characteristics (Table 1).
Table 1
Frequency and demographic distribution of benign brain tumours showing restricted diffusion with possible hypothesis resulting in restricted diffusion based on histopathological correlation
Discussion
DWI provides information about the microarchitecture of a tissue without using ionizing radiation or intravenous contrast administration. This sequence is important and is used routinely for any routine MRI brain study. It allows tissue characterization at a microscopic level, which is based on the Brownian motion of water molecules, when 2 diffusion gradients are added to T2-weighted (T2W) sequences.
Many benign intracranial lesions have high signal on DWI at b = 1000 s/mm2 and low signal intensity on ADC maps, similarly to the behaviour of malignant neoplasms.
Apparent diffusion coefficient (ADC) is a quantitative measure of tissue diffusivity expressed in ×10−3 as mm2/s and derived from the slope of exponential decrease in signal between at least 2 DWI b-values. The accuracy of ADC maps depends on the number of b-values acquired, mis-registration between the b-value acquisitions, and the signal-to-noise ratio (SNR). Ideally, in clinical practice, it is common to acquire 2 b-values, and no more than 3 b-values.
The DWI sequences are always interpreted after correlation with the routine T1W and T2W MR images, because DWI is considered only a complementary sequence.
Meningioma
Meningiomas are extra-axial non-glial neoplasms that arise from arachnoid cap cells and constitute about 14-20% of CNS neoplasms [2]. They commonly occur in the middle-aged population, with a male-to-female ratio of 1 : 2. They are usually benign; however, 10% of the meningiomas are atypical or malignant and have a high rate of recurrence. Hence, distinguishing them before surgery helps to decide the extent of surgical resection [3]. They are typically dural-based lesions, which are isointense to grey matter on T1W and T2W MR images and show homogenous enhancement. Histologically, meningiomas are composed of spindle-shaped tumour cells that show a whorl pattern of arrangement with psammoma bodies. Surov et al. [4] in their study reported that grade II/III meningiomas had statistically significantly lower ADCmean values than grade I meningiomas. They also found that ADCmean values correlated negatively with tumour proliferation index and ADCmin with tumour cell count. Furthermore, these associations were observed to be different in several meningioma grades.
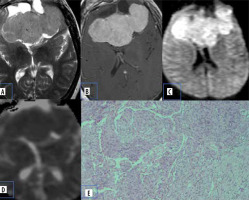
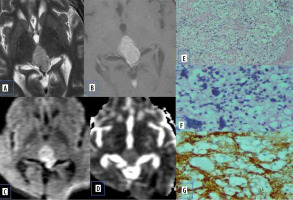
Atypical meningiomas show hyper cellularity, cytologic pleomorphism, increased collagen formation, and fibrosis with less extracellular space, which causes reduced mean ADC values (Figure 1).
Figure 1
Meningioma. A) Axial T2-W MRI shows an extra-axial lesion along the anterior cranial fossa. B) Axial T1-W post-contrast image shows homogeneous enhancement. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion within the lesion. E) Histopathological evaluation shows tumour cells that are largely uniform, with oval nuclei with delicate chromatin that on occasion show central clearing or the formulation of cytoplasmic-nuclear inclusions
Pituitary macroadenoma
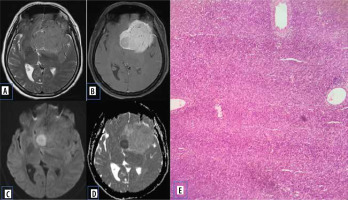
Pituitary adenomas comprise the most common sellar mass. They account for about 10-15% of all intracranial neoplasms and approximately 30-50% of pituitary masses [5]. They can be functioning (secretory ~ 65%) or non-functioning (non-secretory ~ 35%) [6]. Non-functioning usually presents with signs and symptoms due to mass effect. Based on size, they are classified into pituitary micro-adenoma (< 1 cm) and macroadenoma (> 1 cm). Diffusion characteristics of pituitary adenoma are well documented in various studies. Pierallini et al. [7] in their study correlated the collagen content with diffusion pattern and ADC values. Tumours with hard consistency were found to have lower ADC values. This was attributed to the increased cellularity, scant fibrous tissue, and low collagen content (Figure 2). Tumours with hard consistency and with lower ADC values usually require a transcranial surgical approach as opposed to trans-sphenoidal approach, due to difficulty in suction and curettage.
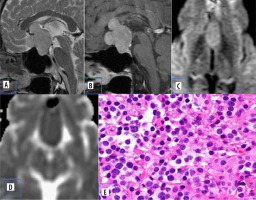
Figure 2
Pituitary adenoma. A) Sagittal T2-W MRI shows isointense sellar-suprasellar lesion, normal pituitary gland is not separately visualized. B) Sagittal T1-W post-contrast image shows homogenous post-contrast enhancement. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. E) Histopathological evaluation shows medium sized cells with chromophobic or slightly acidophilic cytoplasm and a central, oval nucleus
Vestibular Schwannoma
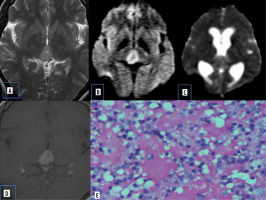
Vestibular schwannomas are benign neoplasms and are the most common type of mass found in the cerebellopontine (CP) angle location. Most of the solitary tumours are sporadic, whereas bilateral vestibular schwannomas are strongly suggestive of type 2 neurofibromatosis. They classically present as solid mass with intracanalicular extension causing widening of porus acousticus with intense post-contrast enhancement. They may show haemorrhage and cystic degeneration. There is no uniform consensus regarding diffusion characteristics of schwannomas. Histologically they consists of fibrous tissue and hypercellular Antoni type A bodies and hypocellular Antoni type B bodies with widely separated cells in reticulated myxoid matrix [8]. Low ADC values in some schwannomas are presumed to be secondary to the predominant hypercellular Antoni type A cells (Figure 3).
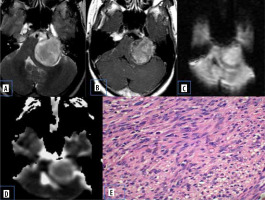
Figure 3
Vestibular schwannoma. A) Axial T2-W MRI shows heterogenous signal intensity lesion in the left CP angle with intracanalicular extension. B) Axial T1-W post-contrast image shows heterogenous enhancement. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. E) Histopathological evaluation shows areas of compact, elongated cells with occasional nuclear palisading (Antoni A pattern) and less cellular, loosely textured cells (Antoni B)
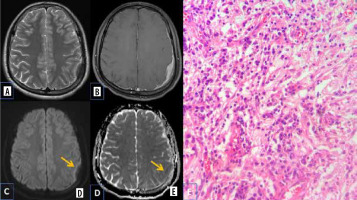
Central neurocytoma
Central neurocytomas are rare WHO grade II neuronal intraventricular tumours, which constitute about 0.25 to 0.5% of all CNS tumours. They most commonly occur in intraventricular locations involving the lateral ventricles and are often attached to the septum pellucidum. They occur in young patients (20-40 years old), who mostly present with features of raised intracranial pressure. On MR imaging, they appear mainly as a solid lesion with intratumoural cystic changes and broad attachment to the septum pellucidum and/or lateral wall of the lateral ventricle [9].
Diffusion characteristics are not well documented in central neurocytomas. The restricted diffusion in a few central neurocytomas could be related to high cell density, which leads to reduced extracellular space and an increased nucleus-to-cytoplasmic ratio, which inhibit free motion of water molecules (Figure 4).
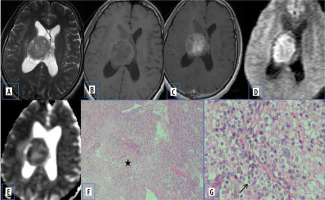
Figure 4
Central neurocytoma. A and B) T2-W axial and T1-W axial MRI shows isointense lesion attached to septum pellucidum. C) Axial T1-W post-contrast image shows heterogenous enhancement. D and E) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. F) Paraffin section shows acellular neuropil like areas (asterisk) with cellular areas. G) High-power view shows round cells with prominent perinuclear halo (arrow) [Haematoxylin and eosin: (F) ×100; (G) ×400]
Pleomorphic xanthoastrocytoma (PXA)
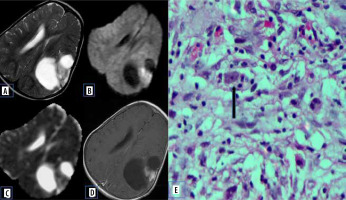
These are rare, WHO grade II (low grade) astrocytomas that occur in young patients (usually less than 30 years old), who often present with temporal lobe epilepsy. They are cortical tumours with solid a enhancing nodule with an eccentric cystic component. They may exhibit dural tail sign on post-contrast images due to their peripheral location with inflammatory reactive response of the adjacent leptomeninges [10]. The lower ADC value could be explained by atypical histological features of PXA, which is usually composed of pleomorphic tumour cells, often large and multinucleated, with an increased nucleus-to-cytoplasmic ratio, which may restrict the movement of water molecules (Figure 5).
Figure 5
Pleomorphic xanthoastrocytoma. A) Axial T2-W MRI shows hyperintense lesion in the posterior third ventricle. B) Axial T1-W post-contrast image shows homogenous enhancement. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. E and F) Histopathological evaluation shows cells showing xanthomatous change with cellular pleomorphism. G) GFAP-positive tumour cells. H) Immuno-negativity for PLAP [Haematoxylin and eosin: (E) ×100, (F) ×400] [Avidin Biotin Complex immunoperoxidase method: (G) ×400]
Solitary fibrous tumour
Solitary fibrous tumours (SFT) are rare mesenchymal tumours that can occur at any age group, predominantly seen at 5-60-year-olds. Most of them are extra axial and dural based. Few intra-axial brain parenchymal cases have also been reported [11]. On MR imaging, they exhibit diverse characteristics, with appearance on the particular tissue components within the tumours. Commonly, they are isointense on T1WI, and iso- to hyperintense on T2WI, with intense heterogeneous or homogeneous enhancement [11]. They are highly cellular tumours composed of spindle cells with ovoid nuclear and less cytoplasm with irregular intercellular collagen bundles, which contributes to its diffusion characteristics (Figure 6).
Figure 6
Solitary fibrous tumour. A) Axial T2-W MRI shows hyperintense lesion in the left parasagittal frontal region. B) Axial T1-W post-contrast image shows heterogenous enhancement. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion at the medial aspect of the lesion. E) Histopathological evaluation shows sheets of uniform homogenous cells with round to oval nucleus, moderate cytoplasm arranged in storiform pattern at places. Intratumoural, thick-walled blood vessels noted
Pineocytoma
Pineocytomas constitute 14-60% of pineal parenchymal neoplasms. They are composed of well-differentiated pineocytes and are considered as slow growing WHO grade I or II tumours. The usual age of presentation is during adulthood (mean age 38 years); however, they can occur throughout life. On magnetic resonance imaging (MRI), pineocytomas are usually low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images. These lesions typically show avid contrast enhancement. Occasionally cystic or partially cystic pineocytomas are also noted, which demonstrate internal or nodular wall enhancement on post-contrast imaging [12]. Major data regarding diffusion and ADC values is yet to be established. Atypical behaviour of pineocytomas causing restricted diffusion can be explained by the compact arrangement of the ganglion cells in this location (Figure 7).
Figure 7
Pineocytoma. A) Axial T2-W MRI shows hyperintense pineal region lesion. B and C: DWI at b 1000 s/mm2 and ADC map show restricted diffusion. D) Axial T1-W post-contrast image shows homogenous enhancement. E) Histopathological evaluation shows small, uniform, mature cells resembling pineocytes
Desmoplastic infantile ganglioglioma
Desmoplastic infantile gangliogliomas are rare WHO grade I intracranial tumours, which have good prognosis despite their aggressive appearance on imaging. They occur in children, usually less than 2 years of age. They are usually large and predominantly cystic tumours with a peri-pheral solid component. The solid portion of the mass is usually located along the cortical margin and shows intense enhancement. They may occasionally show a dural tail [13].
Histologically, these tumours show a poorly differentiated neuroepithelial component with small embryonal cells, which are densely packed in a reticulin free fibrillar matrix, which results in decreased volume of extracellular matrix, thereby causing restricted diffusion (Figure 8). Diffusion findings may mislead the radiologist to other malignant differentials such as embryonal tumours with multi-layered rosettes and atypical teratoid rhabdoid tumours (ATRT).
Figure 8
Desmoplastic infantile ganglioglioma. A) Axial T2-W MRI shows cyst with mural nodule in left temporal lobe without perilesional oedema. B and C) DWI at b 1000 s/mm2 and ADC map show restricted diffusion of the mural nodule. D) Axial T1-W post-contrast image shows enhancement of mural nodule. E) Histopathological evaluation shows neoplastic neurons range from atypical ganglionic cells to small polygonal cell types
Gangliocytoma
Gangliocytomas are purely neuronal tumours without glial tissue. They are frequently encountered in children who present with epilepsy. No malignant change has been observed in the neurons of these tumours. On imaging, they are cortical-based solid tumours without significant perifocal oedema or significant mass effect. They may show calcification and cystic changes. The solid component of these tumours usually shows enhancement [14]. There is a lack of available data in the literature regarding its diffusion and ADC values. Histologically, the tumour cells contain large nuclei with prominent nucleoli usually showing multipolar morphology, which can probably explain the restricted diffusion (Figure 9).
Figure 9
Gangliocytoma. A) Axial T2-W MRI shows hyperintense suprasellar lesion. B) On axial T1-W lesion is isointense. C) Axial T1-W post-contrast image shows heterogenous enhancement. D and E) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. F) Histopathological evaluation shows irregular group of large, multipolar neurons with dysplastic features
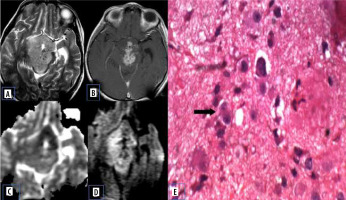
Atypical choroid plexus papilloma
Choroid plexus tumours are rare neuroepithelial intraventricular tumours. Papillomas are typically seen in younger children under the age of 5 years. Atypical papillomas show increased mitotic activity. Compared to the choroid plexus papilloma, atypical papillomas show necrosis, peritumoral oedema, and blurred margins [15]. Jeibmann et al. [16] in their study discussed the significance of atypical histological features in choroid plexus tumours. This probably reflects on the diffusion characteristics of the lesion (Figure 10).
Figure 10
Atypical choroid plexus papilloma. A) Axial T2-W MRI shows posterior third ventricular cystic lesion with small solid component. B) Axial T1-W post-contrast image shows enhancement of the solid component and cyst wall. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion of the solid component. E, F, and G) Histopathological evaluation shows acellular neuropil like areas, high-power view showing round cells with prominent perinuclear halo
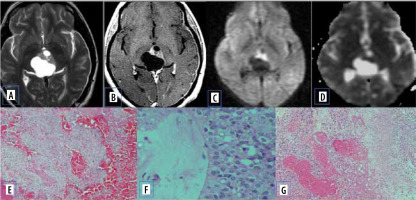
Pilomyxoid astrocytoma
Pilomyxoid astrocytoma (PMA) are rare paediatric solid brain tumours, considered as an aggressive variant of pilocytic astrocytoma. They commonly occur in the hypothalamic-chiasmatic region but can rarely be found elsewhere. They have diverse characteristics on MRI. Usually, they are well circumscribed, have solid or cystic components, and are isointense on T1- and hyperintense on T2-W images. They demonstrate homogeneous or heterogeneous enhancement with contrast. The ADC values and T2 signal intensity are generally higher than in PAs, which indicates the proportion of myxoid matrix. Differentiating pilomyxoid astrocytoma from pilocytic astrocytoma is important because pilomyxoid astrocytomas are relatively aggressive with a higher chance of recurrence. They can also spread by CSF dissemination. Histologically, pilomyxoid astrocytomas show a predominant myxoid matrix and angiocentric pattern of arrangement of bipolar cells, which may inhibit the free movement of water molecules [17]. This is a potential reason for restricted diffusion on MR imaging (Figure 11).
Figure 11
Pilomyxoid astrocytoma. A) Axial T2-W MRI shows hyperintense suprasellar lesion. B and C) DWI at b 1000 s/mm2 and ADC map show restricted diffusion. D and E) Axial and sagittal T1-W post-contrast image show heterogenous post enhancement. F) Histopathological evaluation shows prominent mucoid matrix and angiocentric arrangement of monomorphous, bipolar tumour cells, typically without Rosenthal fibres or eosinophilic granular bodies/hyaline droplets
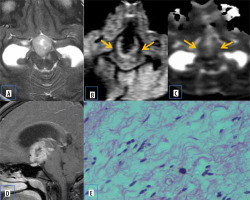
Inflammatory pseudotumor
Primary intracranial pseudo tumours are rare lesions with unknown aetiology. The MRI findings are variable and non-specific. Microscopic examination shows dense chronic inflammatory infiltrate comprising plasma cells, lymphocytes, a few histiocytes, eosinophils, and occasional neutrophils with marked fibrosis [18]. There are few studies that document the role of DWI in inflammatory pseudotumour. Their potential risk for malignant transformation and recurrence necessitate periodic follow-up.
The possible explanation being offered for restricted diffusion in inflammatory pseudotumour is increased cell membrane synthesis and increased cellularity, which is attributed secondarily to hyperplasia of the inflammatory cells (Figure 12).
Figure 12
Primary intracranial pseudo tumour. A) Axial T2-W MRI shows hypointense dural based lesion in the left frontoparietal convexity. B) Axial T1-W post-contrast image shows homogenous enhancement with dural thickening. C and D) DWI at b 1000 s/mm2 and ADC map show restricted diffusion at the periphery of the lesion (arrows). E) Histopathological evaluation shows characteristic emperipolesis intermixed with plasma cells and small lymphocytes
Although there is abundant literature on the role of DWI in high-grade neoplasms, its role and occurrence on benign neoplasms has not been highlighted. Radiologists should be aware of these atypical entities to avoid potential misdiagnosis.
Conclusions
Benign CNS tumours have few characteristic imaging findings, and most of them rarely show restricted diffusion on MRI. But when encountered with restricted diffusion, owing to their innate nature, as evident on histological characteristics, they can be misdiagnosed as malignant neoplasms. With improved understanding through their histological features, we can augment the diagnostic capability and prevent potential misdiagnosis, especially in rare tumours. With upcoming advances in DWI, further research is needed to clarify reasons for the restricted diffusion in benign intracranial neoplasms.


