Received: Fri 21, Oct 2022
Accepted: Mon 07, Nov 2022
Abstract
COVID-19 is identified as primarily a respiratory illness but its impact on other end organs has increasingly been brought to light in literature. In rare occasions, patients develop liver disease that deteriorates to the point of transplant consideration. Mechanism of this physiologic drift is not fully understood. We describe a rare case of COVID-19 in a 57-year-old man whose clinical course was complicated with the development of diffuse intra-hepatic strictures. This report demonstrates the abrupt development of cholestatic liver injury in critically ill COVID-19 patients, adding to the literature on long-term non-respiratory impacts of COVID-19.
Keywords
COVID-19, biliary strictures, liver transplantation, sclerosing cholangitis
1. Introduction
Liver injury is common in patients with COVID-19 infection with about 70% of admitted patients demonstrating a hepatocellular elevation of liver enzymes primarily due to hepatic expression of the Ribonucleic acid (RNA) virus receptor [1, 2]. In most cases the liver injury seen is transient and self-limiting but in some rare situations, critically ill patients with COVID-19 infection develop a persistent cholestatic elevation in liver enzymes despite pulmonary and renal recovery [3, 4]. This case reports the clinical course of a 57-year-old patient with prolonged hospitalization from COVID-19, no prior history of liver disease presenting with abdominal pain, jaundice, dark-coloured urine, and persistent elevation of liver enzymes, which was proved to be secondary to diffuse intrahepatic strictures as noted on endoscopic retrograde cholangiopancreatography (ERCP).
2. Case Presentation
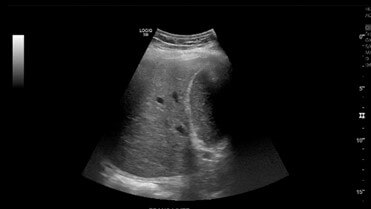
This is a 57-year-old patient with past medical history of COVID-19 pneumonia complicated by prolonged hospitalization with hypoxic respiratory failure requiring intubation was admitted with a chief complaint of abdominal pain with dark-coloured urine and jaundice. Laboratory workup obtained was remarkable for a cholestatic elevation of liver enzymes with Alkaline phosphatase (ALP), Alanine transaminase (ALT), and Aspartate aminotransferase (AST) 900, 100, 85, respectively. The patient denied history of alcohol use. The patient’s elevation of liver enzymes dates as far back as six months prior during his hospitalization for COVID pneumonia. During that time, elevation was believed to be secondary to critical illness as patients required pressors to maintain hemodynamics. Interestingly, even after discharge, liver enzymes remained elevated. At that time, a right upper quadrant abdominal ultrasound (Figure 1) was obtained which noted a normal sonographic appearance of the liver and biliary tree with no intrahepatic and extrahepatic biliary dilatation. Magnetic resonance imaging (MRCP) noted the hydropic appearance of the gallbladder containing sludge, but no biliary ductal dilation or choledocholithiasis was found.
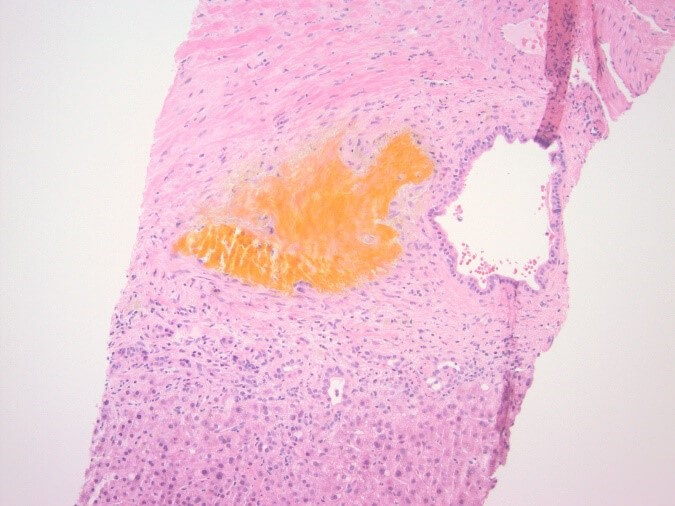
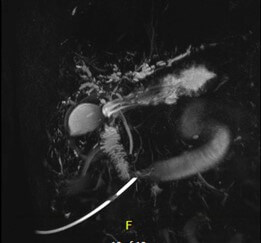
Hepatitis A, B, and C panel, Epstein Barr virus (EBV), cytomegalovirus (CMV), Smooth muscle antibody, and Alpha 1 antitrypsin testing all returned unremarkable. At this point, transaminitis was believed to be secondary to Bactrim. The patient was on for prophylaxis as he was being treated with high-dose steroids for necrotizing myositis. Subsequently liver biopsy was done and the results were remarkable for large bile duct obstruction injury pattern secondary to infection versus ischaemia (Figure 2). The decision was made to repeat MRCP and this time, development of intrahepatic biliary ductal dilation with relative caliber changes at the liver hilum though no obstructing lesion was identified (Figure 3). Endoscopic retrograde cholangiopancreatography (ERCP) was done to correlate with MRCP findings. Multiple severe segmental biliary strictures were found in the left main hepatic duct, right main hepatic duct, and left and right hepatic and all intrahepatic branches, the strictures were fibrotic and inflammatory. The biliary tree was swept, and nothing was found. A biliary sphincterotomy was performed and cells for cytology were obtained from the left and right main hepatic duct which returned all negative for malignant cells. A colonoscopy was performed to rule out underlying inflammatory bowel disease (IBD) and the results were unremarkable. Overall, the patient’s presentation is believed to be a case of COVID-19 – related secondary sclerosing cholangitis with Model for end-stage liver disease (MELD) score >23 and Child-Pugh class B. Unfortunately, the patient was deemed not a candidate for liver transplantation as risks of transplantation outweighed benefits due to the patient being bed bound for months and subsequent development severe muscle wasting and protein calorie malnutrition.
3. Discussion
COVID-related sclerosing cholangitis (CrSC) is a form of cholestatic liver disease seen in critically ill COVID-19 patients with only a few cases recorded worldwide. Due to its rarity, the mechanism of disease development is not fully understood. Circulatory shock in critically ill COVID-19 patients is seen to play a predominant role in the pathogenesis of the disease process because unlike hepatocytes with dual blood supply from the portal vein and hepatic artery, the biliary tree is made of cholangiocytes with biliary epithelium that receives a single blood supply from the peri-biliary plexus making more prone to injury in settings of systemic hypoperfusion [5-7]. When ischaemic occurs, necrosis and subsequent sloughing off of the biliary epithelium is seen.
On the other hand, formation of toxic bile with high acid content is also linked to the development of CrSC [5]. Physiologically, bile acids are secreted by the liver into the biliary system and biliary secretion of bicarbonate helps to counteract the damage this bile acid does to the biliary epithelium. Increased inflammatory response seen in critically ill COVID patients increases hepatic secretion of toxic bile displacing the effect of bicarbonate and subsequently leads to cholangiocyte necrosis [8].
As in our patient, all cases reported of COVID-related sclerosing cholangitis all had long hospital courses requiring mechanical ventilation. Mechanical ventilation with high positive end expiratory pressure (PEEP) usually > 10 cm H20 is seen to lead to microcirculatory ischaemia within the hepato-splanchnic plexus [9] leading the sclerosing cholangitis. The risk also increases in patients with refractory hypoxia needing pronation.
The mortality rate of CrSC in intensive care unit (ICU), patients go as high as 50% [10]. 40% of patients develop stable liver cirrhosis and 20% require transplantation. [11] Overall, the median survival rate without liver transplantation is 12-44 months [12]. For this reason, further investigation is needed in the literature to understand the effect of COVID-19 on the biliary system.
4. Conclusion
COVID-19 related sclerosing cholangitis is a dramatic but yet unfortunate disease process seen in critically ill patients, associated with destruction of the biliary tree with diffuse stricture formation, with major concern being the development of progressive liver failure with potential need for liver transplantation. The pathophysiology of cholangiopathy in COVID-19 patients requires further investigation for better understanding of the disease process and need for prevention.
REFERENCES
[1]
Ling
Xu, Jia Liu, Mengji Lu et al. “Liver injury during highly pathogenic human
coronavirus infections”. Liver Int, vol. 40, no. 5, pp. 998-1004, 2020.
View at: Publisher
Site | PubMed
[2]
Xiaoqiang
Chai, Longfei Hu, Yan Zhang et al. “Specific ACE2 Expression in Cholangiocytes
May Cause Liver Damage After 2019-nCoV Infection”. Bio-Rxiv [Preprint],
2020. View at: Publisher
Site
[3]
Qingxian
Cai, Deliang Huang, Hong Yu et al. “COVID-19: Abnormal liver function tests”. J
Hepatol, vol. 73, pp. 566-574, 2020. View at: Publisher
Site | PubMed
[4]
Oren
K Fix, Bilal Hameed, Robert J Fontana et al. “Clinical Best Practice Advice for
Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD
Expert Panel Consensus Statement”. Hepatology, vol. 72, no. 1, pp.
287-304, 2020. View at: Publisher
Site | PubMed
[5]
Silke
Leonhardt, Wilfried Veltzke-Schlieker, Andreas Adler “Trigger mechanisms of
secondary sclerosing cholangitis in critically ill patients”. Crit Care,
vol. 19, no. 1, pp. 131, 2015. View at: Publisher
Site | PubMed
[6]
L
Laurent, C Lemaitre, A Minello “Cholangiopathy in critically ill patients
surviving beyond the intensive care period: a multicentre survey in liver
units”. Aliment Pharmacol Ther, vol. 46, no. 11-12, pp. 1070-1076, 2017.
View at: Publisher
Site | PubMed
[7]
Pierre
Deltenre, Dominique-Charles Valla “Ischemic cholangiopathy”. J Hepatol,
vol. 44, no. 4, pp. 806-817, 2006. View at: Publisher
Site | PubMed
[8]
Ulrich
Beuers, Simon Hohenester, Lucas J Maillette de Buy Wenniger et al. “The biliary
HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic
aspects of fibrosing cholangiopathies”. Hepatology, vol. 52, no. 4, pp.
1489-1496, 2010. View at: Publisher
Site | PubMed
[9]
Gabriele
I Kirchner, Petra Rümmele “Update on Sclerosing Cholangitis in Critically Ill
Patients”. Viszeralmedizin, vol. 31, no. 3, pp. 178-184, 2015. View at: Publisher Site | PubMed
[10]
T
Voigtländer, A A Negm, A S Schneider et al. “Secondary sclerosing cholangitis
in critically ill patients: model of end-stage liver disease score and renal
function predict outcome”. Endoscopy, vol. 44, no. 11, pp. 1055-1058,
2012. View at: Publisher
Site | PubMed
[11] Ting Lin, Kai Qu, Xinsen Xu et al. “Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience”. Front Med, vol. 8, no. 1, pp. 118-126, 2014. View at: Publisher Site | PubMed
[12] H Kulaksiz, D Heuberger, S Engler et al. “Poor outcome in progressive sclerosing cholangitis after septic shock”. Endoscopy, vol. 40, no. 3, pp. 214-218, 2008. View at: Publisher Site | PubMed
