Pancytopenia with Recurrent Infections in Adolescents and Young Adults Should Remind of GATA2 Deficiency: A Case Report
Letícia Guimarães, Laura Magalhães, Letícia Teixeira, Flávio Guedes, Luciana Werneck Zuccherato and Ricardo Mesquita Camelo*
Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte, Brazil
Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
Faculdade de Ciências Médicas de Minas Gerais, Belo Horizonte, Brazil.
Received Date: 07/06/2021; Published Date: 22/06/2021
*Corresponding author: Ricardo Mesquita Camelo, MD, MBA, MSc. Rua Lorena, 1020/101 Padre Eustáquio – Belo Horizonte-MG Brazil. ZIP 30.730-170. Tel: +55.31.991518362. Email: rmcamelo@hotmail.com, ORCID: https://orcid.org/0000-0001-9025-0289
Abstract
GATA2 is a hematopoiesis regulatory nuclear protein. GATA2 deficiency syndrome is a rare disorder caused by heterozygous mutations leading to GATA2 decreased gene expression. It has several phenotypes, in special immunodeficiency predisposing to myelodysplastic syndrome (MDS). We reported a 19-year-old woman who developed pancytopenia with hypocellular bone marrow of unclear etiology. Subsequently, she presented recurrent and rapidly evolving infections, predominantly bacterial. The association between her clinical and hematological alterations highlighted the likelihood of GATA2 deficiency, later confirmed by the detection of the heterozygous variant c.1084C>T (R362*) in GATA2. Allogeneic bone marrow transplantation was performed with complete engraftment and reversion of hematological and infectious phenotypes. The early diagnosis and treatment were critical to control the progression of MDS and her vulnerability to infections. This case highlights the importance of GATA2 deficiency suspicion in adolescents and young adults with recurrent infections and pancytopenia, especially when bone marrow dysplasia is also diagnosed.
Keywords: GATA2 deficiency; Recurrent infections; Myelodysplastic syndrome; Bone marrow transplantation
Introduction
Guanine-adenine-thymine-adenine sequence linkers type 2 (GATA2) is a regulatory nuclear protein that binds to the consensus T/A(GATA)A/G DNA sequence, located on numerous promoters and enhancers. This protein controls the transition from hemogenic endothelium to hematopoietic stem cells (HSC), essential for the survival and the self-renewal (maintenance) of different blood cell lines [1,2].
GATA2 gene is located on the long arm of chromosome 3 (3q21.3) [1]. GATA2 deficiency is an autosomal dominant syndrome caused by heterozygous GATA2 mutations in germlines, but somatic mutations have also been reported [3]. Most described mutations behave similarly as they reduce/abrogate transcriptional activity [1,3]. Furthermore, almost 50% of individuals with GATA2 mutations develop myelodysplastic syndrome (MDS) associated with fibrosis and megakaryocyte dysplasia [1]. Moreover, monocytopenia, mild chronic neutropenia, and NK lymphocyte deficiency are associated with GATA2 deleterious variants [3-5]. However, many patients experience clinical complications before reaching the criteria for MDS. Treatment initially focuses on infection control, while defining the best time to treat the bone marrow dysfunction.
Case Report
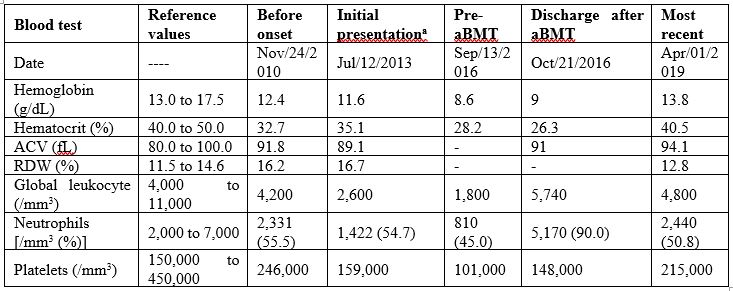
During a routine consultation in 2013, a 19-year-old woman had mild anemia with anisocytosis, poikilocytosis with ovalocytes, dacrocytes, and polychromatophilia, and leucopenia without morphological changes (Figure 1, Table 1). Her previous medical records showed normal blood counts. There was no history of hematological diseases in her family.
Initial investigation ruled out hypovitaminosis, and thyroid, renal and hepatic diseases. Rheumatoid factor, antinuclear factor, serologies for viral hepatitis, human immunodeficiency virus and cytomegalovirus were negative. No clones were detected by flow cytometry. Karyotype was normal, and the chromosomal breakage assay was negative. The investigation for celiac disease was negative, and the immunoglobulin dosage was normal. However, she had altered iron kinetics. No abnormalities were found on chest X-ray, electrocardiogram, transthoracic echocardiography, abdominal ultrasound, and rectosigmoid/colonoscopy. Enanthematous pangastritis associated with H. pylori and erosive duodenitis were treated.
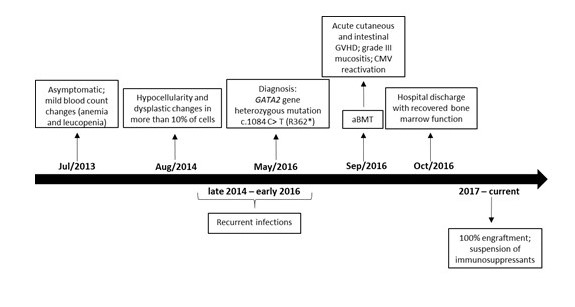
Figure 1: Timeline from asymptomatic period before diagnosis to post-transplant and currently.
aIn the initial presentation, poikilocytosis with ovalocytes, target red blood cells, and dacryocytes with polychromatophilia were also evidenced.
aBMT, allogeneic bone marrow transplantation; GVHD, graft versus host disease; CMV, cytomegalovirus.
Table 1: Blood count evolution from initial presentation to the last follow-up visit.
The bone marrow aspiration in early 2014 showed normocellular erythrocyte series, with dysplastic alterations in more than 10% of the cells evaluated as focal chromatin attenuation, mitosis figures, and hemoglobinization failure; normocellular granulocytic series, with presence of hypo granular cells, and hyper segmented neutrophils; unchanged lymphoplasmacytic series with 3.5% of plasma cells; but with enlarged, hyper lobulated, and dysplastic megakaryocytes, with no evident platelet production (Figure 1). The karyotype was normal. The biopsy showed hypocellularity, but with rare immature CD34+ cells and micro megakaryocytes (Figure 1). Iron staining was negative and revealed no deposit nor reserve in the bone marrow.
In September/2014, a right axillary abscess was drained and treated with cephalexin. In December/2014, a necrotizing Streptococcus pyogenes lymphadenitis after pharyngitis was drained and treated with clindamycin. In March/2015, a pansinusitis was treated with amoxicillin+clavulanate. In October/2015, she had a perianal abscess without fistulation which was drained and treated with ciprofloxacin and metronidazole. In November/2015, a right lower limb lymphadenitis was firstly treated with ceftriaxone and metronidazole, switched to amoxicillin+clavulanate. In February/2016, she had an eye infection with right-sided corneal ulcer, without pathogen identification. She was initially treated with moxifloxacin eye drops and acyclovir eye ointment, later changed to natamycin and amphotericin B eye drops. In September/2016, a human papilloma virus-associated mild cervical dysplasia was cauterized.
The association of hematological alterations of unclear etiology and recurrent infections highlighted the possibility of GATA2 deficiency syndrome. In May/2016, Sanger sequencing from peripheral blood leucocytes confirmed the hypothesis by detecting the heterozygous variant c.1084C>T (p. Arg362Ter) or R362*, rs1553770510[1], in the GATA2 gene, which generates a stop codon in the normal protein sequence (Figure 1) [6]. The mutation was not detected in her first-degree relatives.
Allogeneic bone marrow transplantation (aBMT) was immediately indicated in evidence of pancytopenia (pre-aBMT in Table 1), confirmed by both bone marrow aspiration and biopsy. Immunophenotyping confirmed B-cell and NK lymphopenia. Transthoracic echocardiogram, electrocardiogram, chest computed tomography, and pulmonary function were normal. Since there was not histocompatibility within the family, the HSC were donated by a matched unrelated individual (10/10). The conditioning regimen involved fludarabine 160 mg/m2 and busulfan 16.4 mg/kg (days -5, -4, -3 and -2) [6]. Immune prophylaxis was performed with cyclosporine, methotrexate, and anti-thymocyte globulin 7.5 mg/kg (days -3, -2 and -1). Antimicrobial prophylaxis was performed with sulfamethoxazole+trimethoprim, fluconazole, and acyclovir. On Sep/23/2016, 1.8x108 bone marrow nucleated cells/kg were infused, with 2.0x107 CD3+ cells/kg and 1.6x106 CD34+ cells/kg (Figure 1). No peri-procedure complication was reported (Table 1).
After 15 days, the patient developed grade-III mucositis and watery diarrhea due to graft-versus-host disease (GVHD), which were treated with corticosteroids (Figure 1). On the 22nd day, she developed nonpruritic maculopapular rash in the upper and lower limbs, which also resolved with corticosteroids. The antigenemia for cytomegalovirus reactivated twice, both resolving with intravenous ganciclovir followed by oral valganciclovir (Figure 1). Blood marrow function recovered with neutrophil engraftment on day 16 and platelet engraftment on day 20 post-aBMT. Chimerism analysis on day 28 post-aBMT suggested complete chimera, 100% compatible with donor genotype. The patient was discharged home.
Cyclosporine, methotrexate, and corticosteroids were gradually discontinued. Oral candidiasis, herpes zoster, and herpetic stomatitis were treated as an outpatient. One year later, she was submitted to cervical high-frequency cauterization for moderate dysplasia/squamous metaplasia. Currently, 4.5 years after aBMT, she is healthy, without infections, and in semesterly hematological follow-up (Figure 1). Her last blood count was normal in April/2019 (Table 1).
Discussion and Conclusion
The current report may contribute to a better understanding of the disease. The average age at onset of symptoms of the syndrome is 20 years, ranging from 4 months to 78 years [3,4,7]. Three-quarters of patients develop MDS at this age, mainly starting with cytopenias, immunodeficiency, and hypocellular bone marrow.(8) The affected individuals usually complicate with severe infections [8]. In the case described herein, mild anemia and leucopenia were initially evidenced, evolving to pancytopenia and bone marrow hypocellularity with micro megakaryocytes. Then the patient started rapidly evolving infections, which ultimately led to the diagnosis of GATA2 deficiency.
GATA2 deficiency has a broad phenotypical spectrum with partial penetration [1,9]. Many presentations have been described since 2011: a dominant autosomal form of familial MDS/AML [10]; Emberger syndrome comprising MDS, lymphedema, and warts [11]; MonoMAC syndrome of monocytopenia and Mycobacterium avium [2]; and the deficiency of dendritic cells, monocytes, B lymphocytes and NK cells (DCML) with vulnerability to viral infections [12]. Shortly thereafter, mutations in GATA2 were also recognized in congenital neutropenia and pediatric MDS [1,3,4,7]. Additional findings, such as deafness, erythema nodosum, spontaneous abortion, and hypothyroidism, were reported later [7]. DCML is the condition closest to the clinical presentation of our patient, as she presented B lymphoid cell and NK cytopenias. Although we have no data about dendritic cells profile, both the rarity of viral and the absence of mycobacterial infections she experienced stand against this hypothesis.
The mortality associated with recurrent infections with the risk of severe infections would be enough to justify the BMT [13]. Some authors consider the most important and relevant points for indicating aBMT are the progression of bone marrow dysplasia and cytogenetic abnormalities, due to the tendency of progression to hypocellular MDS and AML [13]. The likelihood of developing MDS/AML increases rapidly with each decade of life, from 20% at the age of 20 to 80% at 40 years, causing a considerable increase in mortality [3,7]. This reinforces the importance of early treatment of the patient. Another indication of BMT is the emergence of severe infections and their recurrences [13], the main reason why our patient was treated with aBMT.
There is no consensus on the optimal conditioning regimen for BMT, which can be nonmyeloablative, reduced-intensity conditioning, and myeloablative, depending on both donor and recipient characteristics. Cuellar-Rodriguez et al. reported six patients who received nonmyeloablative HSC transplantation (HSCT), which corrected monocyte, B-cell and NK counts, as well as the clinical phenotype [14]. Nevertheless, Grossman et al. described 14 transplanted patients, reinforcing the merit of conventional myeloablative conditioning for the elimination of pre-leukemic host cells [13]. Recently, Parta et al. described 13 matched unrelated donor recipients who received a busulfan-based conditioning regimen, from which 3 had clinical features similar to our patient: GATA2 variant, MDS, and bone marrow conditioning [6]. Although 3/13 patients died, all the 3 patients with clinical features similar to ours survived [6].
There are no reliable estimates of the prospective outcomes of these treatments. A survival rate of 54% in four years after HSCT has been reported in 21 patients transplanted for myeloid neoplasia or immunodeficiency [7]. In other study, survival after BMT depends on the age of the patient when performing the treatment, being better when it is performed sooner [3]. The treatment chosen in the respective case was myeloablative, with complete engraftment after 3 years, as reported before [13].
Given the general uncertainty about the progression, early diagnosis and BMT treatment are critical to a good outcome in GATA2 deficiency syndrome. General physicians should be aware of these signs and symptoms, to refer those patients to a specialist. The reported case addresses all these essential treatment milestones. Even among adolescents/young adults, pancytopenia with several rapidly evolving infections associated with bone marrow hypocellularity, GATA2 deficiency syndrome should also be considered. This may be decisive for successful treatment and increased survival of the patient.
Authors' contributions: RMC conceptualized and implemented the project. LG, LM, LT, and FG collected data. LZ analyzed the genetic files. All the authors contributed equally to the text writing and the final version.
Acknowledgments: The authors would like to thank the patient and her family for participating in this study.
Declarations:
Financial Support: This report did not receive any funding or grants to be planned, performed, or published.
Conflicts of Interest/Competing Interests: The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Ethics Aproval: This study was approved by the Committees on Ethics in Research (CAAE 89152518.5.0000.5121, Hospital Luxemburgo/Fundação Mário Penna/Associação Mário Penna; and CAAE 96110718.2.0000.5135, Hospital Vera Cruz - HVC/MG), in compliance with the Declaration of Helsinki and the Resolution 466/12 and Operational Norm 001/2013 of the Brazilian National Council on Health. Patient and her family signed the written informed consent form to participate and to have their data published in the case report.
Consent to Participate: Patient and her family signed the written informed consent form to participate and to have their data published in the case report.
Consent for Publication: Patient and her family signed the written informed consent form to participate and to have their data published in the case report.
Availability of Data and Material: Not applicable.
References
- Hsu AP, McReynolds LJ, Holland SM. GATA2 Deficiency. Curr Opin Allergy Clin Immunol. 2015; 15(1): 104-109.
- Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011; 118(10): 2653–2655.
- Donadieu J, Lamant M, Fieschi C, De Fontbrune FS, Caye A, Ouachee M, et al. Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica. 2018; 103(8): 1278–1287.
- Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014; 123(6): 863–874.
- Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010; 115(8): 1519–1529.
- Parta M, Shah NN, Baird K, Rafei H, Calvo KR, Cole K, et al. Allogeneic Hematopoietic Stem-Cell Transplantation for GATA2 Deficiency Using a Busulfan-Based Regimen Mark. Biol Blood Marrow Transpl. 2018; 24(6): 1250–1259.
- Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014; 123(6): 809–821.
- Wlodarski MW, Hirabayashi S, Pastor V, Stary J, Hasle H, Masetti R, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. 2015; 127(2018): 1387-1397.
- Oleaga-Quintas C, Oliveira-Júnior EB de, Rosain J, Rapaport F, Deswarte C, Guérin A, et al. Inherited GATA2 Deficiency Is Dominant by Haploinsufficiency and Displays Incomplete Clinical Penetrance. J Clin Immunol. 2021; 41: 639-657.
- Hahn CN, Chong C-E, Carmichael CL, Wilkins EJ, Brautigan PJ, Li X-C, et al. Heritable GATA2 Mutations Associated with Familial Myelodysplastic Syndrome and Acute Myeloid Leukemia. Nat Genet. 2012; 43(10): 1012-1017.
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011; 43(10): 929-931.
- Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011; 118(10): 2656-2658.
- Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant [Internet]. 2014; 20(12): 1940-1948. Available from: http://dx.doi.org/10.1016/j.bbmt.2014.08.004
- Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011; 118(13): 3715-3720.

