Individual’s DNA Repair Capacity and COVID-19: Let’s Take One Step Back to understand it
Sneh M Toprani*
Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Received Date: 02/06/2020; Published Date: 07/07/2020
*Corresponding author: Sneh Manishi Toprani, John B Little Center for Radiation Sciences, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA. E-mail: stoprani@hsph.harvard.edu, snehtoprani@gmail.com
Editorial
The outbreak of novel coronavirus from Wuhan, Hubei Province, China has brought turbulence in the entire world. On January 9, 2020; World Health Organization reported that Chinese authorities had identified a novel strain of coronavirus. Each and every country and its authorities are taking major steps to avoid the spread of this pandemic situation. Coronavirus pandemic has disrupted the economy of many countries by hampering business, airline industry, stock market, tourism, and several more. The spread of the virus among humans has demonstrated a wide range of differences in susceptibility. Many individuals get infected by virus or remain asymptomatic or are carriers of it. Immunity, self-hygiene, and civic sense play a pivotal role to avoid getting infected in this scenario of existing overburden in the medical field.
Keywords: COVID-19; Coronavirus DNA Damage; DNA Repair; Human; Respiratory Illness
However, there is a wide variation observed in an individual’s susceptibility towards contracting the COVID-19 infection and their treatment outcomes. Viruses are known to play with the host’s DNA damage responses by either over activating or inhibiting it [1]. There is a need to understand the changes of biological mechanisms undergoing on the viral infection and its impact on the host’s DNA damage and repair responses (Figure 1).
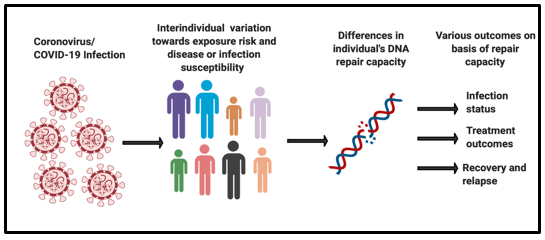
Figure 1: Inter individual variation in DNA repair capacity towards COVID-19 infection outcomes.
Every single minute, humans are exposed to diverse forms of endogenous and exogenous DNA damaging agents which threatens to disrupt the genomic integrity of the cells. Various forms of DNA lesions (single-strand breaks, double-strand breaks, oxidative base damages, abasic sites, mismatch bases, inter DNA crosslink, DNA bulky adducts) are induced by these agents [2,3]. Naturally, existing DNA repair mechanisms (such as base excision repair, non-homologous end joining, mismatch repair, homologous recombination, nucleotide excision repair, direct reversal repair, Fanconi anemia repair, and many others) ensure faithful repair of the DNA lesions to maintain the homeostasis [4,5]. Accumulation of unrepaired DNA lesions and inefficient DNA repair activity can lead to an increase in genomic unstable cells, which if escaped cell death mechanisms, can proliferate to generate damaged daughter cells which can be selective for malignant transformation. These defects have been associated with many adverse reproductive health incidences, cancer risk, disease susceptibility, aging, neurodegenerative disorders, and variation in treatment outcomes [2]. Alterations in DNA damage and repair mechanism can further hamper the multitude downstream cellular processes such as proliferation, inflammatory responses, cell cycle alterations, apoptosis, increase in oxidative stress, metabolic changes, and many others [6]. These alterations in the ancillary cellular processes can have a major impact on the functioning of the organ or the human body. Genetic polymorphism of DNA repair genes, sex, age, lifestyle, body mass index, stress level, ethnic or racial background, family history impacts the individual’s DNA repair capacity which in turn are associated with infection susceptibility or disease risk [7,8].
Understanding the role of DNA damage and repair mechanisms in virus infection and collaborating with the genetic parameters of the individual can shed some light at the end of the dark tunnel. Changes in DNA damage and repair capacity of individuals on getting infected and after treatment can assist to establish the relationship of the underlying potential biological mechanisms. A deeper understanding of the biological basis with genetic factors and environmental exposures can assist to design personalized treatment regime.
References
-
- Xiaofei E, Kowalik TF. The DNA damage response induced by infection with human cytomegalovirus and other viruses. Viruses. 2014;6(5):2155‐2185. DOI:10.3390/v6052155
- Toprani SM. DNA damage and repair scenario in ameloblastoma. Oral oncology.2020;108:104-804. DOI: 10.1016/j.oraloncology.2020.104804.
- Soren DC, Toprani SM, Jain V, Saini D, Das B. Quantitation of genome damage and transcriptional profile of DNA damage response genes in human peripheral blood mononuclear cells exposed in vitro to low doses of neutron radiation. International Journal of Radiation Research 2019;17(1):1-14. DOI: http://ijrr.com/article-1-2453-en.html.
- Toprani SM, Das B. Role of base excision repair genes and proteins in gamma-irradiated resting human peripheral blood mononuclear cells. Mutagenesis. 2015a;30: 247-61. DOI: 10.1093/mutage/geu065.
- Saini D, Shelke S, Mani Vannan A, Toprani S, Jain V, Das B, et al. Transcription profile of DNA damage response genes at G0 lymphocytes exposed to gamma radiation. Mol Cell Biochem. 2012;364: 271–281. DOI: 10.1007/s11010-012-1227-9.
- Sun T, Yang W, Toprani SM, Guo W, He L, De Leo AB, et al. Induction of immunogenic cell death in radiation-resistant breast cancer stem cells by repurposing anti-alcoholism drug disulfiram. Cell communication and signaling. 2020;18:36. DOI: 10.1186/s12964-019-0507-3.
- Toprani, S.M. and Das, B. Radio-adaptive response, individual radio-sensitivity and genetic association of base excision repair gene polymorphism (hOGG1, APE1, XRCC1 and LIGASE1) in human peripheral blood mono-nuclear cells exposed to gamma radiation Environmental and Molecular mutagenesis. Environmental and Molecular Mutagenesis. 2020. DOI:10.1002/em.22383.
- Toprani SM, Das B. Radio-adaptive response of base excision repair genes and proteins in human peripheral blood mononuclear cells exposed to gamma radiation. Mutagenesis 2015b;30: 663-76. DOI: 10.1093/mutage/gev032.

