Published online Nov 26, 2016. doi: 10.4330/wjc.v8.i11.623
Peer-review started: May 3, 2016
First decision: June 17, 2016
Revised: July 7, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: November 26, 2016
Surgical revascularization with coronary artery bypass grafting (CABG) has become established as the most effective interventional therapy for patients with moderately severe and severe stable ischemic heart disease (SIHD). This recommendation is based on traditional 5-year outcomes of mortality and avoidance of myocardial infarction leading to reintervention and/or cardiac death. However, these results are confounded in that they challenge the traditional CABG surgical tenets of completeness of anatomic revascularization, the impact of arterial revascularization on late survival, and the lesser impact of secondary prevention following CABG on late outcomes. Moreover, the emergence of physiologic-based revascularization with percutaneous cardiovascular intervention as an alternative strategy for revascularization in SIHD raises the question of whether there are similar physiologic effects in CABG. Finally, the ongoing ISCHEMIA trial is specifically addressing the importance of the physiology of moderate or severe ischemia in optimizing therapeutic interventions in SIHD. So it is time to address the role that physiology plays in surgical revascularization. The long-standing anatomic framework for surgical revascularization is no longer sufficient to explain the mechanisms for short-term and long-term outcomes in CABG. Novel intraoperative imaging technologies have generated important new data on the physiologic blood flow and myocardial perfusion responses to revascularization on an individual graft and global basis. Long-standing assumptions about technical issues such as competitive flow are brought into question by real-time visualization of the physiology of revascularization. Our underestimation of the impact of Guideline Directed Medical Therapy, or Optimal Medical Therapy, on the physiology of preoperative SIHD, and the full impact of secondary prevention on post-intervention SIHD, must be better understood. In this review, these issues are addressed through the perspective of multi-arterial revascularization in CABG, which is emerging (after 30 years) as the “standard of care” for CABG. In fact, it is the physiology of these arterial grafts that is the mechanism for their impact on long-term outcomes in CABG. Moreover, a better understanding of all of these preoperative, intraoperative and postoperative components of the physiology of revascularization that will generate the next, more granular body of knowledge about CABG, and enable surgeons to design and execute a better surgical revascularization procedure for patients in the future.
Core tip: This review examines the emerging understanding of physiology in revascularization from the preoperative, intraoperative and postoperative perspectives. The particular importance of physiology in arterial revascularization, which is becoming the standard of care, is discussed using novel intraoperative imaging data results. These imaging data objectively confirm certain physiologically-determined outcomes, and highlight inadequacies in a number of long-standing assumptions about surgical revascularization with coronary artery bypass grafting.
- Citation: Ferguson Jr TB. Physiology of in-situ arterial revascularization in coronary artery bypass grafting: Preoperative, intraoperative and postoperative factors and influences. World J Cardiol 2016; 8(11): 623-637
- URL: https://www.wjgnet.com/1949-8462/full/v8/i11/623.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i11.623
Arterial revascularization, and in particular complete arterial revascularization, is a current emerging trend in surgical revascularization with coronary artery bypass grafting (CABG). This Review examines the physiologic aspects of arterial revascularization in light of its documented clinical outcomes benefits (Table 1).
| Arterial revascularization in CABG |
| Emerging “standard of care” for CABG |
| Years of data to document benefits, but slow to adopt |
| Both based on long term survival outcomes |
| Mechanisms for increased survival based on traditional anatomic construct for surgical revascularization |
| Better long-term graft patency |
| Preoperative factors and influences |
| Effectiveness of GDMT - physiologic modulation of underlying ischemia |
| Extent of disease |
| Collateral development |
| Influence on myocardium |
| Impact on subsequent revascularization |
| ISCHEMIA trial |
| Equipoise issue |
| Implications for revascularization |
| Same physiologic principles impacting PCI must also impact CABG |
| Difference in anatomic extent of disease |
| Surgical revascularization not dependent on completeness of (anatomic) revascularization |
| Intraoperative factors and influences |
| Dynamic nature of in situ arterial grafts |
| Competitive flow in arterial grafts (vs vein grafts) |
| Incomplete revascularization vs appropriate incomplete revascularization |
| FFR-based revascularization |
| Postoperative factors and influences |
| Secondary prevention measures in CAD |
| DAPT |
| Secondary prevention efforts following CABG |
Since its inception in the 1960s, the history of CABG has included incremental developments to improve outcomes[1]. Among these, the use of in situ internal mammary artery (IMA) grafting has been documented to have the most profound beneficial effect[2,3]. Placed to the left anterior descending coronary artery (LAD), this intervention is perhaps the most singularly effective in all of ischemic heart disease[4,5]. Multiple studies have documented excellent short-term angiographic results, superior long-term patency vs other conduits, and a direct impact on long-term survival in observational studies[6,7]. Interestingly, this benefit appears to have its maximal impact 10-20 years post-surgery, after most non-arterial conduits have lost their efficacy[8].
Surgical groups with a long-standing interest in multi-arterial grafting have hypothesized about the mechanism(s) for this incremental benefit on survival, based on their excellent observational studies[9-11]. With improvement in techniques such as skeletonization[12], the use of bilateral arterial grafting is being advocated as the new “standard” of care[13,14]. This despite the additional work product and time required for this surgical approach, because of it’s association with significantly better long-term outcomes[15].
The standard explanation for these improved outcomes is the substantial long-term anatomic patency of arterial grafts, both early and late[16]. While this certainly is a factor, the complete mechanism is more complicated. Indeed, as our understanding of the physiologic substrates for stable ischemic heart disease (SIHD) and acute coronary syndrome (ACS) have evolved, it is clear that physiologic factors are as if not more important than anatomic factors, which have for years formed the based of technical surgical revascularization design and execution.
Thus the premise of this review is that the true impact of multi-arterial in situ grafting in CABG results from its impact on the physiology of myocardial revascularization in that patient.
The revascularization strategy that is CABG today should be very different from previous iterations, in order to take advantage of: (1) concomitant developments in ischemic heart disease therapies and their physiologic impact on SIHD and ACS; and (2) the changes in the patient population of those patients coming to surgery, and the changes in the myocardial pathophysiologic substrate in these new patients. Truly innovative improvements in surgical revascularization must address these physiologic and pathophysiologic substrate issues in order to be successful.
Nowhere has this been more evident than in the emergence of optimal medical therapy (OMT), or guideline-directed medical therapy (GDMT), in SIHD[17]. From an afterthought 10-15 years ago, GDMT has emerged as an initial mainstay of therapy for SIHD patients outside the scope of ACS, where emergent intervention can be life-saving[18]. The impact of GDMT on clinical survival outcomes was documented in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and trial sub-studies in patients with mild to moderate ischemia[19-22]. These findings are not without controversy, however[23,24], with particular attention to their impact on early intervention revascularization strategies, mostly for percutaneous coronary intervention (PCI) but also for CABG[17]. The COURAGE population had predominantly mild ischemia symptoms and objective findings, and early PCI in these patients demonstrated no benefit in terms of the primary outcome of death from any cause and non-fatal myocardial infarction (MI)[19].
The importance of preoperative ASA, beta-blockers and statins on CABG surgical outcomes have all been examined as well. Preoperative beta-blockade was documented to positively impact on CABG outcomes in 2002[25], with incorporation into the National Quality Forum Quality Measures for CABG Surgery and the ACCF/AHA Guidelines for CABG[26]. A decade later, this benefit was re-examined in a more contemporary patient population, and no longer found to be a statistically significant influence on survival[27]. Rather than indicating the loss of effectiveness of beta-blocker therapy in CABG patients however, or that the initial studies were flawed, these findings almost certainly reflect the change in the underlying physiologic substrate of patients coming for contemporary CABG[28,29].
In patients with SIHD, GDMT is necessary because it prevents MI and death. The mechanism for the effectiveness of GDMT is the beneficial modulation of the underlying physiologic substrates of hypoperfusion/ischemia, atherosclerosis, myocardial contractility and relaxation, and microvascular and macrovascular myocardial blood flow[30]. Obviously, these same factors greatly influence CABG patients as well.
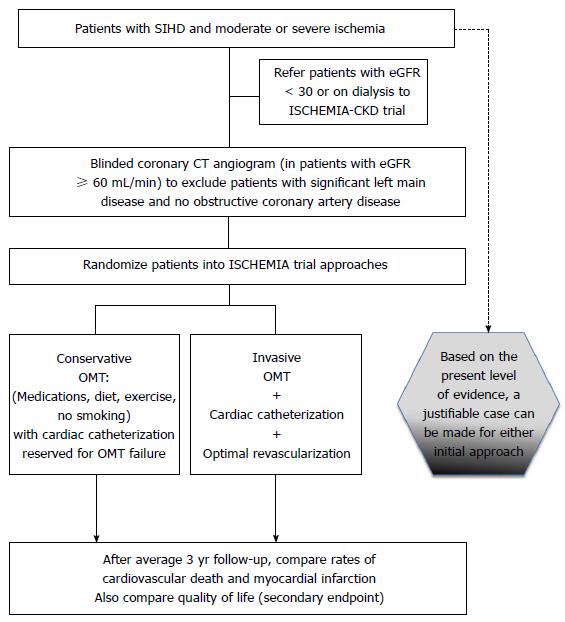
The impact of GDMT with and without early revascularization in patients with moderate to severe ischemia is currently being tested in the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial, (NCT01471522). The aim of the ISCHEMIA trial is to determine whether an initial invasive strategy of cardiac catheterization and optimal revascularization (with PCI or CABG, as determined by the local heart team) plus OMT will reduce the primary composite endpoint of cardiovascular death or nonfatal MI in SIHD with moderate or severe ischemia and medically controllable or absent symptoms, as compared with an initial conservative strategy of OMT alone, with catheterization reserved for failure of OMT (Figure 1). The major secondary endpoint is angina-related QoL. Other important secondary endpoints are health resource utilization, costs and cost effectiveness. The ISCHEMIA study thus aims to address limitations of previous strategy trials by: (1) enrolling patients before catheterization, so that anatomically high-risk patients are not excluded; (2) enrolling a higher-risk patients are not excluded; (3) minimizing crossovers; (4) using contemporary DES and physiologically-guided decision-making [fractional flow reserve analysis (FFR)] to achieve complete ischemic (rather than anatomic) revascularization; and (5) being adequately powered to demonstrate whether routine revascularization reduces cardiovascular death or non-fatal MI in patients with SIHD and at least moderate ischemia. The results of the ISCHEMIA trial will have important implications regarding global guidelines for performance and reimbursement of revascularization procedures in patients with SIHD.
One additional preoperative pathophysiologic substrate that impacts surgical revascularization today much more than before involves the substantial development of the myocardial collateral circulation as a result of the heart’s response to MI or even transient myocardial ischemia[31,32]. According to the STS database, approximately 40% of patients revascularized with CABG have a documented prior MI, with many more lacking that documentation or with a history of multiple episodes of ischemia preoperatively. Thus patients coming to surgery today have much more extensive collateralization than in the past. These collaterals have been directly linked to long-term survival in IHD patients, and recently their importance in patients with diabetic microvascular disease has been established[33,34].
In surgical revascularization with CABG, particularly in patients with extensive anatomic and functional disease, these collaterals impact the effectiveness of each individual bypass graft, depending upon the regional myocardial perfusion substrate supplied by that graft and the surrounding adjacent substrates[35-37].
Several randomized trial post-hoc analyses have produced data to support the importance of this pathophysiologic substrate in contemporary CABG patients. The Project of Ex-Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) documented 12-18 mo angiographic follow-up and 5-year clinical outcomes[38]. Vein graft failure in patients on follow-up angiogram was common (43%); in these patients followed for 4 years, clinical outcomes were associated with repeat revascularization, but not with death and/or MI[39]. These data suggest that the myocardium supplied by these occluded vein grafts had enough other blood flow [from the native target vessel epicardial coronary artery (TVECA) and/or collateral flow] so as not to influence the major outcomes of death and MI. In the SYNergy between PCI with TAXus and cardiac surgery (SYNTAX) trial, (ClinicalTrials.gov number NCT00114972)[40] the better outcomes seen in the surgical arm occurred despite a > 40% incomplete revascularization rate at CABG by SYNTAX anatomic criteria[41,42]. Head et al[43] documented that incomplete revascularization was associated with adverse outcomes in the PCI cohort but not the CABG cohort. This outcome is impacted by the higher incidence of preoperative MI in the CABG group, and by the greater extent of anatomic disease impacting the underlying myocardial pathophysiologic substrate in these patients. While the revascularization was as complete as technically possible, incompleteness by anatomic criteria alone was likely ameliorated by this dynamic collateral exchange of perfusion in these contemporary surgical revascularization patients.
The patient-level benefits of arterial grafting are clear, as IMA grafting to the LAD has been the standard of care for three decades. Despite the overwhelming body of evidence that multi-arterial grafting yields even further benefit for patients, including long-term survival, and freedom from MI, and that bilateral IMA grafting can be safely performed in elderly diabetic patients[44,45], transitioning of the standard of care to this new technical solution has been difficult[11]. Multi-arterial grafting represents a substantial change in the approach and work product of a CABG procedure for the surgeon[9,46]. Therefore, a more thorough understanding of the mechanisms underlying the benefits of multi-arterial grafting is important.
Most randomized trials involving CABG with protocol-specified angiographic follow-up have documented 1 year patency rates for in-situ IMA grafts between 95% and 100% at 12-18 mo, with grafting thresholds for angiographic stenoses of 70% or greater[47-50]. The 5% early attrition rate is widely thought to be due to technical errors at surgery[51,52].
Late graft failure following IMA grafting is much more complex in terms of etiology. The “competitive flow” from moderately stenosed native TVECA has been posited as a major cause for late in situ IMA graft failure. This is in contradistinction to causes for vein graft failures[53]. Additional causes of late arterial graft failure include factors typically attributed to other conduit failures, such as poor run-off of the distal native coronary circulation, thrombogenic factors, and size mis-match might be factors as well[50]. More recently, computer flow dynamic modeling studies have clarified the role of wall shear stress (WSS) on local hemodynamics, where atheroma are inhibited or retarded under conditions of high shear stress but predisposed to occur under conditions of low shear stress[54-56]. Despite the clinical studies associating intermediate coronary stenoses with increased IMA graft failure, Shimizu et al[57] demonstrated that the shear stress of the in situ IMA is maintained despite the flow volume being reduced by flow competition. Ding et al[58] used computerized flow dynamic modeling to study competitive flow in an IMA-LAD graft model. In this study, they correlated the Time-Averaged WSS and the oscillatory shear index (OSI) with TVECA percent stenosis, and found that TAWSS dropped when the stenosis was < 75%; concomitantly, the OSI distribution increased below 75% stenosis, where high OSI predisposes to endothelial dysfunction and atherogenesis[59], while maintained WSS is responsible for normal endothelial function and endogenous vasodilator production such as nitric oxide (NO).
Further complicating this story is the fact that two myocardial factors influence WSS in TVECAs and arterial conduits as well: Myocardial ischemia resulting from a physiologically significant proximal coronary stenosis increases WSS at the anastomosis and in the vessels, and this increased WSS stimulates the development of collateral circulation through arteriogenesis[60,61]. The influence of myocardial vasculature WSS on conduit and TVECA shear stresses has not been well-characterized.
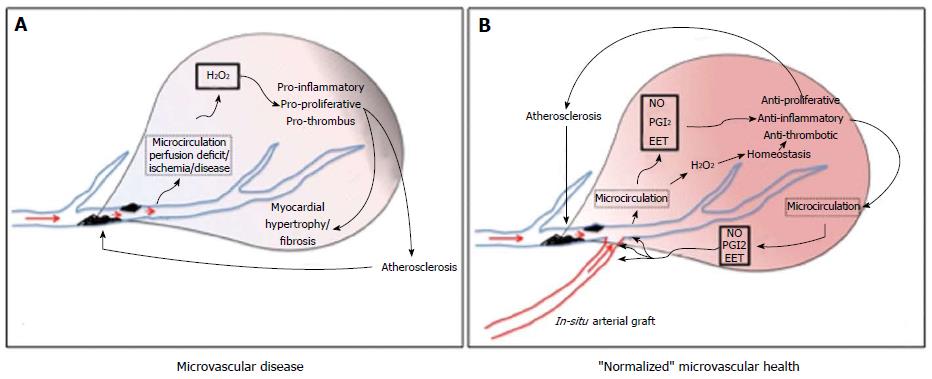
The reasons for improved long-term patency vs other conduits for grafting have been discussed at length in the surgical literature. These include the in situ nature of the conduit, endothelial production of endogenous vasodilators NO, prostacyclin (PGI2) and epoxyeicosatrienoic acids (EETs) to dilate the conduit and protect against the development of atherosclerotic disease in the vessel, better diameter matching between the graft and the TVECA, the absence of atherosclerotic disease and disease progression, and others. In a recent article, Gutterman et al[62] characterized the differences between microvascular health and microvascular disease. Figure 2 adapts this concept to the CABG setting, including pre-grafting ischemia (Figure 2A) and post-grafting regional myocardial status (Figure 2B). In “normalized” microvascular health (such as a non-diseased IMA graft in a CABG patient), atherostasis is achieved by predominant endothelial production of these vasodilators (NO, PGI2 and EET), with anti-proliferative, anti-inflammatory, and anti-thrombotic effects. In disease (such as SIHD), microcirculatory production of reactive oxygen species (H2O2) maintains dilation but at the expense of pro-inflammatory, pro-proliferative, and pro-thrombotic responses that contribute to atherosclerosis in TVECAs and hypertrophy and fibrosis in the myocardium. In arteries from healthy subjects, normal WSS activates production of NO to stimulate dilation and vascular homeostasis. Abnormal WSS, vascular stress or the presence of coronary artery disease stimulates the pathological basal level of oxidants and initiates a switch in the mediator of flow-induced dilation from NO to H2O2; dilation is maintained (for a time) but at the expense of vascular inflammation and its consequences[62].
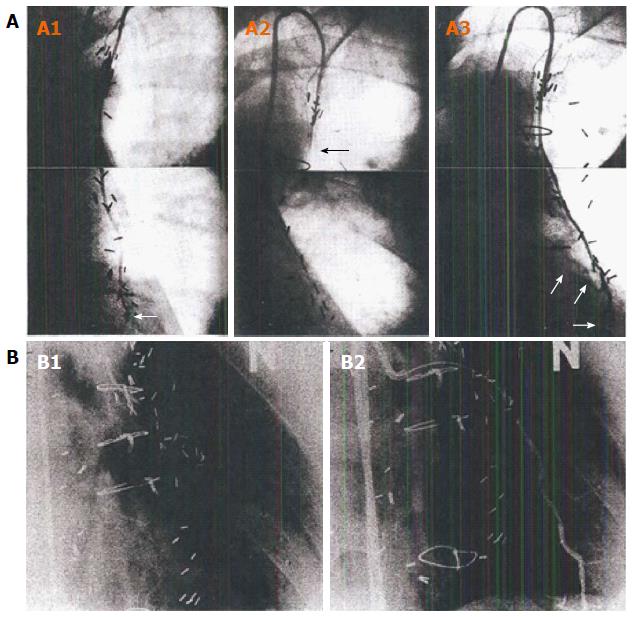
Among these, specific relevance to CABG is the endogenous production of vasodilators is believed to be the most important[59,63]. The powerful influence of these physiologic processes has been documented in studies illustrating serial angiographic follow-up after in situ IMA grafting. Hartman et al[64] and Akasaka et al[65] both documented progression from a normal-sized in situ conduit, to a string sign several years later, and finally to a supra-normal conduit, as the native coronary circulation and non-arterial bypass grafts developed progressive disease (Figure 3). It is clear from the above discussion that this string sign is not the product of irreversible microvascular disease from vascular inflammation. Rather, it must be an exogenously-stimulated normal endothelial response that can change over time.
In fact, many studies have documented that there is a predictable physiologic response in size and conduit flow in normal in-situ arterial grafts not compromised by technical errors over time. In their studies, Shimizu et al[57] and Akasaka et al[66] hypothesized that competitive flow between the TVECA and the in situ graft created the angiographic string sign - a technically angiographically patent graft, where conduit flow was minimal, but which could respond over time by increasing diameter and decreasing flow velocity, improving flow capacity, due to endothelial response triggers.
We now understand more clearly the role that the distal myocardium plays in influencing IMA conduit flow. Our near-infrared fluorescence (NIRF) imaging studies at the time of off-pump beating heart coronary artery bypass (OPCAB) quantified the change in regional myocardial perfusion, if any, associated with anatomically patent bypass grafting, including IMA, vein, and radial arterial grafts (Figure 4). Overall, in 80% of anatomic grafts to arteries with a minimum 70% proximal stenosis, there was a real-time increase in quantified regional perfusion when supplied by the TVECA and the graft conduit, vs the TVECA alone[35,37,67]. We believe this perfusion increase was dependent on the physiologic status of the distal myocardium in terms of tissue oxygen and blood flow demand. In the same way, this myocardial status impacts the dynamic flow characteristics of the in situ arterial conduits. Beginning with a technically adequate patent IMA graft, the functionality of the proximal TVECA stenosis (beyond anatomic severity alone) will influence subsequent IMA graft behavior. Early on, the perfusion status of the myocardium impacts early WSS and flow[57]. The pressure drop across the stenosis, if functionally significant, increase shear stress in the myocardial collateral vessels; both ischemia and increased shear stress promote the development of collateralization in the myocardium[60]. In the TVECA, the diminution of flow decreases WSS. If myocardial ischemia is relieved by the combined IMA/TVECA flow, then TVECA WSS is normalized, and the IMA graft accommodates flow velocity and flow capacity according to its contribution to myocardial demand relief[66]. If the proximal stenosis in the TVECA is not functionally significant, such as described by Ding et al[58], then time-averaged WSS of the graft falls, and OSI increases, contributing to the development of a string sign configuration angiographically. This IMA conduit, under new conditions of ischemia, can physiologically respond accordingly to meet this perfusion demand deficit[64]. Over time, as the native TVECA and graft disease progressed, the IMA conduit was driven to supply more and more blood flow to that regional myocardium, resulting in significant vasodilation of the in situ conduit. Importantly, this in situ conduit likely is supplying blood flow to other regions of the heart as well, given the extent of angiographic disease at this later stage and the assumed presence of extensive collaterals.
From a physiologic perspective, this dynamic nature of in situ IMA grafts, coupled to the functional status of the TVECA regional myocardium in a heart with extensive coronary disease and significant collateralization, is a likely physiologic-based explanation for the long-term clinical outcomes benefit from IMA grafting.
Competitive flow is the term used to describe flow interaction between the graft conduit and the TVECA, presumed to occur to a greater extent as the angiographic stenosis in the TVECA lessens. Thus it is presumed that there is more competitive flow to a TVECA with a 50% proximal stenosis than a 70% proximal stenosis. Glineur has reported that this situation of arterial graft competitive flow occurs when conductance (the ability of fluid to transmit through materials) of the graft closely matches that of the native circulation, and is mainly dependent on stenosis severity and on graft diameter and length[68]. However, because intraoperative conventional coronary angiography has not been widely available, and because coronary angiography per se does not represent true physiologic conditions, our knowledge about competitive flow in arterial grafts is limited. Moreover, since the behavior of these grafts changes over time, documentation of competitive flow at the time the bypass is created would be useful for understanding its true physiologic impact[69].
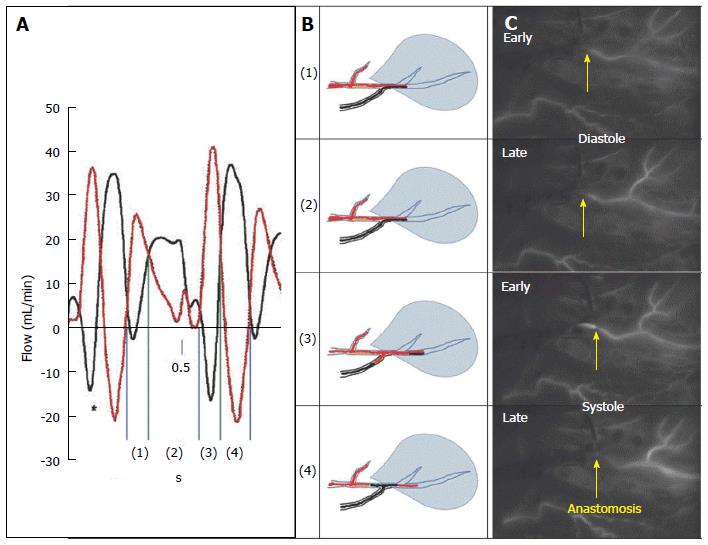
Pagni et al[70,71] performed a series of animal studies assessing the flow patterns in the graft conduit (IMA and/or vein grafts) and the unobstructed native TVECA. These studies documented four characteristic flow phases during systole and diastole associated with actual competitive flow between the IMA conduit and the TVECA in this experimental setting, without a proximal stenosis (fully competitive flow): In phases 1 and 2, during diastole, there is antegrade flow in both the TVECA and the arterial graft. In early systole, there is antegrade flow in the TVECA but retrograde flow in the distal arterial graft, which reverses in late systole, where there is retrograde flow in the TVECA and antegrade flow in the arterial conduit[70].
The actual flow patterns that occur with more significant proximal stenoses and more severe distal disease are less understood. Gould et al[72] demonstrated the relationship between coronary flow reserve (CFR) and isolated coronary stenoses, where CFR was maintained until the stenosis reached 70% or greater. With diffuse anatomic disease, however, this relationship degrades, and “critical” coronary flow reduction becomes prognostic[73]. Ding’s simulated competitive flow results in models of the IMA-LAD anastomosis are similar to Gould and Pagni, with a dependency on the degree of proximal stenosis[58]. Using angiographic characteristics, however, Berger[50] concluded that minimal competitive flow occurs until the proximal stenoses is greater than 70% angiographically.
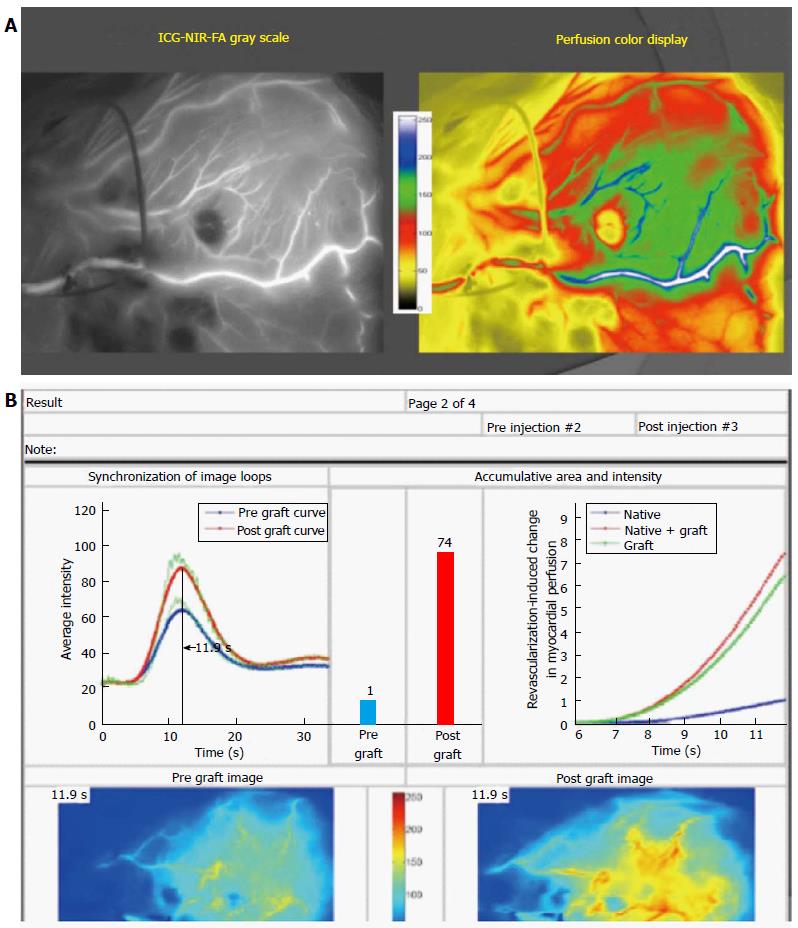
Our extensive studies of bypass grafting in off-pump CABG, where the actual physiology of blood flow in grafts and perfusion to the myocardium has been studied in over 1000 patients on a per graft basis, documented exactly this same reversal of flow pattern as Pagni in angiographically widely-patent IMA grafts to the LAD[67]. However, all these TVECAs (LAD or circumflex marginal branches) had a minimum of 70% proximal stenoses by preoperative angiography (Figure 5). The real-time intraoperative imaging technique was NIRF angiography (SPY, Novadaq Technologies, Toronto, Ontario, CA), coupled with the Complex Angiography and Perfusion Analysis analysis platform developed and patented in our Imaging Laboratory[35]. Importantly, this technology in OPCAB images blood flow and perfusion simultaneously in the TVECAs and graft conduits, and where there are physiologic conditions of coronary flow, coronary pressure, and myocardial functional performance[36].
These clinical studies uncovered two important factors. First, competitive flow was not ever documented angiographically in non-arterial conduits to TVECAs, regardless of the degree of TVECA proximal stenosis, similar to Glineur’s data[68]. Presumably, at the time of surgery, the flow down vein grafts free of technical problems is so dominant that even with intermediate (40%-70%) stenosis in the TVECA, competitive flow doesn’t occur. Second, in a sub-study of in situ IMA conduits to TVECAs with a 70% or greater proximal stenosis on the L side of the heart (LAD, circumflex marginal branches), a total of 23% of IMA grafts did not improve regional myocardial perfusion. That is, the post vs pre quantified distal regional myocardial perfusion (Figure 4) didn’t increase, despite a widely patent anastomosis angiographically and the absence of any clinical signs of incomplete relief of regional hypoperfusion/ischemia, or hemodynamic instability[67]. Examination of the real-time image sequences from these 23% in situ IMA grafts documented that > 80% had NIRF angiography documented competitive flow by these Pagni criteria.
These objective, imaging-based data document that competitive flow in arterial grafts does occur with proximal stenoses of 70% or greater severity at a much higher frequency that presumed based on indirect data as reported[74,75]. These data also strongly support the concept that the flow interaction between the in situ conduit and the TVECA is in fact less influenced by the proximal stenosis severity (anatomy) and more influenced by the physiologic status of the distal regional myocardium in terms of perfusion deficit and regional myocardial ischemia. As discussed above, based on these physiologic factors the arterial conduits will adapt to meet these demands over time and to the degree possible[66,69]. Nordgaard et al[76] demonstrated in an experimental model that WSS and OSI of an IMA graft was affected by the degree of competitive flow, where high competitive flow produced unfavorable WSS conditions consistent with endothelial dysfunction and subsequent graft narrowing and failure. However, the severity of competitive flow was based on percent proximal stenosis, and did not account for the functionality status of the stenosis.
These intraoperative imaging findings, in fact, are supported by the critically important developments over the past decade in PCI revascularization, based on the numerous studies with FFR and instantaneous wave free (iFR) studies[77-82]. In the FFR vs Angiography for Multivessel Evaluation (FAME) (ClinicalTrials.gov, No. NCT00267774) 1 study, 20% of angiographic stenoses between 70% and 90% were determined to be non-functional stenoses, consistent with our OPCAB studies[77]. Moreover, as presented by Stone et al[17], the physiologic status of the myocardium in patients with SIHD, and the ability of OMT to influence that status, creates equipoise in determining a conservative vs interventional therapeutic approach to patients, even with documented moderate-to-severe ischemia prior to therapy initiation. Importantly, in this context PCI and CABG are considered alternative forms of revascularization intervention determined by current RCT data from the head-to-head SYNTAX[42] and Future Revascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) (ClinicalTrials.gov number NCT00086450) trials[83]. However, the fact that they are alternative revascularization strategies means that the physiologic substrate for that intervention is equivalent, from a physiologic perspective. Our data strongly support the importance of physiology in determining short-term and long-term outcomes from surgical revascularization, in parallel to this experience in percutaneous revascularization[36]. In addition, others have described the current potential role of FFR-guided CABG, further emphasizing the emerging importance of physiology in revascularization[84].
In addition, recent data from SYNTAX examining the causes of death following PCI vs CABG in complex CAD emphasized the importance of the physiological impact of revascularization on the myocardial substrate in complex CAD[85]. CABG was associated with a significantly reduced rate of MI-related death, indicating that the anatomically-incomplete but functionally complete CABG revascularization provided sustained global perfusion and protection from subsequent ischemic events (e.g., MI), in part because of the collateralization associated with more extensive severity of CAD.
Importantly, the emerging call for multi-arterial revascularization to become the “standard of care” for contemporary CABG fits tightly into this strategy. Because of the findings outlined here, the concept of “complete anatomic revascularization” must be revised into “reasonable incomplete revascularization”[86,87]. A multi-arterial strategy may be initially thought to limit the number of potential grafts, producing incomplete revascularization, at least from an anatomic perspective. However, recognizing the importance of physiology in surgical revascularization, including the functional nature of the proximal stenoses, physiologic status of the distal regional myocardium vs assumed competitive flow, understood dynamic nature of the in situ arterial conduit, and the existence of collateral flow in the myocardial substrate being operated upon, allows for more specific design of a revascularization strategy using arterial conduits that will remain beneficial over the long-term.
The current results, based on clinical outcomes, from CABG intervention in patients with moderate and severe SIHD by anatomic criteria are excellent, and clearly are preferable to OMT and PCI interventions in the correct patient population[88]. Overall, however, the 25-plus year decline in risk-adjusted 30-d mortality for CABG, despite the concomitant increase in predicted operative risk, has plateaued over the past 5 years (STS database) at approximately 2%. This plateau is so distinct from the prior trend that it is appropriate to query why this might be the case[89]. Is 2% “as low at is feasible”, given the current population coming to CABG? Is the relative impact of collecting and sharing clinical outcomes data on continuous quality improvement efforts lessened at this level of high-performance? Or is risk-adjusted mortality as a benchmark for quality no longer effective at this high-performance level[45]?
An alternative perspective is that current standard outcomes are not granular enough to drive further quality improvements, as has been demonstrated previously with the infrastructure of the STS National Database[90,91]. Other metrics, in addition to existing ones, are needed to further drive clinical improvements in outcomes. It may well be that these physiologic aspects of revascularization (preoperative, intraoperative and postoperative), along with intraoperative documentation of technical quality and the absence of surgeon error represent the new metrics needed to drive quality improvement in the future.
One area where the impact of quality improvement interventions remains to demonstrate its effectiveness is in the area of secondary prevention of SIHD following CABG. Based on then-contemporary data from other areas of cardiovascular medicine therapy, we documented in the largest randomized clinical trial of continuous quality improvement to date the effectiveness of dissemination of information, local quality improvements and the infrastructure of a national database the increased adoption of secondary prevention measures [ASA, beta-blocker, statin, and ACE inhibitor therapy (in appropriate patients)] following CABG[91]. This study covered a time interval that was relatively early in the statin era, and while the adoption at > 400 surgical centers across the United States increased for each measure and the composite of all measures, the adoption of post-operative statin therapy was the most dramatic.
Since the Achilles’ heel of CABG has been the late development of atherosclerotic disease in vein graft conduits (in patients operated upon 15-50 years ago)[53], the full effect of this postoperative statin intervention still remains to be evaluated. Importantly, the benefits of statin therapy in non-surgical patients with SIHD are incontrovertible[92]. However, if the anti-atherosclerotic effects of statins in CABG mirror the effects in other settings of IHD, this intervention should impact long-term outcomes by retarding the development of disease progression in CABG patients[93]. Other pleiotropic effects of statins have been advocated as beneficial in CABG patients as well[26,92].
In an important recent study, however, room for improvement and justification for that improvement was highlighted. Iqbal et al[94] studied the use of OMT in patients with complex coronary disease undergoing revascularization in SYNTAX, and addressed the long-term significance of these use patterns. OMT was defined as the combination of at least one antiplatelet drug, statin, beta-blocker and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker. OMT was underused in all revascularization patients, especially in the CABG group. In five-year outcomes analyses, OMT was an independent predictor of survival, including mortality and the composite end-point of death/MI/stroke. The treatment effect with OMT (36% relative reduction over 5 years) was greater than the treatment effect of the revascularization strategy (26% relative reduction in mortality with CABG vs PCI over 5 years). All components of OMT were important for reducing adverse outcomes in both revascularization strategies. Clearly, contemporary cardiac surgery must continue to aggressively incorporate this life-sustaining physiologic intervention in post-CABG patients, no only for the first six weeks but work with all cardiovascular providers to make sure this intervention is sustained indefinitely following CABG[17].
One area of continuous evolution in secondary prevention is the utilization of dual anti-platelet therapy (DAPT) following CABG, and in particular in the approximately 18% of CABG cases performed using the OPCAB technique. The AHA/ACC/STS recently updated the guidelines for Secondary Prevention Following CABG, in particular with reference to DAPT. At the same time, the Guidelines on duration of DAPT in patients with Coronary Artery Disease has been updated as well[95]. The recent introduction of other new anti-platelet agents may result in the emergence of an improved DAPT strategy. Thus this post-operative secondary prevention arena following CABG will continue to evolve, as the full impact of statin therapy and DAPT therapy becomes evident. Again, the effects of these agents in modifying the physiology of atherosclerosis and platelet actions drives new potential improvements in clinical outcomes in CABG[95].
The STS has recently published Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting[96]. This excellent, technically and anatomically focused Guideline recommending that use of arterial grafts (specific targets, number, and type) should be a part of the discussion of the heart team in determining the optimal approach for each patient. This physiologic discussion provides in part the underlying scientific support for that recommendation.
Finally, this emerging granularity of the physiologic circumstances and effects of revascularization in CABG promises to have a similar impact as FFR/iFR have had on PCI intervention: The generation and incorporation of an entire body of new knowledge, which has benefitted both revascularization strategies. Thus far, these data presented here have produced a new definition for the goal of CABG, namely, to relentlessly restore blood supply, by both anatomic and physiologic criteria, to all areas of myocardium possible for the longest interval of time possible. “What we don’t know” represents the future[97].
A portion of the near-infrared fluorescence data analysis was supported by a Sponsored Research Agreement between ECU and Novadaq Technologies, Inc., Toronto, Ontario, Canada.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chawla M, Pocar M, Petix NR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Favaloro RG. Critical analysis of coronary artery bypass graft surgery: a 30-year journey. J Am Coll Cardiol. 1998;31:1B-63B. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Ferguson TB, Coombs LP, Peterson ED. Internal thoracic artery grafting in the elderly patient undergoing coronary artery bypass grafting: room for process improvement? J Thorac Cardiovasc Surg. 2002;123:869-880. [PubMed] [Cited in This Article: ] |
| 3. | Aranki SF, Tatooles AJ. Disconnect between vein graft failure and clinical events after coronary artery bypass graft surgery. Circulation. 2014;130:1439-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gersh BJ, Frye RL. Methods of coronary revascularization--things may not be as they seem. N Engl J Med. 2005;352:2235-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LA, Gill CC, Taylor PC, Sheldon WC. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1932] [Cited by in F6Publishing: 1815] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 6. | Head SJ, Kieser TM, Falk V, Huysmans HA, Kappetein AP. Coronary artery bypass grafting: Part 1--the evolution over the first 50 years. Eur Heart J. 2013;34:2862-2872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Head SJ, Börgermann J, Osnabrugge RL, Kieser TM, Falk V, Taggart DP, Puskas JD, Gummert JF, Kappetein AP. Coronary artery bypass grafting: Part 2--optimizing outcomes and future prospects. Eur Heart J. 2013;34:2873-2886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Taggart DP. Thomas B. Ferguson Lecture. Coronary artery bypass grafting is still the best treatment for multivessel and left main disease, but patients need to know. Ann Thorac Surg. 2006;82:1966-1975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Taggart DP. Bilateral internal mammary arteries: a very important missing trick for coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;41:776-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Raja SG, Benedetto U, Husain M, Soliman R, De Robertis F, Amrani M. Does grafting of the left anterior descending artery with the in situ right internal thoracic artery have an impact on late outcomes in the context of bilateral internal thoracic artery usage? J Thorac Cardiovasc Surg. 2014;148:1275-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Puskas JD, Lazar HL, Mack MJ, Sabik JF, Paul Taggart D. State-of-the-art coronary artery bypass graft. Semin Thorac Cardiovasc Surg. 2014;26:76-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lytle BW. Skeletonized internal thoracic artery grafts and wound complications. J Thorac Cardiovasc Surg. 2001;121:625-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Buxton BF, Shi WY, Tatoulis J, Fuller JA, Rosalion A, Hayward PA. Total arterial revascularization with internal thoracic and radial artery grafts in triple-vessel coronary artery disease is associated with improved survival. J Thorac Cardiovasc Surg. 2014;148:1238-1243; discussion 1243-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Shi WY, Hayward PA, Tatoulis J, Rosalion A, Newcomb AE, Fuller JA, Buxton BF. Are all forms of total arterial revascularization equal? A comparison of single versus bilateral internal thoracic artery grafting strategies. J Thorac Cardiovasc Surg. 2015;150:1526-1533, 1534e1-1534e3, discussion 1533-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Puskas JD, Williams WH, Mahoney EM, Huber PR, Block PC, Duke PG, Staples JR, Glas KE, Marshall JJ, Leimbach ME. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes: a randomized trial. JAMA. 2004;291:1841-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 449] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Stone GW, Hochman JS, Williams DO, Boden WE, Ferguson TB, Harrington RA, Maron DJ. Medical Therapy With Versus Without Revascularization in Stable Patients With Moderate and Severe Ischemia: The Case for Community Equipoise. J Am Coll Cardiol. 2016;67:81-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Giugliano RP, Braunwald E. The year in acute coronary syndrome. J Am Coll Cardiol. 2014;63:201-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3259] [Cited by in F6Publishing: 3059] [Article Influence: 179.9] [Reference Citation Analysis (0)] |
| 20. | Shaw L, Berman DS, Maron DJ, Hachamovitch R, Hartigan PM, Min JK, Sedlis SP, Dada M, Mancini EGJ, O’Rourke RA. Impact of pretreatment ischemia on therapeutic risk reduction and long-term prognosis in patients with stable angina: Results from courage trial. J Am Coll Cardiol. 2010;55:A98.E925. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Shaw LJ, Weintraub WS, Maron DJ, Hartigan PM, Hachamovitch R, Min JK, Dada M, Mancini GB, Hayes SW, O’Rourke RA. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, Maron DJ, Teo K, Sedlis SP, Chaitman BR. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Williams DO, Vasaiwala SC, Boden WE. Is optimal medical therapy “optimal therapy” for multivessel coronary artery disease? Optimal management of multivessel coronary artery disease. Circulation. 2010;122:943-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Thomas S, Gokhale R, Boden WE, Devereaux PJ. A meta-analysis of randomized controlled trials comparing percutaneous coronary intervention with medical therapy in stable angina pectoris. Can J Cardiol. 2013;29:472-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Ferguson TB, Coombs LP, Peterson ED. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221-2227. [PubMed] [Cited in This Article: ] |
| 26. | Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123-e210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 575] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 27. | Brinkman WT, Herbert MA, Prince SL, Magee MJ, Dewey TM, Smith RL, Edgerton JR, Head SJ, Ryan WH, Mack MJ. Preoperative beta-blocker usage: is it really worthy of being a quality indicator? Ann Thorac Surg. 2011;92:788-795; discussion 795-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Shin J, Johnson JA. Pharmacogenetics of beta-blockers. Pharmacotherapy. 2007;27:874-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, Ohman EM, Cannon CP, Smith SC, Zeymer U. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 549] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 31. | Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34:2674-2682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33:614-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Ferguson TB, Chen C, Buch AN. Fractional flow reserve-guided coronary bypass surgery: should surgeons use it? Curr Opin Cardiol. 2013;28:654-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 36. | Buch AN, Chen C, Ferguson Jr TB. Revascularization for stable ischemic heart disease: Are there new parallels beetween percutaneous coronary intervention and coronary artery bypass grafting? Interv Cardiol. 2015;7:149-167. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Ferguson TB, Chen C. Does the concept of functional stenosis exist in surgical revascularization with cabg? J Am Coll Cardiol. 2012;59:E1453. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Lopes RD, Williams JB, Mehta RH, Reyes EM, Hafley GE, Allen KB, Mack MJ, Peterson ED, Harrington RA, Gibson CM. Edifoligide and long-term outcomes after coronary artery bypass grafting: PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) 5-year results. Am Heart J. 2012;164:379-386.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Lopes RD, Mehta RH, Hafley GE, Williams JB, Mack MJ, Peterson ED, Allen KB, Harrington RA, Gibson CM, Califf RM. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation. 2012;125:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Davierwala P, Mohr FW. Five years after the SYNTAX trial: what have we learnt? Eur J Cardiothorac Surg. 2013;44:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Mohr FW, Rastan AJ, Serruys PW, Kappetein AP, Holmes DR, Pomar JL, Westaby S, Leadley K, Dawkins KD, Mack MJ. Complex coronary anatomy in coronary artery bypass graft surgery: impact of complex coronary anatomy in modern bypass surgery? Lessons learned from the SYNTAX trial after two years. J Thorac Cardiovasc Surg. 2011;141:130-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Morel MA, Van Dyck N. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1214] [Cited by in F6Publishing: 1158] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 43. | Head SJ, Mack MJ, Holmes DR, Mohr FW, Morice MC, Serruys PW, Kappetein AP. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg. 2012;41:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 44. | Raza S, Sabik JF, Ainkaran P, Blackstone EH. Coronary artery bypass grafting in diabetics: A growing health care cost crisis. J Thorac Cardiovasc Surg. 2015;150:304-2.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Raza S, Sabik JF, Masabni K, Ainkaran P, Lytle BW, Blackstone EH. Surgical revascularization techniques that minimize surgical risk and maximize late survival after coronary artery bypass grafting in patients with diabetes mellitus. J Thorac Cardiovasc Surg. 2014;148:1257-1264; discussion 1264-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu LM, Campbell H, Flather M. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J. 2010;31:2470-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 47. | Alexander JH, Ferguson TB, Joseph DM, Mack MJ, Wolf RK, Gibson CM, Gennevois D, Lorenz TJ, Harrington RA, Peterson ED. The PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial: study rationale, design, and baseline patient characteristics. Am Heart J. 2005;150:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446-2454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 458] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 49. | Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361:1827-1837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 761] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 50. | Berger A, MacCarthy PA, Siebert U, Carlier S, Wijns W, Heyndrickx G, Bartunek J, Vanermen H, De Bruyne B. Long-term patency of internal mammary artery bypass grafts: relationship with preoperative severity of the native coronary artery stenosis. Circulation. 2004;110:II36-II40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Harskamp RE, Williams JB, Halkos ME, Lopes RD, Tijssen JG, Ferguson TB, de Winter RJ. Meta-analysis of minimally invasive coronary artery bypass versus drug-eluting stents for isolated left anterior descending coronary artery disease. J Thorac Cardiovasc Surg. 2014;148:1837-1842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Hashimoto H, Isshiki T, Ikari Y, Hara K, Saeki F, Tamura T, Yamaguchi T, Suma H. Effects of competitive blood flow on arterial graft patency and diameter. Medium-term postoperative follow-up. J Thorac Cardiovasc Surg. 1996;111:399-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Kim FY, Marhefka G, Ruggiero NJ, Adams S, Whellan DJ. Saphenous vein graft disease: review of pathophysiology, prevention, and treatment. Cardiol Rev. 2013;21:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Gambaruto AM, Peiró J, Doorly DJ, Radaelli AG. Reconstruction of shape and its effect on flow in arterial conduits. Int J Numer Meth Fl. 2008;57:495-517. [DOI] [Cited in This Article: ] |
| 55. | Varshney G, Katiyar VK. Computational study of steady blood flow simulation in a complete coronary artery bypass anastomosis model. Can J Pure and Applied Sci. 2007;1:103-109. [Cited in This Article: ] |
| 56. | Sankaran S, Esmaily Moghadam M, Kahn AM, Tseng EE, Guccione JM, Marsden AL. Patient-specific multiscale modeling of blood flow for coronary artery bypass graft surgery. Ann Biomed Eng. 2012;40:2228-2242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Shimizu T, Ito S, Kikuchi Y, Misaka M, Hirayama T, Ishimaru S, Yamashina A. Arterial conduit shear stress following bypass grafting for intermediate coronary artery stenosis: a comparative study with saphenous vein grafts. Eur J Cardiothorac Surg. 2004;25:578-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Ding J, Liu Y, Wang F, Bai F. Impact of competitive flow on hemodynamics in coronary surgery: numerical study of ITA-LAD model. Comput Math Methods Med. 2012;2012:356187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 766] [Cited by in F6Publishing: 769] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 60. | Simic I, Zdravkovic V, Davidovic G, Iric-Cupic V, Vucic R, Tasic M, Ignjatovic V. Fractional flow reserve of intermediate lesions on collateral donor coronary arteries after myocardial infarction. Arch Biol Sci. 2013;65:571-576. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 61. | Schaper W, Buschmann I. Collateral circulation and diabetes. Circulation. 1999;99:2224-2226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The Human Microcirculation: Regulation of Flow and Beyond. Circ Res. 2016;118:157-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 63. | Deussen A, Ohanyan V, Jannasch A, Yin L, Chilian W. Mechanisms of metabolic coronary flow regulation. J Mol Cell Cardiol. 2012;52:794-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Hartman J, Kelder H, Ackerstaff R, van Swieten H, Vermeulen F, Bogers A. Preserved hyperaemic response in (distal) string sign left internal mammary artery grafts. Eur J Cardiothorac Surg. 2007;31:283-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S, Nasu M, Yoshikawa J. Flow dynamics of angiographically no-flow patent internal mammary artery grafts. J Am Coll Cardiol. 1998;31:1049-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Akasaka T, Yoshikawa J, Yoshida K, Maeda K, Hozumi T, Nasu M, Shomura T. Flow capacity of internal mammary artery grafts: early restriction and later improvement assessed by Doppler guide wire. Comparison with saphenous vein grafts. J Am Coll Cardiol. 1995;25:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Ferguson TB, Chen C, Babb JD, Efird JT, Daggubati R, Cahill JM. Fractional flow reserve-guided coronary artery bypass grafting: can intraoperative physiologic imaging guide decision making? J Thorac Cardiovasc Surg. 2013;146:824-835.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Glineur D, Hanet C. Competitive flow and arterial graft a word of caution. Eur J Cardiothorac Surg. 2012;41:768-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Nakajima H, Kobayashi J, Toda K, Fujita T, Shimahara Y, Kasahara Y, Kitamura S. Angiographic evaluation of flow distribution in sequential and composite arterial grafts for three vessel disease. Eur J Cardiothorac Surg. 2012;41:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Pagni S, Storey J, Ballen J, Montgomery W, Chiang BY, Etoch S, Spence PA. ITA versus SVG: a comparison of instantaneous pressure and flow dynamics during competitive flow. Eur J Cardiothorac Surg. 1997;11:1086-1092. [PubMed] [Cited in This Article: ] |
| 71. | Pagni S, Storey J, Ballen J, Montgomery W, Qaqish NK, Etoch S, Spence PA. Factors affecting internal mammary artery graft survival: how is competitive flow from a patent native coronary vessel a risk factor? J Surg Res. 1997;71:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 325] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 73. | Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2:1009-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Sabik JF, Lytle BW, Blackstone EH, Khan M, Houghtaling PL, Cosgrove DM. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg. 2003;76:1490-1496; discussion 1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Sabik JF, Blackstone EH. Coronary artery bypass graft patency and competitive flow. J Am Coll Cardiol. 2008;51:126-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Nordgaard H, Swillens A, Nordhaug D, Kirkeby-Garstad I, Van Loo D, Vitale N, Segers P, Haaverstad R, Lovstakken L. Impact of competitive flow on wall shear stress in coronary surgery: computational fluid dynamics of a LIMA-LAD model. Cardiovasc Res. 2010;88:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2974] [Cited by in F6Publishing: 2851] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 78. | Pijls NH. Fractional flow reserve to guide coronary revascularization. Circ J. 2013;77:561-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1837] [Cited by in F6Publishing: 1831] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 80. | Davies JE, Sen S, Broyd C, Hadjiloizou N, Baksi J, Francis DP, Foale RA, Parker KH, Hughes AD, Chukwuemeka A. Arterial pulse wave dynamics after percutaneous aortic valve replacement: fall in coronary diastolic suction with increasing heart rate as a basis for angina symptoms in aortic stenosis. Circulation. 2011;124:1565-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Carabello BA. Understanding coronary blood flow: the wave of the future. Circulation. 2006;113:1721-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Berger A, Botman KJ, MacCarthy PA, Wijns W, Bartunek J, Heyndrickx GR, Pijls NH, De Bruyne B. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol. 2005;46:438-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375-2384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1325] [Cited by in F6Publishing: 1246] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 84. | Casselman F, Van der Merwe J, Ferrara A, Barbato E. The present day potential role of fractional flow reserve-guided coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2016;151:926-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Milojevic M, Head SJ, Parasca CA, Serruys PW, Mohr FW, Morice MC, Mack MJ, Ståhle E, Feldman TE, Dawkins KD. Causes of Death Following PCI Versus CABG in Complex CAD: 5-Year Follow-Up of SYNTAX. J Am Coll Cardiol. 2016;67:42-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 86. | Dauerman HL. Reasonable incomplete revascularization. Circulation. 2011;123:2337-2340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Taggart DP. Incomplete revascularization: appropriate and inappropriate. Eur J Cardiothorac Surg. 2012;41:542-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Boden WE, Mancini GB. CABG for Complex CAD: When Will Evidence-Based Practice Align With Evidence-Based Medicine? J Am Coll Cardiol. 2016;67:56-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | LaPar DJ, Filardo G, Crosby IK, Speir AM, Rich JB, Kron IL, Ailawadi G. The challenge of achieving 1% operative mortality for coronary artery bypass grafting: a multi-institution Society of Thoracic Surgeons Database analysis. J Thorac Cardiovasc Surg. 2014;148:2686-2696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Ferguson TB, Peterson ED, Coombs LP, Eiken MC, Carey ML, Grover FL, DeLong ER. Use of continuous quality improvement to increase use of process measures in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. JAMA. 2003;290:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Williams JB, Delong ER, Peterson ED, Dokholyan RS, Ou FS, Ferguson TB. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation. 2011;123:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Drozda JP, Ferguson TB, Jneid H, Krumholz HM, Nallamothu BK, Olin JW, Ting HH. 2015 ACC/AHA Focused Update of Secondary Prevention Lipid Performance Measures: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2016;67:558-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1083] [Cited by in F6Publishing: 1115] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 94. | Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP, Feldman T, Stahle E, Escaned J, Banning AP. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation. 2015;131:1269-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 95. | Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 990] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 96. | Aldea GS, Bakaeen FG, Pal J, Fremes S, Head SJ, Sabik J, Rosengart T, Kappetein AP, Thourani VH, Firestone S. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann Thorac Surg. 2016;101:801-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 97. | Ferguson TB. Off-pump coronary artery bypass grafting versus conventional coronary artery bypass grafting: What we don’t know. J Thorac Cardiovasc Surg. 2016;151:893-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Dincer B, Barner HB. The “occluded” internal mammary artery graft: restoration of patency after apparent occlusion associated with progression of coronary disease. J Thorac Cardiovasc Surg. 1983;85:318-320. [PubMed] [Cited in This Article: ] |
| 99. | Kitamura S, Kawachi K, Seki T, Sawabata N, Morita R, Kawata T. Angiographic demonstration of no-flow anatomical patency of internal thoracic-coronary artery bypass grafts. Ann Thorac Surg. 1992;53:156-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |