Published online Jul 26, 2021. doi: 10.4330/wjc.v13.i7.211
Peer-review started: October 15, 2020
First decision: March 31, 2021
Revised: April 12, 2021
Accepted: April 29, 2021
Article in press: April 29, 2021
Published online: July 26, 2021
Left ventricular (LV) noncompaction cardiomyopathy is a rare cardiomyopathic subtype that has been recognized in recent years and is being diagnosed at an increased rate. There is no consensus regarding the diagnosis of the disease, and increased trabeculation rates that meet the existing diagnostic criteria may even be present in healthy asymptomatic people. This indicates that differentiating criteria for diagnosis are needed.
To examine the increase in myocardial trabeculation and the change in left ventricular global and regional functions.
This retrospective study included 65 patients (28 females, 37 males) diagnosed with LV noncompaction cardiomyopathy who underwent cardiac magnetic resonance imaging between January 2011 and August 2016 and had a noncompacted/compacted myocardial thickness ratio of over 2.3 in more than one segment in the left ventricle. The distribution and ratios of trabeculations in apical, midventricular, and basal regions were examined in short-axis images obtained from cardiac magnetic resonance. In addition, by using short-axis cine images, regional ejection fraction (EF) and global EF were calculated using the Simpson method in the left ventricle at apical, basal, and midventricular levels.
While the number of trabeculated segments were similar at the apical (3.2 ± 1.0) and midventricular levels, a statistically significant level of involvement was not observed at the basal level (0.4 ± 0.9) (P > 0.05). The highest noncompacted/com
No global or regional relationship was observed between LV dysfunction and trabeculation rate or the number of trabeculated segments. This limits the usefulness of change in LV functions in the differentiation between normal and pathological trabeculation.
Core Tip: With the advancements in cardiac magnetic resonance, there is increased frequency of left ventricular noncompaction cardiomyopathy diagnosis; meanwhile, increased trabeculation that meet diagnostic criteria may also be observed in healthy asymptomatic individuals. This suggests the need for new diagnostic criteria of the disease. In this regard, our retrospective study investigates the impact of the number of trabeculated segments and the trabeculation ratio on regional and global left ventricular functions.
- Citation: Yildirim G, Dursun M, Arslan R. Effect of trabeculated myocardial mass on left ventricle global and regional functions in noncompaction cardiomyopathy. World J Cardiol 2021; 13(7): 211-222
- URL: https://www.wjgnet.com/1949-8462/full/v13/i7/211.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i7.211
Left ventricular noncompaction cardiomyopathy (LVNC) is a form of cardiomyopathy that has been classified as primary genetic cardiomyopathy by the World Health Organization since 2006[1,2]. It is characterized by phenotypically prominent trabeculations, deep intertrabecular recesses, and thick noncompacted endocardium vs thin compacted epicardium. Etiologic factors of the disease are not fully clear. Genetic transmission may occur in sporadic or familial forms[3,4]. Several hypotheses have been prepared to explain the development mechanism of LVNC. However, there is a consensus of ideas that the left ventricle is the result of abnormal morphogenesis. In the 8th wk of gestation, myocardial trabeculations begin to be compacted starting from the basal region of the heart (compaction: Compacting of the trabeculations)[5]. Ventricular compaction advances from the epicardium towards the endocardium, from the base of the heart towards the apex and from the septum towards the free wall[6]. In the fetal heart, ventricular myocardial compaction is completed in the 4th mo. If the compaction process is interrupted, non-compaction cardiomyopathy occurs and the clinical manifestation varies depending on the stage of interruption. In addition, autopsy studies have shown that prominent trabeculations arising from the embryonal development process can also be found in high rates in the hearts of normal people and can be observed in the apex[7-9].
The actual prevalence of non-compaction cardiomyopathy is not fully known; however, one single-study in the literature reported that it varies between 0.014%-1.3%[10]. The most important diagnostic parameter is the ratio of non-compacted layer in relation to compacted layers of myocardium; this ratio should be over 2 in echocardiography and over 2.3 in cardiac magnetic resonance (CMR) imaging[11,12]. Different criteria have been proposed for diagnosis, the most important of which is to decide whether the trabeculations observed in imaging are normal, a normal variant, or pathological. The existing diagnostic criteria based on echocardiographic and CMR findings have certain limitations[13]. Recent studies have shown that increased trabeculation ratios may also be present in healthy asymptomatic individuals, leading to increased rates of LVNC diagnosis and indicating that new diagnostic criteria are needed. In addition, LVNC may be complicated by heart failure and result in cardiac transplantation, while the existing diagnostic criteria cannot determine or predict this process[6,14].
In this retrospective study, we aimed to investigate the effect of increased myocardial trabeculation on left ventricular global and ejection fraction (EF). In this regard, changes in EF can be included among the diagnostic criteria of the disease, preventing misdiagnosis, and can be used in the follow-up of the disease.
A total of 65 patients between ages 18-65 who were diagnosed with LVNC and referred to our department between January 2011 and August 2016 were included in our study. Patients were diagnosed by experienced cardiologists according to Jenni echocardiographic criteria. In the retrospective evaluations, the most important inclusion criteria independent from age and gender was CMR noncompacted/com
The cardiac magnetic resonance imaging (MRI) protocol for LVNC in our clinic was applied to the patient group. Imaging was conducted in supine position with a 1.5 Tesla (Philips Achieva; Philips Medical Systems, Amsterdam, Netherlands) MRI device. The SENSE-XL-Torso cardiac coil was placed on the anterior chest wall, and a signal acquisition system using electrocardiogram and respiratory trigger was applied. Initially, cardiac survey imaging was performed for the coronal, axial, and sagittal planes with Single Shot Balanced transcription factor E sequence. From the survey images, the plane formed between the mitral valve and apex in the axial plane and long axis two-chamber cine images from the Balanced transcription factor E sequence were obtained. Long axis four-chamber cine and short-axis (SA) cine images were obtained from these images. Since equal breath-hold is important for image quality, patients were asked to hold their breath at the end of expiration.
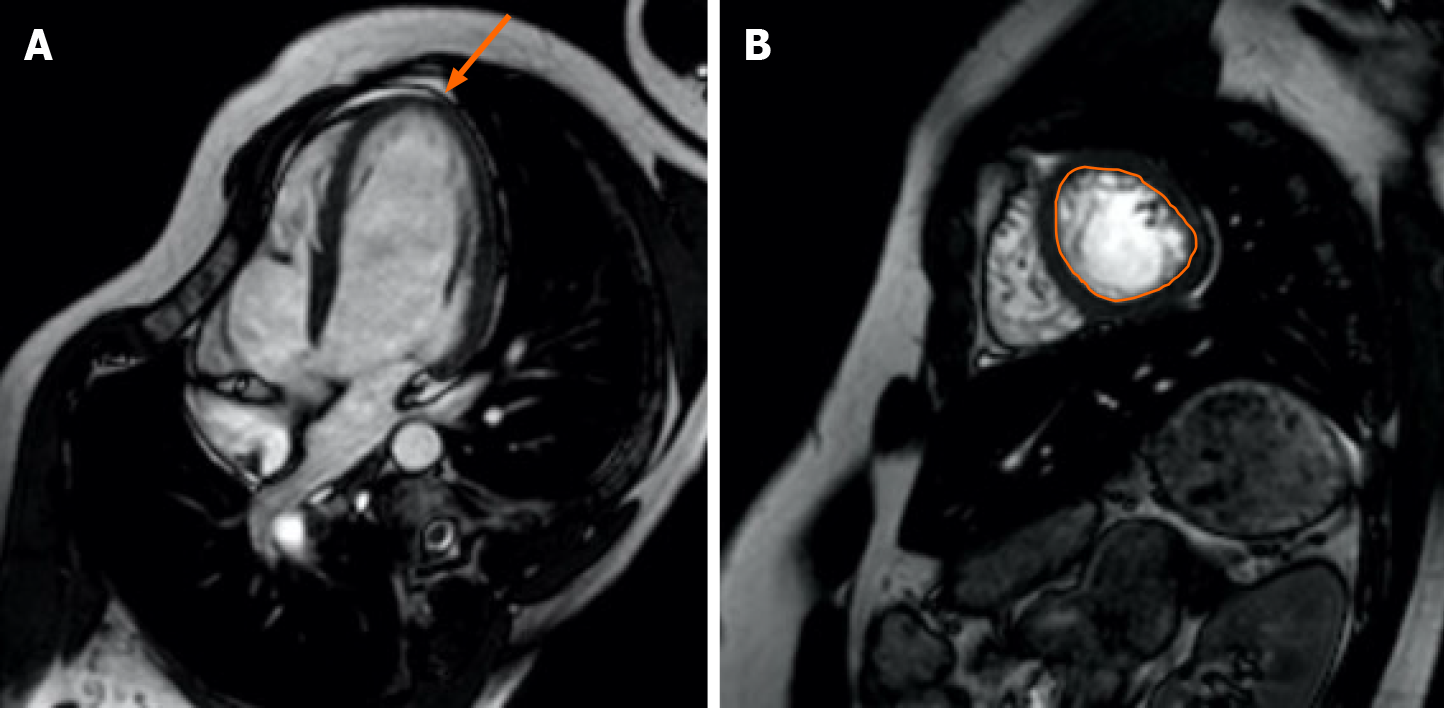
Cardiac MRI images were retrospectively evaluated by two radiologist who were experienced with cardiac images together using the standard approach on a Philips workstation. In the analyses, first the segment’s non-trabeculated segment ratio was measured according to the American Heart Association’s 16-segment model based on the ratio of trabeculated segment to non-trabeculated segment in the left ventricle (LV) at end-diastole[15]. Segments above the 2.3 cutoff value in all three SA planes (apical, mid, and basal), and the segment with the highest ratio in each axis was determined when making measurements. When distinguishing segmentation, the papillary muscle level was specified for the midventricular segment, the superior of the papillary muscles for the basal segment, and inferior of the papillary muscles as the apical segment. When determining the basal regions, the short axis section where more than half of the lumen was surrounded by myocardium was chosen. According to the current protocol, trabeculations were determined as regions with muscular structure moving together with the inner myocardium border, extending towards the cardiac region, and not accompanying the papillary muscles at the end of systole. The border between compacted and trabeculated myocardium in each segment was chosen outside of the line where trabeculation was not prominent (Figure 1). In the second step, systolic function analysis was performed separately for all three axes and globally for the entire LV. Simpson method was used for analysis. In the calculation of EF values, diastole and end-systolic endocardial boundaries were drawn manually for all three axes in short axis images, and finally, global EF values were calculated by drawing systole and end-diastolic endocardial boundaries in all sections from the apex to the base. The narrowest and widest ventricular cavity size was used at the mid-ventricular region to determine the systole and end-diastolic phases, respectively. Endocardial boundaries were drawn using the difference between the hyperintensity of the blood-filled cavity and the moderate intensity of the myocardium[16]. Papillary muscles were included in the ventricular cavity since they did not affect mass and cavity volume change in EF measurements.
Statistical analyses were performed using the SPSS 22.0 package program (Armonk, NY, United States). Mean, standard deviation, median, lowest-highest frequency, and percentage values were used in the descriptive statistics of the data. The distribution of variables was measured with the Kolmogorov-Smirnov test. Mann-Whitney U test was used in the analysis of quantitative independent data. Chi-square test was used to analyze qualitative independent data, and Fischer’s exact test was used when Chi-square test conditions were not met. Spearman’s rank correlation coefficient was used for correlation analysis.
We reviewed the electronic medical records and the baseline clinical characteristics, and cardiovascular compliments (congestive heart failure, syncope, embolic events, and any arrhythmias) of the patient population are summarized in Table 1. Also, in the study group none of the patients had diabetes mellitus and dyslipidemia. However, 5 patients had hypertension.
| Clinical characteristics | mean ± SD, n (%) |
| Age in yr | 31.09 ± 10.22 |
| Male gender, n (%) | 37 (56.9) |
| Syncope | 5 (7.7) |
| Arrhytmia | 2 (3.1) |
| Embolic events | 1 (1.5) |
| CHF | 0 |
| Asymptomatic | 57 (87.7) |
All patients who were included in the study met echocardiographic and CMR diagnostic criteria. All 65 patients had trabeculation ratio over 2.3 in at least three segments and at least two regions (apical, midventricular, basal). The number of trabeculated segments were high and similar at apical (3 ± 1) and midventricular (3 ± 1.4) regions, while there was no statistically significant involvement in basal regions. According to segmental distribution, trabeculation was most frequently observed in the apical-lateral (93.9%) and apical-anterior (89.4%) segments (P < 0.001). The highest trabeculation ratio was seen in the apical region (3.6 ± 1.4) (Tables 2 and 3).
| Min-Max | Media | mean ± SD | |
| Highest ratio | |||
| Apical, global | 0.4-9.0 | 3.6 | 3.9 ± 1.4 |
| Midventricular | 1.7-6.5 | 3.2 | 3.3 ± 0.9 |
| Basal | 2.0-4.0 | 2.6 | 2.7 ± 0.6 |
| Number of segments | |||
| Total | 0.0-11.0 | 7.0 | 6.3 ± 2.2 |
| Apical total | 0.0-4.0 | 3.0 | 3.2 ± 1.0 |
| Mid total | 0.0-5.0 | 3.0 | 2.8 ± 1.4 |
| Basal total | 0.0-3.0 | 0.0 | 0.4 ± 0.9 |
| n | % | |
| Apical | ||
| Septal | 31 | 47.0 |
| Anterior | 56 | 84.8 |
| Lateral | 62 | 93.9 |
| Inferior | 59 | 89.4 |
| Midventricular | ||
| Anterior | 42 | 63.6 |
| Anterolateral | 52 | 78.8 |
| Inferolateral | 49 | 74.2 |
| Inferior | 30 | 45.5 |
| Inferoseptal | 7 | 10.6 |
| Anteroseptal | 3 | 4.5 |
| Basal | ||
| Anterior | 7 | 10.6 |
| Anterolateral | 10 | 15.2 |
| Inferolateral | 7 | 10.6 |
| Inferior | 2 | 3.0 |
| Inferoseptal | 0 | 0.0 |
| Anteroseptal | 1 | 1.5 |
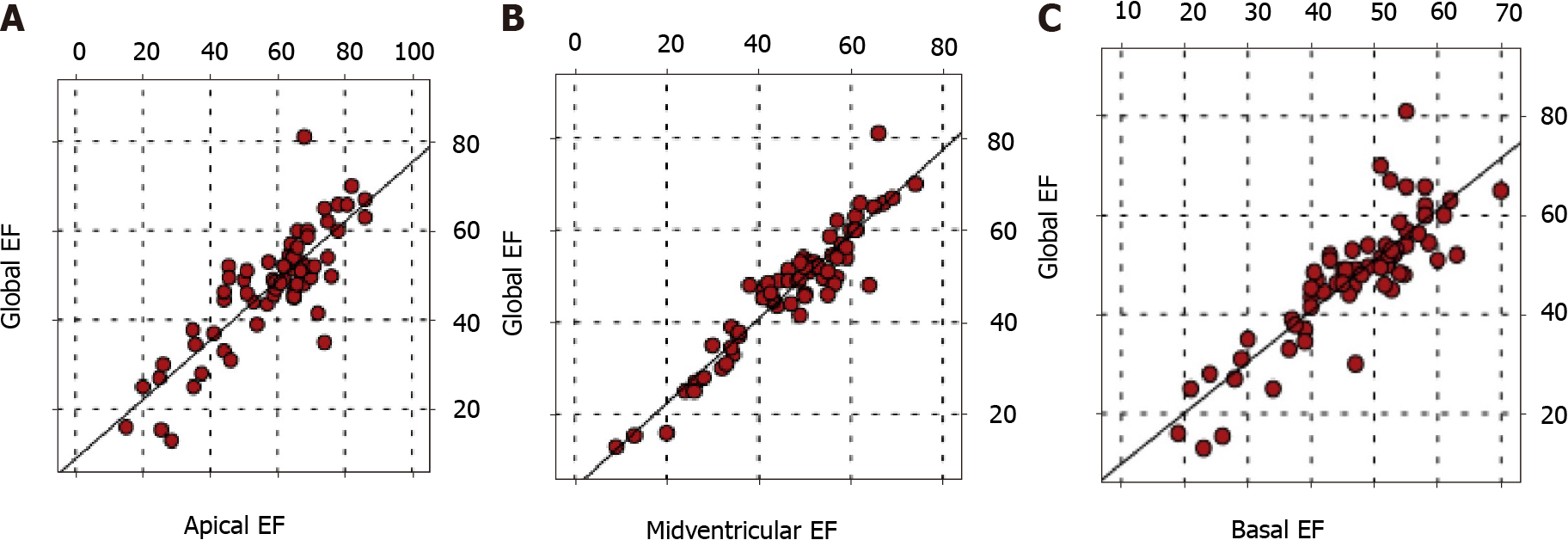
There was a positive correlation between global EF and apical, midventricular, and basal EFs (P < 0.05). In addition, there was a positive correlation between the EFs of all three regions (P < 0.05) (Figure 2).
There was no correlation between global EF and total number of segments involved and highest trabeculation rates for all three apical, midventricular, and basal regions (P > 0.05). No correlation was found between the regional EF values of the apical, midventricular, and basal regions and the number of trabeculated segments and the highest trabeculation ratio in these regions (P > 0.05) (Tables 4 and 5).
| Highest trabeculation ratio | ||||
| Apical | Midventricular | Basal | ||
| Global EF | r | 0.072 | -0.054 | -0.128 |
| P value | 0.573 | 0.687 | 0.707 | |
| Apical EF | r | 0.028 | ||
| P value | 0.825 | |||
| Midventricular EF | r | -0.022 | ||
| P value | 0.873 | |||
| Basal EF | r | -0.91 | ||
| P value | 0.789 | |||
| Number of segments | |||||
| Total | Apical | Midventricular | Basal | ||
| Global EF | r | 0.075 | -0.034 | 0.146 | -0.169 |
| P value | 0.555 | 0.246 | 0.179 | ||
| Apical EF | r | 0.120 | 0.048 | ||
| P value | 0.340 | 0.704 | |||
| Midventricular EF | r | 0.173 | 0.225 | ||
| P value | 0.168 | 0.072 | |||
| Basal EF | r | 0.083 | -0.229 | ||
| P value | 0.510 | 0.066 | |||
Noncompaction cardiomyopathy is a form of cardiomyopathy characterized by a bilayered compacted and noncompacted myocardium model with prominent myo
In order to diagnose LVNC, the main defined structural features must be revealed with imaging. The most important parameter in diagnosis is the ratio of the non-compacted layer to the compacted layers. This ratio must be more than 2 in echocardiography, and more than 2.3 in CMR. Echocardiography and CMR are the main imaging modalities used to diagnose the disease. Echocardiography is widely used due to its availability, common use, low cost, and absence of radiation exposure[22]. Chin et al[11] initially proposed evaluating the ratio of X to Y for echocardiographic diagnosis, in which X refers to the distance from the epicardial surface to the deepest part of the trabecular recess, and Y is the distance from the epicardial surface to the peak of the trabeculation. An X/Y ratio up to 0.5 at end-diastole is enough for diagnosis. Chin et al[11] also acknowledged the relationship between the clinical presentation of the disease and genetically transmitted cardiac diseases[11].
Jenni et al[23] specified diagnostic criteria as NC/C > 2 measured at end-systole and recommended additional morphological criteria including visualization of deep perfused intertrabecular recesses on color flow Doppler, involvement predominately in the lateral, apical, or inferior walls of the LV, and absence of additional cardiac anomaly[23]. The contribution of Stöllberger et al[12] to echocardiographic diagnostic criteria was > 3 trabeculations apically to the LV papillary muscles, apart from aberrant bands and false tendons[12]. However, there are many studies on the pitfalls of echocardiographic diagnostic criteria. One such was a study by Ottaviani et al[24], who measured trabeculations in the explanted hearts of 105 patients diagnosed as LVNC by echocardiography and who underwent orthotopic heart transplant. NC/C ratio was found as 1.7/1 ± 0.2, demonstrating the discordance between echocardiography and pathology[24]. Echocardiography has several limitations, such as such as being operator dependent, inadequate apex evaluation, difficulty in distinguishing the bilayer appearance, and inadequate short axis imaging. Therefore, the weak cor
Regarding CMR based diagnostic criteria, Petersen et al[27] conducted a study evaluating trabeculation distribution and NC/C ratios in different patient groups, such as dilated cardiomyopathy, hypertrophic cardiomyopathy, as well as 7 patients with LVNC; they concluded that trabeculation ratio > 2.3 (NC/C > 2.3) measured in end-diastole SA supported non-compaction cardiomyopathy diagnosis with 86% sensitivity and 99% sensitivity[27]. Fazio et al[29] performed end-diastole measure
Ventricular myocardium in LVNC has varying morphology ranging from increased spongiosis to dysplastic appearance. Changes in coronary microcirculation together with myocardial abnormalities are thought to play a role in the development of fibrosis. Late gadolinium enhancement may reveal findings of subendocardial and trabecular fibrosis. Although fibrosis may emerge as a follow-up parameter due to increased fibrosis occurrence in adult patients and advanced disease, recent studies have shown that late contrast enhancement may also occur in normally compacted myocardium[32,33]. Similar to a study by Nucifora et al[34], the presence and preva
In our study, we investigated the relationship between and global and regional EF of apical, midventricular, and basal regions and the trabeculation ratio and number of trabeculated segments of these regions in 65 patients diagnosed with LVNC. We did not find any concordance between EF and trabeculated segment number and ratio for all three regions or global LV. Choudhary et al[36] investigated the relationship between LV systolic function and global and regional LVNC mass. Contrary to our study, a significant inverse correlation was found between global EF and NC/C ratios in basal, apical, and midventricular regions in end-systolic measurements, and a significant inverse correlation was observed between end-systolic NC/C ratio and regional EF[36]. Dellegrottaglie et al[37] used qualitative and quantitative methods such as wall motion score and fractional wall thickening to measure regional systolic function in 16 adult patients. In measurements of regional systolic functions, an inverse relationship was found between NC/C ratio and fractional wall thickening, and a direct relationship with wall motion score. In other words, a positive correlation was found between regional function and NC/C myocardial ratio. While no significant correlation could be found between the number of noncompacted segments and left ventricular EF, it was considered an indicator of global left ventricular dysfunction[37].
Choi et al[38] conducted a study on 145 people [24 isolated LVNC, 33 non-isolated LVNC, 30 dilated cardiomyopathy with noncompaction, 27 dilated cardiomyopathy, and 31 healthy controls] and, similar to our study, did not find a significant correlation between left ventricular trabeculation volumes and EF in isolated LVNC patients compared to the control group[38].
Fazio et al[29] evaluated the relationship between ventricular dysfunction and the number of noncompacted segments in noncompaction cardiomyopathy and found that 122 of 238 patients (mean EF 44.39%) diagnosed with isolated LVNC had ventricular dysfunction with mean EF as 34.6%. However, they did not find a correlation between the number of affected segments and systolic dysfunction[29].
The severity and extent of myocardial involvement in patients with LVNC varies according to the stage of the disease and is heterogeneous. While some studies have found a significant relationship between trabeculation ratio and change in global ventricular function, other studies did not find a significant correlation between ventricular function and the ratio and frequency of trabeculation, as in our study. In addition, most of the studies conducted to date have focused on the relationship between trabeculation volume and global LV systolic function (EF). The limited number of studies on the impact to segmental function report varying results. In our study, we did not find a significant relationship between segmental function and the number of trabeculated segments or trabeculation ratio (P > 0.05).
The main limitations of this study are the lack of control group and its retrospective design. In addition, cardiac biopsies and genetic factors were not available for the included patients. Histopathological findings and genetic factors could help to provide more accurate diagnoses for LVNC.
In conclusion, there is a need for large cohort studies that include healthy subjects in order to utilize changes in left ventricular function as a criterion in the diagnosis of LVNC. There is not enough evidence yet for the use of segmental and global functions in the diagnosis and follow-up of the disease.
The frequency of left ventricular noncompaction cardiomyopathy (LVNC) diagnosis is increasing day by day due to inadequate diagnostic criteria. In addition, the criteria used cannot determine the prognosis and stage of the disease.
New cardiac magnetic resonance (CMR) criteria that can be used in the diagnosis of LVNC can explain when the increased trabeculation rate in healthy individuals can be called disease. In this context, left ventricular function changes due to increased trabeculation may be a parameter.
In our study, it was aimed to evaluate the relationship between LV global and regional function and trabeculation increase. A new parameter that can be used in diagnosis and follow-up will increase the diagnostic specificity of LVNC.
The distribution and ratios of trabeculations in apical, midventricular, and basal regions were examined in CMR. In addition, by using short-axis cine images, regional ejection fraction (EF) and global EF were calculated with the Simpson method in the left ventricle (LV) at apical, basal, and midventricular levels.
Global EF was correlated with apical, midventricular, and basal regional EF, but there was no significant correlation between global EF and the number of trabeculated segments or trabeculation ratio in the global LV. Also, there was no significant correlation between regional EF and the number of trabeculated segments or trabeculation ratio in all three regions.
Global and regional EF changes may be new diagnostic criteria in the diagnosis of LVNC and in the follow-up of the disease.
Studies on the relation of LV segmental functions with trabeculation are limited. Studies with larger cohorts and control groups should be conducted.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Radiology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Falconi M, Iacoviello M, Yang F S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1735] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 2. | Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology; Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2284] [Cited by in F6Publishing: 2118] [Article Influence: 117.7] [Reference Citation Analysis (0)] |
| 3. | Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | Finsterer J, Stöllberger C, Blazek G, Sehnal E. Familal left ventricular hypertrabeculation (noncompaction) is myopathic. Int J Cardiol. 2013;164:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Stöllberger C, Winkler-Dworak M, Blazek G, Finsterer J. Left ventricular hypertrabeculation/noncompaction with and without neuromuscular disorders. Int J Cardiol. 2004;97:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Boyd MT, Seward JB, Tajik AJ, Edwards WD. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two-dimensional echocardiography. J Am Coll Cardiol. 1987;9:323-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Zambrano E, Marshalko SJ, Jaffe CC, Hui P. Isolated noncompaction of the ventricular myocardium: clinical and molecular aspects of a rare cardiomyopathy. Lab Invest. 2002;82:117-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wengrofsky P, Armenia C, Oleszak F, Kupferstein E, Rednam C, Mitre CA, McFarlane SI. Left Ventricular Trabeculation and Noncompaction Cardiomyopathy: A Review. EC Clin Exp Anat. 2019;2:267-283. [PubMed] [Cited in This Article: ] |
| 9. | Bartram U, Bauer J, Schranz D. Primary noncompaction of the ventricular myocardium from the morphogenetic standpoint. Pediatr Cardiol. 2007;28:325-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Stanton C, Bruce C, Connolly H, Brady P, Syed I, Hodge D, Asirvatham S, Friedman P. Isolated left ventricular noncompaction syndrome. Am J Cardiol. 2009;104:1135-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 912] [Cited by in F6Publishing: 856] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol. 2002;90:899-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 364] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Tamborini G, Pepi M, Celeste F, Muratori M, Susini F, Maltagliati A, Veglia F. Incidence and characteristics of left ventricular false tendons and trabeculations in the normal and pathologic heart by second harmonic echocardiography. J Am Soc Echocardiogr. 2004;17:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, Deveci B, Sahin O, Kisacik HL, Korkmaz S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4399] [Cited by in F6Publishing: 4282] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 16. | Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Singh DP, Patel H. Left Ventricular Non-compaction Cardiomyopathy. 2020 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021. [PubMed] [Cited in This Article: ] |
| 18. | van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck-Zwarts KY, Baas AF, Boekholdt SM, van Melle JP, Teske AJ, Asselbergs FW, Backx APCM, du Marchie Sarvaas GJ, Dalinghaus M, Breur JMPJ, Linschoten MPM, Verlooij LA, Kardys I, Dooijes D, Lekanne Deprez RH, IJpma AS, van den Berg MP, Hofstra RMW, van Slegtenhorst MA, Jongbloed JDH, Majoor-Krakauer D. Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J Am Coll Cardiol. 2018;71:711-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Tariq M, Ware SM. Importance of genetic evaluation and testing in pediatric cardiomyopathy. World J Cardiol. 2014;6:1156-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Dellefave LM, Pytel P, Mewborn S, Mora B, Guris DL, Fedson S, Waggoner D, Moskowitz I, McNally EM. Sarcomere mutations in cardiomyopathy with left ventricular hypertrabeculation. Circ Cardiovasc Genet. 2009;2:442-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 22. | Mavrogeni SI, Markousis-Mavrogenis G, Vartela V, Manolopoulou D, Abate E, Hamadanchi A, Rigopoulos AG, Kolovou G, Noutsias M. The pivotal role of cardiovascular imaging in the identification and risk stratification of non-compaction cardiomyopathy patients. Heart Fail Rev. 2020;25:1007-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1026] [Cited by in F6Publishing: 986] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 24. | Ottaviani G, Segura AM, Rajapreyar IN, Zhao B, Radovancevic R, Loyalka P, Kar B, Gregoric I, Buja LM. Left ventricular noncompaction cardiomyopathy in end-stage heart failure patients undergoing orthotopic heart transplantation. Cardiovasc Pathol. 2016;25:293-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Diwadkar S, Nallamshetty L, Rojas C, Athienitis A, Declue C, Cox C, Patel A, Chae SH. Echocardiography fails to detect left ventricular noncompaction in a cohort of patients with noncompaction on cardiac magnetic resonance imaging. Clin Cardiol. 2017;40:364-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 804] [Cited by in F6Publishing: 767] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 28. | Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol. 2014;64:1840-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Fazio G, Novo G, D'Angelo L, Visconti C, Sutera L, Grassedonio E, Galia M, Ferrara F, Midiri M, Novo S. Magnetic resonance in isolated noncompaction of the ventricular myocardium. Int J Cardiol. 2010;140:367-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010;31:1098-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 31. | Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, Gutberlet M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol. 2012;22:2699-2709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Dursun M, Agayev A, Nisli K, Ertugrul T, Onur I, Oflaz H, Yekeler E. MR imaging features of ventricular noncompaction: emphasis on distribution and pattern of fibrosis. Eur J Radiol. 2010;74:147-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Sato Y, Matsumoto N, Matsuo S, Kunimasa T, Yoda S, Tani S, Kasamaki Y, Kunimoto S, Saito S. Myocardial perfusion abnormality and necrosis in a patient with isolated noncompaction of the ventricular myocardium: evaluation by myocardial perfusion SPECT and magnetic resonance imaging. Int J Cardiol. 2007;120:e24-e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail. 2011;13:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Dawson DK, Maceira AM, Raj VJ, Graham C, Pennell DJ, Kilner PJ. Regional thicknesses and thickening of compacted and trabeculated myocardial layers of the normal left ventricle studied by cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2011;4:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Choudhary P, Hsu CJ, Grieve S, Smillie C, Singarayar S, Semsarian C, Richmond D, Muthurangu V, Celermajer DS, Puranik R. Improving the diagnosis of LV non-compaction with cardiac magnetic resonance imaging. Int J Cardiol. 2015;181:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Dellegrottaglie S, Pedrotti P, Roghi A, Pedretti S, Chiariello M, Perrone-Filardi P. Regional and global ventricular systolic function in isolated ventricular non-compaction: pathophysiological insights from magnetic resonance imaging. Int J Cardiol. 2012;158:394-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Choi Y, Kim SM, Lee SC, Chang SA, Jang SY, Choe YH. Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: evaluation of trabecular volume and refined semi-quantitative criteria. J Cardiovasc Magn Reson. 2016;18:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |