Published online Sep 26, 2018. doi: 10.4330/wjc.v10.i9.87
Peer-review started: February 8, 2018
First decision: April 4, 2018
Revised: April 6, 2018
Accepted: April 18, 2018
Article in press: April 22, 2018
Published online: September 26, 2018
This review article aims to: (1) discern from the literature the immune and inflammatory processes occurring in the pericardium following injury; and (2) to delve into the molecular mechanisms which may play a role in the progression to constrictive pericarditis. Pericarditis arises as a result of a wide spectrum of pathologies of both infectious and non-infectious aetiology, which lead to various degrees of fibrogenesis. Current understanding of the sequence of molecular events leading to pathological manifestations of constrictive pericarditis is poor. The identification of key mechanisms and pathways common to most fibrotic events in the pericardium can aid in the design and development of novel interventions for the prevention and management of constriction. We have identified through this review various cellular events and signalling cascades which are likely to contribute to the pathological fibrotic phenotype. An initial classical pattern of inflammation arises as a result of insult to the pericardium and can exacerbate into an exaggerated or prolonged inflammatory state. Whilst the implication of major drivers of inflammation and fibrosis such as tumour necrosis factor and transforming growth factor β were foreseeable, the identification of pericardial deregulation of other mediators (basic fibroblast growth factor, galectin-3 and the tetrapeptide Ac-SDKP) provides important avenues for further research.
Core tip: Constrictive pericarditis arises as a complication of pericarditis from a wide range of aetiologies. A comprehensive understanding of the fibrotic process eventually leading to pathological symptoms is currently lacking.Through this review of the literature, we have identified various molecular mediators which are likely to play a role in the establishment of constriction and which warrant further studies.
- Citation: Ramasamy V, Mayosi BM, Sturrock ED, Ntsekhe M. Established and novel pathophysiological mechanisms of pericardial injury and constrictive pericarditis. World J Cardiol 2018; 10(9): 87-96
- URL: https://www.wjgnet.com/1949-8462/full/v10/i9/87.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i9.87
Pericarditis describes the clinical syndrome that occurs in response to injury of the pericardium. Following an episode of pericarditis, the natural history of the disease is variable and unpredictable. In a significant proportion of patients there is progression from acutely inflamed pericardium to chronic thickening, fibrosis, and fusion of the two pericardial layers with often dire consequences for patients. The molecular mechanisms involved in the inflammatory and immune mediated injury during pericarditis and the mechanisms involved in progression to constrictive pericarditis are poorly understood[1]. Such an understanding may be important to the design and development of interventions which are able to interrupt and prevent maladaptive and deleterious pericardial responses.
We conducted a comprehensive review of the available literature to summarize what is currently known about (1) immune and inflammation-mediated pericardial injury in a range of different causes of pericarditis; and (2) the molecular mechanisms involved in both pericarditis and subsequent post inflammatory progression to fibrosis and constrictive pericarditis.
The literature search was conducted in Pubmed, Embase, ScienceDirect and Google Scholar to identify journal articles for the review that had been published up to July 2017. In order to identify papers describing inflammatory and fibrotic processes occurring in the different types of pericarditis, the following search terms were used: “Pericarditis”and (“inflammation” or “fibrosis” or “constrictive” or “constriction”). The search was repeated for the common types of pericarditis described using the following search terms in the search criteria described above: “(uraemic or uremic)”, “tuberculous”, “malignant”, “radiation”, “autoimmune”, “viral”, “infectious”, “post surgery” and “myocardial infarction”[2]. Literature regarding different animal models of pericarditis was obtained using the search terms: “animal model” and “pericarditis”. Papers were then filtered according to their titles and content for the identification of relevant literature.
The pericardium is double layered flask-like sac which encloses the heart through its attachments to the great vessels, namely the vena cava, aorta, and pulmonary artery and vein. It is lined on the outside by the parietal pericardium, which is a fibrous layer of connective tissue rich in elastic fibres and collagenous fibres. This fibrosa is supplied by a network of blood and lymphatic vessels that contains macrophages and fibroblasts. The inner visceral pericardium is a single serous layer composed of flat, irregular, ciliated mesothelial cells resting on a thin basement membrane and separated from the fibrous layer by a thin sub-mesothelial space[3,4]. These two layers of the pericardium are 1-2 mm thick and give rise to a cavity which contains on average 15 to 35 mL of pericardial fluid, under normal physiological conditions[5]. Pericardial fluid is formed from ultrafiltration of plasma and comprises largely globular proteins, phospholipids and surfactant-like prostaglandins[6].
The pathophysiological response of the pericardium to injury is characterized by intense inflammation with or without effusion, and the clinical syndrome of pericarditis. Pericarditis is a common disorder[7] that can result from both an infectious and non-infectious aetiology and presents clinically as pericarditis with and without effusion. Causes of pericarditis include viral, bacterial, fungal, uraemic, post-acute myocardial infarction, neoplastic, post-cardiac surgery, following mediastinal irradiation and as a consequence of systemic autoimmune diseases[2]. The major complications of pericarditis are cardiac tamponade with and without hemodynamic instability in the short term, constrictive pericarditis in the long term and death[5]. The latter is usually the consequence of chronic inflammation, thickening, adhesion, fibrosis and obliteration of the pericardial space.
Uraemic pericarditis is a complication of acute and chronic renal failure, which can arise prior to, and on dialysis treatment. The condition was prevalent before the widespread use of dialysis and was commonly associated with a poor prognosis and high mortality. Currently, with modern dialysis, it has a highly improved prognosis and survival rate[8]. The mechanism of the development of pericarditis in uraemic disease is poorly understood. Although pericarditis is more frequent in cases of severe uraemia, there is no correlation between blood urea and creatinine levels and the appearance of pericarditis[9]. Uraemic pericarditis is usually exudative with protein and large numbers of mononuclear cells in the pericardial fluid[10]. Serous or haemorrhagic effusions are common and typically evolve into a fibrinous state. This fibrotic state often manifests as a rough granular surface with irregular, scattered adhesions between parietal and visceral pericardium in a “bread and butter” pattern[8,11]. However, densely adherent pericarditis and gross pericardial thickening with organizing fibrinous pericarditis have been found at autopsy in cases of uraemic disease[12].
Radiation pericarditis occurs as a complication of radiation therapy of malignant mediastinal tissues and organs, most commonly breast cancer or mediastinal Hodgkin’s disease. Radiation pericarditis was a common adverse outcome when large areas of the heart were exposed to high doses of radiation therapy, but the advent of chemo- and immuno- therapy has decreased the incidence of the condition[13]. Both acute and late post radiation pericardial injuries have been described. Acutely, radiation toxicity can cause micro-vascular damage and episodic pericardial ischemia, which in turn leads to permeable neovascularization and fibrous deposition. Later, activated fibroblasts express increasing type I collagen levels, with subsequent focal massive hyalinization, adhesions of the epicardium and thickening pericardium[14,15]. There is also evidence of impaired drainage of extracellular fluid from the pericardium, a chronic fibrinous exudative pericarditis and vascular and lymphatic fibrosis[16].
The degree of inflammation and thickening in radiation pericarditis corresponds to the X-ray exposure, with marked thickening observed more at the site of irradiation. This suggests a cellular injury and necrosis induced inflammatory response as a result of the acute radiation of actively proliferating cells, potentially mesothelial cells of the pericardium. On a molecular level, increased collagen synthesis and the pathological remodelling of the pericardium post radiation have been associated with the activation of various growth factors and cytokines including transforming growth factor β(TGF-β) and connective tissue growth factor (CTGF)[17].
Pericardial involvement can arise in various autoimmune diseases, most commonly systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and systemic sclerosis (SSc)[2].
SLE is a chronic inflammatory disease with a broad range of clinical manifestations and a variable disease course. The exact aetiology of SLE is still unclear, but it is likely to be mediated by antibodies and immune complexes (IC) which typically contribute to the clinical manifestation of SLE. Immune complexes can result in complement activation and inflammation and they have been detected in the pericardial fluid in SLE[18]. While pericarditis is the most common cardiac manifestation of SLE, constrictive pericarditis is a rare occurrence[19].
RA is a chronic inflammatory disorder that primarily affects joints. Symptomatic pericarditis arises in less than 10% of patients with severe disease and is often associated with a poor prognosis[20]. The pericardial involvement is usually a diffuse pericardial effusion, sometimes associated with leukocyte infiltration and often positive for rheumatoid factor and immune complexes. Constrictive pericarditis is not common in RA and can arise despite second-line therapy. Thickened pericardia with collagenous fibrous tissue and organising fibrin, fibrinous exudate and leukocyte infiltration have been described[21]. Asymptomatic pericardial effusions occur in upto 30%-50% of patients with RA and represents the most common cardiac manifestation of the disease.
Systemic sclerosis is a systemic autoimmune disease characterized by aberrant fibroblast activity resulting in dense fibrosis of visceral organs and skin. Pericardial manifestations include pericardial effusions, fibrous pericarditis, pericardial adhesions or constrictive pericarditis. Clinical manifestations of pericardial pathology are apparent in over 5%-16% of cases. The pathogenesis of pericardial effusions in SSc is believed to differ from the inflammatory pathway triggered by auto-antibodies and immune complexes of SLE and RA as evidenced by the “non-inflammatory” profile of the pericardial fluid. Instead, the release of basic fibroblast growth factor (bFGF) and histamine by mast cells may contribute to the pathophysiological manifestations[22,23].
The inflammatory basis of autoimmune pericarditis, centered around the role of the inflammasome, has recently been reviewed by Xu et al[24].
Pericarditis is a common sequelae of transmural myocardial infarction (MI) and arises “early” as pericarditis epistenocardica or as a “delayed” presentation in the form of Dressler syndrome[25].
The acute form of pericarditis is often diagnosed 1-4 d post MI[26] and is sometimes accompanied by a pericardial effusion. Vascular injury and myocardial necrosis have been associated with increased incidence of pericarditis, suggesting an inflammatory response to injury[27,28]. Fibrous deposits and adhesions often develop in the visceral and parietal pericardium covering the area of infarction but may also involve wider and more diffuse pericardial surfaces[29].
Dressler syndrome (DS) commonly arises around two weeks post MI. It is an uncommon presentation since the advent of early reperfusion therapy with thrombolytic therapy and primary percutaneous intervention, and with the widespread use of heparin. DS is presumably a recurrent immune-inflammatory syndrome arising from the release of auto-myocardial antigens from necrosis of myocardial tissues. The formation of immune complexes are believed to trigger a hypersensitivity reaction from molecular mimicry and cross-reactions[25,30]. Indeed, the presence of increased anti-myocardial antibodies following myocardial injury has been previously suggested and supports a possible autoimmune pathogenesis[31].
Pericarditis can present as a midterm or late complication of cardiac surgery. Post surgery pericarditis often bears restrictive haemodynamic characteristics despite an open pericardium and can occur as early as 2 wk following surgery[32]. Adhesions and fibrous patches in the pericardium lead to constrictive pericarditis and cause symptoms of dyspnoea, and signs of congestive cardiac failure. Whilst the exact mechanism for the development of pericardial fibrosis following surgery is obscure, the presence of blood in the pericardial cavity may play a role, with failure to drain bloody effusions being a risk factor for the development of fibrosis. Blood in the pericardium may result in irritation of the serosal layer and inflammation[33]. However, pericardial fibrosis in the absence of bloody effusions after surgery has also been documented[34].
A range of infectious organisms can affect the pericardium, but the most common causes are viruses (coxsakie viruses, influenza virus and enteric cytopathogenic human orphan virus, among others) and bacteria (Staphylococcus and Streptococcus, Haemophilus, and M. tuberculosis).
Bacterial (purulent) pericarditis which is a life threatening condition is characterised by gross pus in the pericardium or microscopically purulent effusion[35]. It is an uncommon occurrence in the developed world, due to widespread antibiotic usage. However, tuberculous pericarditis is a leading cause of pericarditis in Sub Saharan Africa and is discussed separately below[36].
Viral pericarditis, on the other hand, is a common manifestation and is often self-limiting, in that only a small number of patients develop fibrous complications. Viral antigens lead to an inflammatory response of lymphocytic predominance which often results in effusions. Cytotoxic and T and/or B cell-driven immune-mediated mechanisms of inflammation have been described in different types of viral infections[1]. Increased levels of IL-6 and IL-8 have also been described in both serum and pericardial fluid in viral pericarditis, with a marked local increase in the pericardial cavity[37,38]. An increase in pericardial TNF-α levels has also been measured in the pericardial fluid by Ristić et al[37], whilst TGF-β levels were only elevated in serum. However, conflicting Interferon-γ (IFN-γ) deregulation has been reported by the two studies, with a strongly elevated levels described by Pankuweit et al[38], whilst no differences were reported by Ristić et al[37]. This is probably due to the small sample size used in the latter study. Further, Karatolios et al[39] measured increased pericardial and serum levels of vascular endothelial growth factor (VEGF) in viral pericarditis as well as decreased bFGF levels in the pericardial fluid. Elevated serum cardiac troponin I (cTnI) levels have been observed in viral pericarditis and have been associated with ST-segment elevation, and pericardial effusion. Whilst, this increase is often more pronounced with increased myocardial inflammation, it did not affect the prognosis and the development of tamponade and fibrosis[40].
Tuberculous (TB) pericarditis accounts for roughly 4% of cases of acute pericarditis in the developed world. However, in developing countries with a high prevalence of tuberculosis, around 70% of cases of large pericardial effusion are attributable to TB[41,42]. Further, HIV co-infection has not only increased the number of TB pericarditis cases, but has also changed its clinical manifestations and therapeutic considerations[43].
The spread of Mycobacterium tuberculosis (MTb) to the pericardium occurs either through retrograde lymphatic spread or through haematogenous spread from primary sites of infection[36,44]. The inflammatory process in TB pericarditis follows a sequence of pathological events. An early fibrinous exudate is formed with leucocytosis, and early granuloma formation as a response to the high mycobacterial abundance, followed by a sero-sanguineous effusion with a predominantly lymphocytic exudate. The effusion gradually recedes whilst the granulomatous architecture is organised to restrict mycobacterial spread. Fibrin, collagen and extracellular matrix (ECM) deposition lead to pericardial thickening and fibrosis[36].
Infection of the pericardium with the bacilli elicits an immune response, stimulating lymphocytes to release cytokines which activate macrophages and influence granuloma formation. This initial reaction presents pathologically with polymorphonuclear leucocytosis and granuloma formation[45]. Marked elevations of IL-10 and IFN-γ accompanied by low levels of bioactive TGF-βlevels in tuberculous pericardial fluid suggest a Th-1 mediated delayed type hypersensitivity response to the pathogen[46]. Similarly, Reuter et al[47] measured significantly increased IFN-γ levels in the pericardial fluid and observed large numbers of mesothelial cells in tuberculous pericardial aspirates.
A role for complement fixing antimyolemmal antibodies has also been suggested in the development of exudative tuberculous pericarditis through cardiocyte cytolysis[48]. More recently, it was shown that the tetrapeptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) and galectin-3 could be detected in tuberculous pericardial fluid. The reduction in Ac-SDKP levels in TB pericardial effusion has been suggested to contribute to the development of fibrosis associated with TB pericarditis[49].
Elevated pericardial adenosine deaminase (ADA) activity and lysozyme levels have also been associated with TB pericarditis, and are of significant value in the diagnosis of TB pericarditis[47]. High ADA levels are also prognostic for the development of constrictive pericarditis[50].
Both primary (mesotheliomas, sarcomas, fibromas) and secondary (carcinoma, lymphoma, and carcinoid) neoplasms can be accompanied by pericardial inflammation. However, neoplastic pericarditis arises mostly from secondary disorders as a result of tumour spread and metastasis through lymphatic and haematogenous spread[51]. Effusions are common in neoplastic pericarditis and can be bloody. Malignant cells can also be present in the pericardium but almost 50% of symptomatic pericarditis cases have negative cytological results for malignant cells[52]. However, malignancies are commonly widespread when pericardial symptoms become apparent and malignant invasion of the heart and the deposition of fibrous tissue often lead to constriction[53]. Sub-acute inflammation with lymphocytic accumulation and mesothelial hyperplasia has been described in primary pericardial mesothelioma[54].
Ristić et al[37] measured elevated serum and pericardial levels of IL-6 and TGF-β in malignant pericarditis as compared to bypass surgery controls. TNF-α and IFN-γ levels were however not affected. A study by Pankuweit et al[38], found that IFN-γ levels in effusions were found to be slightly lower than in the serum. In accordance with Ristić et al[38] IL-6 and IL-8 levels were markedly increased in pericardial fluid as compared to the serum, suggesting a local initiation of the inflammatory response. Cardiac embryonic antigen is useful in the diagnosis of malignant pericarditis and levels above 5 ng/mL are found in the majority of cases[55].
ANIMAL MODELS OF PERICARDITIS
Several animal models of pericarditis have been described whereby the onset of inflammation was triggered by diverse mechanisms. A severe inflammatory reaction has been described in the pericardium of sheep injected with a bacterial toxin and Freund’s adjuvant. A cellular mesothelial response was observed with changes to the morphology and disturbance to the architecture, followed by detachment from neighbouring cells and desquamation. An accompanying increase in vascular permeability resulted in the accumulation of large numbers of inflammatory cells and the exudation of fibrin. An increased collagen turnover was apparent after 6 d and the appearance of adhesions occurred as early as 2 wk post injection[56].
Coxsackie B viruses are known to cause perimyocarditis, an acute inflammation of the pericardium and the underlying myocardium[57]. In Coxsackie B3 induced perimyocarditis in mice, an early onset of myocardial injury and necrosis has been observed, followed by marked pericardial fibrosis[58]. In this particular study, the sub-epicardial myocardial tissues appeared to mostly contribute to the fibrotic process, with infiltration by macrophages, lymphocytes and polymorphonuclear leukocytes observed in the myocardial layer. However, in Coxsackie B4 pericarditis in mice, fibrotic lesions occurred independently of, or in conjunction with adjacent myocardial lesions. Similar patterns of inflammation were observed in the mesothelial cells with necrosis, cellular infiltration, inflammatory cell infiltration and fibrinous exudate. The inflammatory processes are likely to be as a result of infection of mesothelial cells by the virus, since viral antigens have been detected in the mesothelial cells[59]. Interleukin-33 (IL-33) induced eosinophilic pericarditis has also been implicated in Coxsackie B infection[60].
IFN-γ and TGF-βknockout (KO) mice bear gross histological and haemodynamic characteristics of pericarditis. Pericarditis in the IFN-γ mice presented as a thick and stiff pericardium which formed adhesions to surrounding structures. Mesothelial hyperplasia in the pericardium was accompanied by a morphological change to a cuboidal shape. In addition to the predominant mononuclear cell infiltration, the pericardial inflammatory infiltrate of the IFN-γ KO mice had marked eosinophilia. Similarly, cardiac myocytes bordering areas of inflammation in the TGF-β KO mice presented with eosinophilic inclusions and contained large nucleoli[61,62].
Constrictive pericarditis is a clinical syndrome, characterised by a thickened and non-compliant pericardium, which restricts cardiac filling[63]. The most apparent pathological features of constrictive pericarditis are inflammation and fibrotic thickening of the thin and elastic parietal and visceral pericardial linings. The pericardium commonly bears areas of inflammation of the serosa, scarring, and fibro-calcification[63].
Constrictive pericarditis may result from severe acute inflammation or recurrent less severe inflammatory events over a highly variable time course from the period of injury[64]. While the predictors of progression to constriction following acute pericarditis are poorly understood the incidence of constrictive pericarditis is significantly dependent on the aetiology of the pericarditis. Whilst idiopathic and viral pericarditis have a low incidence of constrictive complications (0.8/1000 person-years), tuberculous (31.7/1000 person-years) and purulent pericarditis (52.74/1000 person-years) are associated with the highest rates of progression to pericardial constriction[7].
Although the molecular processes leading to fibrogenesis are likely to be unique for different pathologies, some key mechanisms and pathways are common to most fibrotic events[65]. The fibrotic cascade of events is triggered upon insult to epithelial or endothelial cells which results in the activation of the coagulation cascade. The initial inflammatory response is characterised by the release of various pro-inflammatory cytokines, including tumour necrosis factor-α, TNF-α[66,67]. TNF-α is a pleiotropic cytokine with a central role in the activation and recruitment of immune cells and the regulation of pro-inflammatory cytokine production[68]. Activated leukocytes then proceed to release pro-fibrotic cytokines such as IL-13 and TGF-β which drive EMT and ECM component production. TGF-β, is a key mediator of the fibrotic response and it acts via canonical (Smad-dependent) and non-canonical (non-Smad-based) signalling pathways to coordinate an ECM accumulation through increased synthesis as well as a decreased degradation of ECM components[69-71].
Molecular mechanisms of pericardial constriction remain to be fully elucidated but are likely to follow a classical pattern of pericardial inflammation mediated by various cytokines (Table 1), including TNF-α, followed by abnormal healing with an exaggerated TGF-β mediated profibrotic response leading to pericardial fibrosis. Both experimental mice models of acute pericarditis and pericardial fluid from patients with tuberculous effusive constrictive pericarditis (associated with a high incidence of pericarditis), demonstrate a mixed picture of both pro-inflammatory IFN-γ, and anti inflammatory cytokines IL-8, and IL-10[72], but their exact roles are as yet unclear.
| Inflammatory/ fibrotic mediator | Major roles in Inflammation and fibrosis | References |
| TGF-β | Anti-inflammatory mediator | [17,37,46,61] |
| ECM deposition and remodelling | ||
| CTGF | Myofibroblast activation | [17] |
| ECM deposition and remodelling | ||
| TNF-α | Inducer and regulator of inflammation | [37,38] |
| Macrophage and Natual Killer cell recruitment | ||
| IL-6 | Late role in inflammatory cascade | [37,38] |
| Adaptive Immune system activation | ||
| IL-8 | Later role in inflammatory cascade Neutrophil cell recruitment | [37,38] |
| IL-10 | Inflammatory mediator | [46] |
| IFN-γ | Immune response modulation Macrophage and Natual Killer cell activation Anti-fibrotic | [37-39,46,62] |
| VEGF | Angiogenesis and fibrosis promotion Fibrosis resolution | [39] |
| bFGF | ECM deposition | [23,39] |
| Ac-SDKP | Major role in the inhibition of fibrosis | [49] |
| Galectin-3 | Myofibroblast activation ECM deposition | [49] |
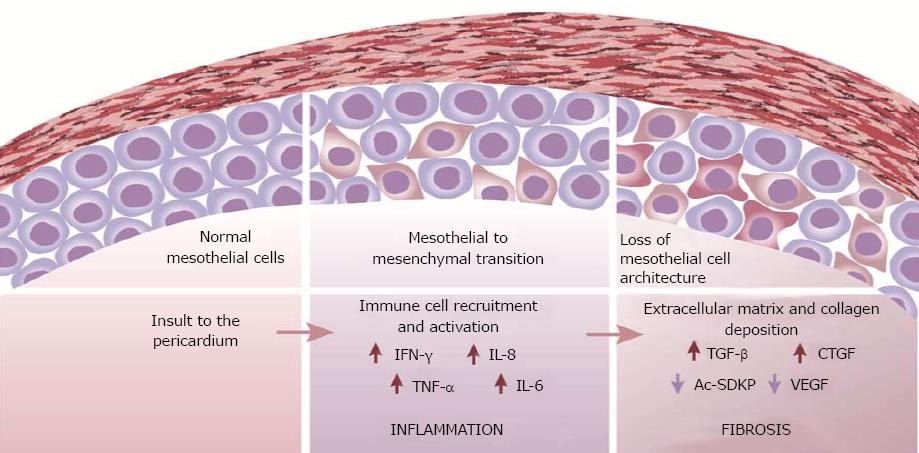
Patterns of inflammation and fibrosis in the pericardium suggest that both myocardial and pericardial cells play a role in the pathogenesis of pericarditis and constriction. A change in mesothelial cell morphology has been consistently described in various forms of pericarditis. Further, a loss of the mesothelial cell architecture, as well as mesothelial desquamation often accompanies constrictive pericarditis (Figure 1). The transition from a “flat” to a “cuboidal” shape has been associated with an “activation” of mesothelial cells and a distinct enzymatic profile of the cells with functions being geared towards oxidative stress and inflammatory responses[73,74]. Activated mesothelial cells secrete chemokines and adhesion molecules to aid in the recruitment and migration of leukocytes across the mesothelium. They are also known to mediate the inflammatory process and produce ECM components[75]. Further, mesothelial cells can undergo phenotypic changes similar to epithelial-to-mesenchymal transition to adopt fibroblast-like morphology and function in the healing serosa[4,76]. The active regulation of both pro- and anti-inflammatory mediators by mesothelial cells suggests a key role for the cells in maintaining pericardial homeostasis and also in the pathogenesis of pericardial fibrosis. Pericardial interstitial cells (PICs) have also been implicated in the production of ECM and calcification in the pericardium[77].
PICs have a comparable immune-phenotype to mesenchymal stem cells. PICs cultured from fibrocalcific human samples could be differentiated into myofibroblasts and osteoblasts which are central to the development of fibrosis and the production of extra-osseous calcification. TGF-β and bone morphogenetic protein 2 (BMP-2) were associated with the trans-differentiation process. TGF-β increased PIC mRNA expression of collagens I and III whilst decreasing the matrix metalloprotease-2 and -9 mRNA levels which are important for elastin degradation, thus regulating the fibrotic process by modulating fibrosis related gene expression[77].
TGF-β is a master regulator of extracellular matrix component expression and the development of fibrosis. Increased pericardial fluid and serum levels of TGF-β have been described in various forms of pericarditis and have been associated with increased collagen synthesis. It is thus safe to say that TGF-β might play a key role in the development of fibrosis in the pericardium. Interestingly, Ristic et al[37] did not detect any increase in TGF-β levels in viral pericardial fluid and this could account for the low proportion of constrictive pericarditis arising in this group.
Ac-SDKP, which is known to decrease TGF-β signalling by decreasing TGF-β transcription and the phosphorylation of Smad2 and Smad3 and their translocation to the nucleus[78-81], may play a role in the pathway to pericardial fibrosis. Patients with TB pericarditis, have been found to have diminished Ac-SDKP levels compared to particpants without pericarditis undergoing cardiac sugery[49]. Lowered Ac-SDKP levels could arise from an increase in angiotensin converting enzyme (ACE) activity, which is known to degrade Ac-SDKP[82,83]. An increase in ACE serum levels has been reported in granulomatous conditions including Mtb infection, and is believed to arise from an overflow of ACE production by the macrophages and phagocytes in the granulomatous lesions into the circulation. Thus increased ACE levels, resulting in increased enzymatic cleavage of Ac-SDKP and a subsequent dampening in its anti-fibrotic potential, could potentially contribute to the pathogenesis of constriction.
Finally, an increase in CTGF was associated with ECM deposition and pericardial remodelling[17]. This is not surprising as CTGF expression is known to be induced by TGF-β in cardiac fibroblasts and cardiac myocytes, whereby it contributes to the expression of fibronectin, collagen type I and plasminogen activator inhibitor-1[84]. Interestingly a decrease in VEGF was observed in viral pericarditis which rarely results in a constrictive pericarditis. Whilst VEGF mediated angiogenesis is known to be important for the promotion of fibrosis, it also plays a role in fibrosis resolution[85]. Indeed, an angio-fibrotic switch of VEGF and CTGF has been described in proliferative diabetic retinopathy, whereby the VEGF to CTGF ratio closely dictates the progression to fibrosi[86,87]. CTGF has also been shown to bind to VEGF and to inhibit its angiogenic functions[88]. Hence, it is possible that such CTGF-VEGF interplay is also involved in the progression to fibrosis in the pericardium. This would further explain the high VEGF levels coinciding with low levels of bFGF in viral pericardial fluid.
In this review, we have highlighted that the pericardium is subjected to noxious injury from a wide spectrum of infections and non infections causes and that the pathogenesis of pericarditis in each instance may differ in significant ways. Importantly the progression from pericarditis, to the development of pericardial fibrosis varies significantly by etiology. A classical pattern of inflammation in the pericardium mediated by various cytokines is likely to occur as a result of most types of insult. However, the unfolding of events leading to the development of fibrosis post-inflammation is harder to accurately predict. Nevertheless, this review has allowed us to postulate various cellular events and signalling cascades which are likely to contribute, albeit to different extents in varying types of pericarditis, to the pathological fibrotic phenotype. Whilst the role of common players such as TGF-β and TNF-α in the inflammatory process can be quite easily predicted, their complex range of functions makes them unattractive targets in the management and treatment of constrictive pericarditis. However, the identification of other pro-and anti-fibrotic mediators such as Galectin-3, Ac-SDKP and bFGF, with a narrower range of functions could represent new avenues for the treatment of pericarditis. Research aimed at developing a better understanding of molecular mechanisms involved in the progression of pericarditis to fibrosis may be able to (1) identify high risk patients for progression to constrictive pericarditis through novel markers of fibrosis; and (2) identify novel targets for therapy to interrupt the progression to fibrosis and prevent the development of constrictive pericarditis.
Research in MN’s, BMM’s and EDS’s research groups is supported by the National Research Foundation, South Africa. We would like to acknowledge S.L. Schwager for crtical reading of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: South Africa
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Movahed A, Sabate M S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). G Ital Cardiol (Rome). 2015;16:702-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 2. | Maisch B, Seferović PM, Ristić AD, Erbel R, Rienmüller R, Adler Y, Tomkowski WZ, Thiene G, Yacoub MH; Task Force on the Diagnosis and Management of Pricardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25:587-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 732] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 3. | Ishihara T, Ferrans VJ, Jones M, Boyce SW, Kawanami O, Roberts WC. Histologic and ultrastructural features of normal human parietal pericardium. Am J Cardiol. 1980;46:744-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Mutsaers SE. Mesothelial cells: their structure, function and role in serosal repair. Respirology. 2002;7:171-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 277] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Little WC, Freeman GL. Pericardial disease. Circulation. 2006;113:1622-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Spodick DH. Macrophysiology, microphysiology, and anatomy of the pericardium: a synopsis. Am Heart J. 1992;124:1046-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Imazio M, Gaita F, LeWinter M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA. 2015;314:1498-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | de Gouveia RH, Santos C, Santi R, Nesi G. Lessons from the past: Uremic pericarditis. Cor Vasa. 2018;60:e101-e103. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kumar S, Lesch M. Pericarditis in renal disease. Prog Cardiovasc Dis. 1980;22:357-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Reyman TA. Subacute constrictive uremic pericarditis. Am J Med. 1969;46:972-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Lindsay J Jr, Crawley IS, Callaway GM Jr. Chronic constrictive pericarditis following uremic hemopericardium. Am Heart J. 1970;79:390-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Bailey GL, Hampers CL, Hager EB, Merrill JP. Uremic pericarditis. Clinical features and management. Circulation. 1968;38:582-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Botti RE, Driscol TE, Pearson OH, Smith JC. Radiation myocardial fibrosis simulating constrictive pericarditis. A review of the literature and a case report. Cancer. 1968;22:1254-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Morton DL, Glancy DL, Joseph WL, Adkins PC. Management of patients with radiation-induced pericarditis with effusion: a note on the development of aortic regurgitation in two of them. Chest. 1973;64:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 17. | Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 18. | Quismorio FP Jr. Immune complexes in the pericardial fluid in systemic lupus erythematosus. Arch Intern Med. 1980;140:112-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Jacobson EJ, Reza MJ. Constrictive pericarditis in systemic lupus erythematosus. Demonstration of immunoglobulins in the pericardium. Arthritis Rheum. 1978;21:972-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Mankad R, Ball CA, Myasoedova E, Matteson EL. Non-atherosclerotic cardiac manifestations of rheumatoid arthritis [Internet]. Handbook of Cardiovascular Disease Management in Rheumatoid Arthritis. Springer; 2017; 19-38. Available from: http://link.springer.com.ezproxy.uct.ac.za/content/pdf/10.1007/978-3-319-26782-1_2.pdf. [Cited in This Article: ] |
| 21. | Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford). 2006;45 Suppl 4:iv4-iv7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol. 2014;6:993-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 23. | Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis. 1997;56:393-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Xu B, Harb SC, Cremer PC. New Insights into Pericarditis: Mechanisms of Injury and Therapeutic Targets. Curr Cardiol Rep. 2017;19:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Feola A, De Stefano N, Della Pietra B. Pericarditis Epistenocardica or Dressler Syndrome? An Autopsy Case. Case Rep Med. 2015;2015:215340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 27. | Dorfman TA, Aqel R. Regional pericarditis: a review of the pericardial manifestations of acute myocardial infarction. Clin Cardiol. 2009;32:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Sugiura T, Iwasaka T, Takayama Y, Matsutani M, Hasegawa T, Takahashi N, Inada M. Factors associated with pericardial effusion in acute Q wave myocardial infarction. Circulation. 1990;81:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Roberts WC. Pericardial heart disease: its morphologic features and its causes. Proc (Bayl Univ Med Cent). 2005;18:38-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Bendjelid K, Pugin J. Is Dressler syndrome dead? Chest. 2004;126:1680-1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Robinson J, Brigden W. Immunological Studies in the Post-Cardiotomy Syndrome. Br Med J. 1963;2:706-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Cohen MV, Greenberg MA. Constrictive pericarditis: early and late complication of cardiac surgery. Am J Cardiol. 1979;43:657-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Yoshioka T, Tokuda Y, Ueda Y. Clinical characteristics of patients with constrictive pericarditis after coronary bypass surgery. Jpn Circ J. 2001;65:480-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Gaudino M, Anselmi A, Pavone N, Massetti M. Constrictive pericarditis after cardiac surgery. Ann Thorac Surg. 2013;95:731-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Pankuweit S, Ristić AD, Seferović PM, Maisch B. Bacterial pericarditis: diagnosis and management. Am J Cardiovasc Drugs. 2005;5:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation. 2005;112:3608-3616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Ristić AD, Pankuweit S, Maksimović R, Moosdorf R, Maisch B. Pericardial cytokines in neoplastic, autoreactive, and viral pericarditis. Heart Fail Rev. 2013;18:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Pankuweit S, Wädlich A, Meyer E, Portig I, Hufnagel G, Maisch B. Cytokine activation in pericardial fluids in different forms of pericarditis. Herz. 2000;25:748-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Karatolios K, Moosdorf R, Maisch B, Pankuweit S. Cytokines in pericardial effusion of patients with inflammatory pericardial disease. Mediators Inflamm. 2012;2012:382082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Imazio M, Demichelis B, Cecchi E, Belli R, Ghisio A, Bobbio M, Trinchero R. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42:2144-2148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 141] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 41. | Syed FF, Mayosi BM. A modern approach to tuberculous pericarditis. Prog Cardiovasc Dis. 2007;50:218-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Fowler NO. Tuberculous pericarditis. JAMA. 1991;266:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 117] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Ntsekhe M, Mayosi BM. Tuberculous pericarditis with and without HIV. Heart Fail Rev. 2013;18:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Myers RB, Spodick DH. Constrictive pericarditis: clinical and pathophysiologic characteristics. Am Heart J. 1999;138:219-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Mutyaba AK, Ntsekhe M. Tuberculosis and the Heart. Cardiol Clin. 2017;35:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Ntsekhe M, Matthews K, Syed FF, Deffur A, Badri M, Commerford PJ, Gersh BJ, Wilkinson KA, Wilkinson RJ, Mayosi BM. Prevalence, hemodynamics, and cytokine profile of effusive-constrictive pericarditis in patients with tuberculous pericardial effusion. PLoS One. 2013;8:e77532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Reuter H, Burgess L, van Vuuren W, Doubell A. Diagnosing tuberculous pericarditis. QJM. 2006;99:827-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Maisch B, Maisch S, Kochsiek K. Immune reactions in tuberculous and chronic constrictive pericarditis. Clinical data and diagnostic significance of antimyocardial antibodies. Am J Cardiol. 1982;50:1007-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Ntsekhe M, Matthews K, Wolske J, Badri M, Wilkinson KA, Wilkinson RJ, Sturrock ED, Mayosi BM. Scientific letter: Ac-SDKP (N-acetyl-seryl-aspartyl-lysyl-proline) and Galectin-3 levels in tuberculous pericardial effusion: implications for pathogenesis and prevention of pericardial constriction. Heart. 2012;98:1326–1328. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Burgess LJ, Reuter H, Carstens ME, Taljaard JJ, Doubell AF. The use of adenosine deaminase and interferon-gamma as diagnostic tools for tuberculous pericarditis. Chest. 2002;122:900-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Wilkes JD, Fidias P, Vaickus L, Perez RP. Malignancy-related pericardial effusion. 127 cases from the Roswell Park Cancer Institute. Cancer. 1995;76:1377-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 53. | Thurber DL, Edwards JE, Achor RW. Secondary malignant tumors of the pericardium. Circulation. 1962;26:228-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Smets P, Guettrot-Imbert G, Hermet M, Delevaux I, Kemeny JL, Aumaître O, André M. Recurrent pericarditis related to primary pericardial malignant mesothelioma. Rev Med Interne. 2013;34:573-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Tatsuta M, Yamamura H, Yamamoto R, Ichii M, Iishi H, Noguchi S. Carcinoembryonic antigens in the pericardial fluid of patients with malignant pericarditis. Oncology. 1984;41:328-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Leak LV, Ferrans VJ, Cohen SR, Eidbo EE, Jones M. Animal model of acute pericarditis and its progression to pericardial fibrosis and adhesions: ultrastructural studies. Am J Anat. 1987;180:373-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Smith WG. Coxsackie B myopericarditis in adults. Am Heart J. 1970;80:34-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 150] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Matsumori A, Kawai C. Coxsackie virus B3 perimyocarditis in BALB/c mice: experimental model of chronic perimyocarditis in the right ventricle. J Pathol. 1980;131:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Tsui CY, Burch GE. Coxsackie virus B4 pericarditis in mice. Br J Exp Pathol. 1971;52:47-50. [PubMed] [Cited in This Article: ] |
| 60. | Abston ED, Barin JG, Cihakova D, Bucek A, Coronado MJ, Brandt JE, Bedja D, Kim JB, Georgakopoulos D, Gabrielson KL. IL-33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function. Circ Heart Fail. 2012;5:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Afanasyeva M, Georgakopoulos D, Fairweather D, Caturegli P, Kass DA, Rose NR. Novel model of constrictive pericarditis associated with autoimmune heart disease in interferon-gamma-knockout mice. Circulation. 2004;110:2910-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, Gress RE, Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995;146:264-275. [PubMed] [Cited in This Article: ] |
| 63. | Goldstein JA. Cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. Curr Probl Cardiol. 2004;29:503-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--a curable diastolic heart failure. Nat Rev Cardiol. 2014;11:530-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1786] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 66. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1075] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 67. | Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2745] [Cited by in F6Publishing: 3035] [Article Influence: 189.7] [Reference Citation Analysis (0)] |
| 68. | Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1006] [Cited by in F6Publishing: 934] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 69. | Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 70. | Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359-4369. [PubMed] [Cited in This Article: ] |
| 71. | Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1242] [Cited by in F6Publishing: 1325] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 72. | Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883-1894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Vogiatzidis K, Zarogiannis SG, Aidonidis I, Solenov EI, Molyvdas PA, Gourgoulianis KI, Hatzoglou C. Physiology of pericardial fluid production and drainage. Front Physiol. 2015;6:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Whitaker D, Papadimitriou JM, Walters MN. The mesothelium: a cytochemical study of “activated” mesothelial cells. J Pathol. 1982;136:169-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Mutsaers SE, Birnie K, Lansley S, Herrick SE, Lim CB, Prêle CM. Mesothelial cells in tissue repair and fibrosis. Front Pharmacol. 2015;6:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 76. | Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 553] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 77. | Liu X, Tan M, Gong D, Han L, Lu F, Huang S, Xu Z. Characteristics of pericardial interstitial cells and their implications in pericardial fibrocalcification. J Mol Cell Cardiol. 2012;53:780–789. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Castoldi G, di Gioia CR, Bombardi C, Perego C, Perego L, Mancini M, Leopizzi M, Corradi B, Perlini S, Zerbini G. Prevention of myocardial fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in diabetic rats. Clin Sci (Lond). 2009;118:211-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-Acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-beta-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Pokharel S, van Geel PP, Sharma UC, Cleutjens JP, Bohnemeier H, Tian XL, Schunkert H, Crijns HJ, Paul M, Pinto YM. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation. 2004;110:3129-3135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Lin CX, Rhaleb NE, Yang XP, Liao TD, D’Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H1253-H1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Azizi M, Junot C, Ezan E, Ménard J. Angiotensin I-converting enzyme and metabolism of the haematological peptide N-acetyl-seryl-aspartyl-lysyl-proline. Clin Exp Pharmacol Physiol. 2001;28:1066-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Inoue K, Ikemura A, Tsuruta Y, Watanabe K, Tsutsumiuchi K, Hino T, Oka H. Quantification of N-acetyl-seryl-aspartyl-lysyl-proline in hemodialysis patients administered angiotensin-converting enzyme inhibitors by stable isotope dilution liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2011;54:765-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 85. | Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, Mukhopadhyay D, Schuppan D, Bi Y, Simonetto D. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339-1350.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 86. | Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 88. | Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |