Published online Feb 28, 2016. doi: 10.4254/wjh.v8.i6.322
Peer-review started: June 30, 2015
First decision: October 6, 2015
Revised: January 7, 2016
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: February 28, 2016
AIM: To investigate effects of severe burn injury (BI) in rat liver through the histopathological and inflammatory markers analysis.
METHODS: Forty-two male Wistar rats were distributed into two groups, control (C) and subjected to scald BI (SBI). The animals were euthanized one, four and 14 d post sham or 45% of the total body surface BI. Liver fragments were submitted to histopathological, morphoquantitative (hepatocyte area and cell density), ciclooxigenase-2 (COX-2) immunoexpression, and gene expression [real-time polymerase chain reaction for tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS) and caspase-3] methods.
RESULTS: Histopathological findings showed inflammatory process in all periods investigated and hepatocyte degeneration added to increased amount of connective tissue 14 d post injury. Hepatocyte area, the density of binucleated hepatocytes and density of sinusoidal cells of SBI groups were increased when compared with control. COX-2 immunoexpression was stronger in SBI groups. No differences were found in TNF-α, iNOS and caspase-3 gene expression.
CONCLUSION: BI induces histopathological changes, upregulation of COX-2 immunoexpression, and cell proliferation in liver of rats.
Core tip: Severe burn injuries result in serious complications that involve host response related to inflammation and multiple organ dysfunction. The goal of this study was to investigate the temporal effects of extensive experimental burn injury (BI) in rat liver through the histopathological and morphoquantitative aspects, immunoexpression of ciclooxigenase-2 (COX-2) and liver gene expression of tumor necrosis factor-α, inducible nitric oxide synthase and caspase-3. Our results revealed that BI induces histopathological changes, upregulation of COX-2 immunoexpression, and cell proliferation in liver of rats.
- Citation: Bortolin JA, Quintana HT, Tomé TC, Ribeiro FAP, Ribeiro DA, de Oliveira F. Burn injury induces histopathological changes and cell proliferation in liver of rats. World J Hepatol 2016; 8(6): 322-330
- URL: https://www.wjgnet.com/1948-5182/full/v8/i6/322.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i6.322
Burn injuries (BIs) represent one of the greatest public health problems, which induce to significant patient morbidity and mortality[1]. Scalds are most common cause of BI and preferentially occurs in children under the five years[2]. In pediatric patient population persistent protein catabolism may lead to delay in growth for up to 2 years after burn[3].
Severe BI greater than 40% is a process that involves several host responses, including organ damage by inflammation and immune response[4]. Hypermetabolism is characterized by the inflammatory response, negative nitrogen balance, increase in resting energy consumption, and alterations in glucose and lipid metabolism[5]. Excessive systemic inflammatory response syndrome (SIRS) following burns to damage distant organ and provoke multiple organ dysfunction syndrome[6].
According to Jeschke et al[7] liver has been shown to play a crucial role after a BI. In a study of 102 children, the authors found that liver size and weight increased during the first week post BI, peaked at 2 wk post burn, and remained increased at 6, 9 and 12 mo after trauma. In autopsy of severely burned pediatric patients hepatomegaly with fatty infiltration was related to elevated occurrence of sepsis and mortality[8]. Jeschke et al[9] showed liver weight was increased by 140% to 150% compared with estimated liver weight at 6, 9 and 12 mo post BI, indicating prolonged alterations in hepato-structure.
Apoptosis from liver cells was evaluated by terminal deoxyuridine nick end labeling (TUNEL) assay in rats that have severe BI greater than 40% total body surface area[10]. Moreover, compensatory hepatocyte proliferation is detected due to increased apoptosis[7].
Severe BI is associated with host responses related to inflammation and apoptotic process and liver clearly plays an important role in metabolic processes post burn. The present study proposes to investigate effects of severe BI in liver of rats through the histopathological and morphoquantitative aspects, immunoexpression of ciclooxigenase-2 (COX-2) and liver gene expression of tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS) and caspase-3.
Male Wistar rats (n = 42), Rattus Norvegicus, with 21-d-old was chosen in the present study to mimic a developing organism. The rats were individually housed cages for five days, distributed into two groups: Control (C) and subjected to scald BI (SBI). The temperature room was controlled (22 °C) with regular light-dark cycle with 12 h, water and food were offered ad libitum. On the sixth day, the animals were anesthetized with an intraperitoneal (IP) injection of Ketamine (50 mg/mL) and Xilazyne (10 mg/mL) and dorsal and ventral hair were removed. The group SBI (n = 21) was submitted to nonlethal scald BI by immersing 45% of each rat’s body, in 87 °C water as described by Walker et al[11]. The C group (n = 21) were submitted to sham of the SBI. Each animal had 30% of its dorsal and 15% of ventral area exposed to SBI for 10 and 3 s, respectively[12]. The rats in both groups were subcutaneously injected with the analgesic Buprenorphine (0.2 mg/kg) immediately after sham or SBI and again 24 h later. One, 4 and 14 d following the SBI, all animals from each group were euthanized with a lethal IP injection of Ketamine (150 mg/kg) and Xilazyne (30 mg/kg).
All institutional and national guidelines for the care and use of laboratory animals were followed. The procedures were approved by the Committee of Ethics and Research from Federal University of São Paulo (protocol No. 329/12).
Liver of euthanized rats from SBI and C groups were examined. The specimens was immediately fixed in 10% formalin phosphate buffer for 24 h for histological analyzes and routinely embedded in paraffin blocks and cut in transversal sections (4 μm). The slides were stained with hematoxylin and eosin (H and E) and Sirius Red[13], whose photomicrographs were made under normal and polarized light to differentiate type I (red and yellow) and III (green) collagen.
The hepatocyte area (μm2) was determined from the measurement of 50 cells stained with H and E per animal. These fibers were randomly chosen from each animal comprising each experimental group. The cell density (number of cells/mm2) was determined as described by Mandarim-de-Lacerda et al[14]. For this purpose, it was used five sections chosen randomly and stained with H and E and two fields of each section was analyzed, totaling ten photomicrographs per animal. It was determinate the density of mononucleated hepatocytes, binucleated hepatocytes and sinusoidal cells. For to investigate the hepatocyte area and density, it was used a computerized imaging equipment (Axio Visio 4.5 - Zeiss®) attached to a binocular microscope (Axio Observer D1, Zeiss®) with a 63 × objective.
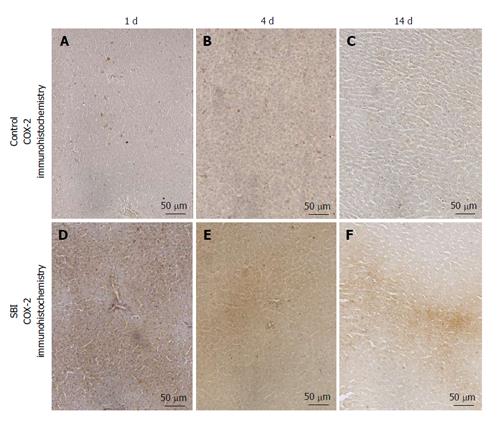
The paraffin of liver sections (4 μm) was removed with xylene and cuts were rehydrated in graded ethanol, after pre-treated with 0.01 mol/L citric acid buffer (pH 6) in a microwave for 15 min at 850 W for antigen retrieval. The sections were pre-incubated for 5 min in 0.3% hydrogen peroxide in phosphate buffered saline (PBS) solution to inactive the endogenous peroxidase. Then the material blocked was with 5% normal goat serum in PBS solution for 10 min and then incubated with anti-COX-2 polyclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA), at concentration of 1:200. Sections were incubated overnight at 4 °C in a refrigerator. After this was washes in PBS and incubated with biotin conjugated secondary antibody anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at a concentration of 1:200 in PBS for 30 min, washed with PBS: Followed by the application of avidin-biotin complex conjugated to peroxidase (Vector Laboratories) for 30 min. Then continued with the application of a 0.05% solution of 3-3-diaminobenzidine solution and counterstained with Harris hematoxylin (Merck).
Liver of animals was homogenized with 1 mL Trizol® (Invitrogen®, CA, United States), was added chloroform, isopropanol and ethanol 75% and centrifuged. In 40 μL of DEPC-treated water the pellet formed was re-suspended. The RNA purity and integrity were guaranteed by optical density (260/280 nm ratio > 1.9; Nanodrop® 2000 c, Thermo Scientific, Canada). Successively, the samples were kept in -80 °C. And were treated with DNAase (deoxyribonuclease I Amp Grade®, Invitrogen®, CA, United States) as fixed by the producer. The total RNA extraction was according with the protocol adapted by Chomczynski et al[15].
The total RNA was treated with DNAase and was built the cDNA by reverse transcriptase [real-time polymerase chain reaction (RT-PCR)], with the High-Capacity cDNA kit Reverser Transcription® (Applied Biosystems®, United States). The primers previously designed for genes of interest and endogenous control (Glyceraldehyde-3-Phosphate Dehydrogenase) were used for gene expression analysis and the detection of amplification was through intercalating DNA (Sybr Green®, Applied Biosystems®, United States). The primers sequences are show in the Table 1.
| Gene | Forward | Reverse |
| TNF-α | 5’-CCCAGAAAAGCAAGCAACCA-3’ | 5’-GCCTCGGGCCAGTGTATG-3’ |
| Caspase-3 | 5’-TCTACCGCACCCGGTTACTA-3’ | 5’-TGTCGTCATGTCCACCACTG-3’ |
| GAPDH | 5’-GCTCTCTGCTCCTCCCTGTTC-3’ | 5’-GACGCTGGCACTGCACAA-3’ |
The samples in duplicate are pipetted on the equipment RT-PCR 7500 Fast (Applied Biosystems®, United States) and subsequent the program of cycling was selected: Holding stage -95 °C for 10 min, 30 cycles of 15 s at 95 °C and 60 °C for 1 min, finish with the melting curve: 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s. The results were acquired by relative quantification (method2-ΔΔCt) at which the Cycle-threshold (Ct) values obtained for C group was compared to the SBI group.
Statistical analysis for hepatocyte area, cell density and RT-PCR were evaluated by analysis of variance with two factors (group and time), and followed with a Tukey’s test for multiple comparisons, when necessary. P < 0.05 was considered to statistical significance.
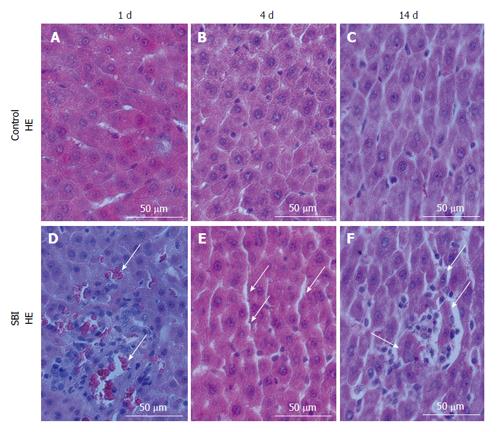
Liver cuts from Control group revealed hepatocytes arranged equidistantly with sinusoidal cells distributed in sinusoidal space (Figure 1A-C). Histopathological evaluation of SBI group investigated one day post injury revealed the presence of erythrocytes in sinusoidal space associated with inflammatory infiltrate (Figure 1D). Four days after injury, an increased sinusoidal space persisted (Figure 1E) and fourteen days post injury, liver sections showed inflammatory cells rounding hepatocytes in degeneration (Figure 1F).
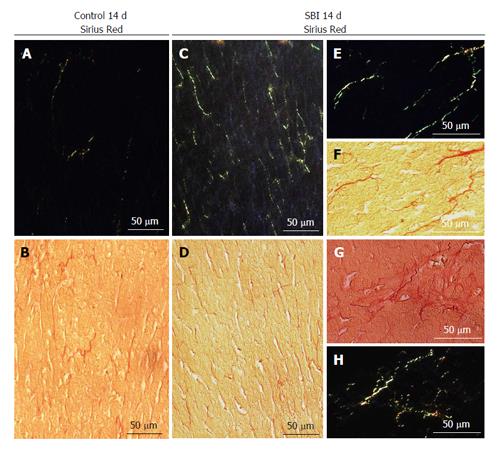
SBI group following 14 d after lesion exhibited increase in connective tissue in the hepatic parenchyma (Figure 2C and D) when compared with controls (Figure 2A and B). The data concerning SBI group 1 and 4 d post BI not have been present for the reason that similar to control groups. Under polarized light connective tissue analysis showed type III collagen (green) preponderance. Further details in increased magnification of SBI group are demonstrated in Figure 2E-H.
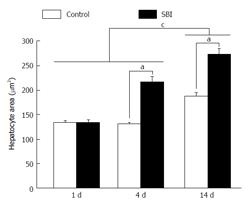
Hepatocytes area was significantly higher in the SBI group investigated 4 and 14 d after BI (Figure 3). Moreover, the mean of cells area in animals with 14 d was statistically larger than 1 and 4 d post BI.
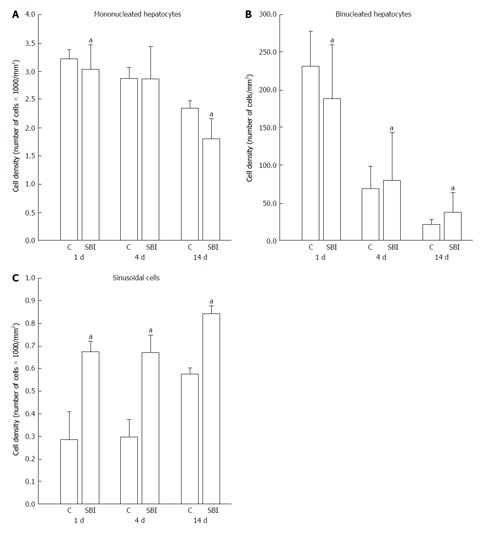
In relation to cell density (cells number/mm2) represented in Figure 4, mononucleated hepatocyte density decrease in SBI groups 1 and 14 d post BI (P < 0.05). Binucleated hepatocyte density in SBI groups showed significantly decreased cell density one day post injury and increased cell density in 4 and 14 d after BI when compared with respectively controls. Sinusoidal cells presented significantly increased cell density for SBI group in all periods investigated.
COX-2 immunoexpression was encountered in the cytoplasm of hepatocytes. Control groups presented weak immunoexpression for all periods investigated in this setting. However a strong and focal immunoexpression was detected in SBI groups after 1 and 4 d and persisted weakly 14 d post BI (Figure 5).
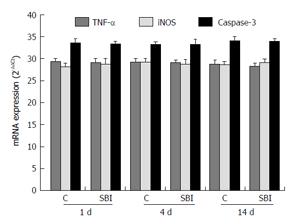
TNF-α and iNOS, related to inflammation, and caspase-3 related to apoptosis, were evaluated in liver (Figure 6). The results showed no statistically differences between control and SBI groups for all periods investigated.
Extensive burn injuries outcomes in critical complications that involve host response related to SIRS and multiple organ dysfunction being liver as a putative target-organ. The dynamic of organism adaptation as a result of liver response of hypermetabolism has been recognized in numerous studies but molecular and morphological occurrences still need to be better clarified. The aim of this paper was to investigate effects of severe BI in rat liver through the histopathological and morphoquantitative aspects, immunoexpression of COX-2 and liver gene expression of TNF-α, iNOS and caspase-3.
The results showed morphological alterations in liver such as sinusoidal space filled by erythrocytes and inflammatory infiltrate associated with hepatocytes in degeneration following 14 d post injury. Damage resulting from severe BI initiates a SIRS as far as serious metabolic disturbances. Systemic signs manifested in the first hours post severe burns is related to enlarged systemic capillary permeability with protein escapement into the interstitial space[16]. Burns greater than 40% of body surface area commonly are followed by stress, inflammation, hypermetabolism, in addition the circulatory response associated to altered glycolysis, proteolysis, glycogenolysis, gluconeogenesis and lipolysis[9]. Our current results are consistent with this stress response of liver after BI.
BI causes liver injury which persists over a prolonged time. In children, at 6, 9 and 12 mo post burn, liver weight was incremented by 140% to 150% compared with estimated liver weight, showing longstanding alterations in liver morphology up to 12 mo after BI[7,9]. Morphoquantitative aspects on hepatocytes investigated in this setting detected increased hepatocyte area 4 and 14 d after BI in SBI group. Additionally, hepatocyte proliferation was present as result of increased binucleated hepatocyte density (number of cells/mm2) 4 and 14 d post BI. These data show that despite liver increase weight gain is caused by edema formation[7], hepatocyte area gain and proliferation should be an important factor for hepatomegaly in burns. The compensatory hepatic cell proliferation are related to liver necrosis and liver apoptosis[10] but the underlying mechanisms, in which extensive burn provoke apoptosis in hepatocytes are not established so far[7]. This requires further study.
Regarding binucleated hepatocyte density, it is important to emphasize that because mitotic figures do not occur in adult liver, binucleated cells are usually assumed to be result of amitosis which implies splitting of the nucleus and these amitosis has been associated both with replacement of aged cells in abnormal tissue and with regenerative growth after injury[17]. Although binucleated hepatocytes are present in both groups (C and SBI), the presence of binucleated hepatocytes in SBI groups are related to regenerative growth as a response of skin BI. Interestingly mononucleated hepatocyte density decrease in SBI groups 1 and 14 d after trauma probably related to degeneration process showed in histopathological findings.
To further elucidate the molecular mechanisms induced by cutaneous BI, caspase-3 gene expression was evaluated in liver and no remarkable differences between groups were detected. Studies using TUNEL assay in liver of post BI experimental models showed apoptosis process[9,10]. Jayaraman et al[18] related that the up-regulation of some acute-phase genes as STAT3, leptin receptor and HNF4α, are related to infection 7 d post injury in 20% of total body surface area in rats, suggesting bacterial infection of the wound post BI in animals. These authors showed up-regulation of Birc4, a protein that block caspase-3 and caspase-7 that are associated to apoptosis post burn. In this way, the present study suggests that the proliferation of hepatocytes cells was due to inflammatory process following necrosis. Following hepatocyte death, growth factors are secreted, and hepatocyte proliferation is trigged in liver.
Post BI, liver modulates the immune responses and the inflammatory processes. Protein catabolism owing to extensive BI is all guided by systemic inflammatory response, with enhanced activation of pro-inflammatory cytokines[19]. In a local investigation of liver, inflammatory infiltrate was viewed in histopathological findings and confirmed with immunohistochemical investigation a result of strong and focal immunoexpression COX-2 in SBI group. COX-2 is responsible for the conversion of arachidonic acid to prostaglandins[20]. Although severe burn in children is related to increased blood cytokine levels[21], experimental studies with murine model submitted to BI for water vapor of 18% of the body surface showed enhanced expression of TNF-α and iNOS in initial stages of BI evaluated by means of peritoneal fluid of RT-PCR analysis[22]. Conversely, the present investigation of these inflammatory mediators (directly on the liver and not in a corporal fluid evaluation) showed no differences between groups.
Liver sinusoidal endothelial cells (LSECs) provide liver regeneration post injury of this organ[23]. In the sinusoidal space, there are four cell types: LSECs, Kupffer cells, stellate cells and pit cells. Kupffer cells generate cytokines and pro-inflammatory factors that stimulate neutrophils activation and change sinusoids porosity and may lead to cirrhosis[24]. Herein, LSECs and Kupffer cells constitute the hepatic reticuloendothelial system[25]. In the present study the density of sinusoidal cells was significantly increased in SBI group for all periods when compared with controls. This should be associated to phagocytic activity of Kupffer cells since local inflammation, erythrocytes invasion activation of neutrophils and disturbance of porosity in the sinusoids walls leading to erythrocytes invasion of sinusoidal space. Severe haemolysis was observed immediately after BI[26]. LSECs are separated from liver parenchyma by space of Disse, which is represented for a perisinusoidal extravascular space. Space of Disse contains collagen type I, III, V and VI and the changes related with perisinusoidal basal lamina in livers, should increase collagen deposition in the space of Disse[25]. Furthermore, agglomeration of connective tissue inside the space of Disse may obstruct the normal traffic between blood and hepatocytes, reducing the release of macromolecules, difficulting the interation between cells and leading to a liver dysfunction[27].
Hepatocyte growth factor (HGF) develops a key function in cell regeneration, motility, growth and morphogenesis. Hepatic stellate cells provide as the main source of HGF in liver, however, after lesion, HGF expression is increased in LSECs[23,28]. In addition, hepatic stellate cells activated may initiate liver fibrosis process. Healthy LSECs inhibit the activation of hepatic stellate cells[23]. In this study, increased density of sinusoidal cells should be related with enhanced of accumulation of the collagen, specially type III, in hepatic parenchyma in SBI animals when compared with controls 14 d after injury.
In conclusion, severe burn in greater than 40% of the body surface induces, in liver, histopathological changes, inflammation related to COX-2 immunoexpression, and cell proliferation not related to caspase-3 expression. Because modulating function of liver after burn injuries, the treatment of severe burns can be focused in liver disarrangements.
Scalds are most common cause of burn injury (BI) and preferentially occur in children under the five years. The persistent protein catabolism may lead to delay in growth for up to 2 years after injury. In addition, severe burn injuries result in serious complications that involve host response related to inflammation and multiple organ dysfunctions, including liver damage.
Studies involving autopsy of severely burned pediatric patients showed data about liver weight increased with fatty infiltration, but molecular and morphological investigation in vivo is necessary to elucidate better the liver damage process during great BI. For this, the present study investigated effects of severe BI in liver of young rats through the histopathological and morphoquantitative aspects, immunoexpression of COX-2 and liver gene expression of tumor necrosis factor-α, inducible nitric oxide synthase and caspase-3.
The liver damage process during severe BI are related with histopathological and morphoquantitative changes such as presence of erythrocytes in sinusoidal space associated with inflammatory infiltrate and inflammatory cells rounding hepatocytes in degeneration. Moreover, increased connective tissue, hepatocyte area larger than control, altered binucleated hepatocyte and sinusoidal cells density, were described in the present study.
Emphasize the importance of global treatment in burn great than 40% of total body surface area mainly in children. Highlight the damage caused in liver morphology clarifying morphological changes to possible treatments to prevent major consequences of BI.
Severe BI greater than 40% in children causes chronic morphological liver consequences ignored in numerous treatment centers. To emphasize the liver dysfunction as a result of extensive skin BI because hypermetabolic consequences was the purpose of the present paper.
The aim of the authors was to investigate the temporal effects of extensive experimental burn injury in rat liver. The are some studies focused on this issue. This is well designed study. This article will provide new information about liver problems developing after BI.
P- Reviewer: Akarsu M, Morales-Gonzalez J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 514] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Krishnamoorthy V, Ramaiah R, Bhananker SM. Pediatric burn injuries. Int J Crit Illn Inj Sci. 2012;2:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 408] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Sun BW, Sun Y, Sun ZW, Chen X. CO liberated from CORM-2 modulates the inflammatory response in the liver of thermally injured mice. World J Gastroenterol. 2008;14:547-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Izamis ML, Sharma NS, Uygun B, Bieganski R, Saeidi N, Nahmias Y, Uygun K, Yarmush ML, Berthiaume F. In situ metabolic flux analysis to quantify the liver metabolic response to experimental burn injury. Biotechnol Bioeng. 2011;108:839-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Dahiya P. Burns as a model of SIRS. Front Biosci (Landmark Ed). 2009;14:4962-4967. [PubMed] [Cited in This Article: ] |
| 7. | Jeschke MG, Micak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma. 2001;51:736-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Jeschke MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med. 2009;15:337-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Jeschke MG, Low JF, Spies M, Vita R, Hawkins HK, Herndon DN, Barrow RE. Cell proliferation, apoptosis, NF-kappaB expression, enzyme, protein, and weight changes in livers of burned rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1314-G1320. [PubMed] [Cited in This Article: ] |
| 11. | Walker HL, Mason AD. A standard animal burn. J Trauma. 1968;8:1049-1051. [PubMed] [Cited in This Article: ] |
| 12. | Newman JJ, Strome DR, Goodwin CW, Mason AD, Pruitt BA. Altered muscle metabolism in rats after thermal injury. Metabolism. 1982;31:1229-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1745] [Cited by in F6Publishing: 1765] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 14. | Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75:469-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1288] [Cited by in F6Publishing: 1276] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 16. | Farina JA, Rosique MJ, Rosique RG. Curbing inflammation in burn patients. Int J Inflam. 2013;2013:715645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | De Handt HA, Elwi AM, Soliman MA. Observations of the binucleate cells of the liver. Nature. 1966;212:827-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Jayaraman A, Maguire T, Vemula M, Kwon DW, Vannucci M, Berthiaume F, Yarmush ML. Gene expression profiling of long-term changes in rat liver following burn injury. J Surg Res. 2009;152:3-17,17.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Merritt EK, Thalacker-Mercer A, Cross JM, Windham ST, Thomas SJ, Bamman MM. Increased expression of atrogenes and TWEAK family members after severe burn injury in nonburned human skeletal muscle. J Burn Care Res. 2013;34:e297-e304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Paiotti AP, Marchi P, Miszputen SJ, Oshima CT, Franco M, Ribeiro DA. The role of nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors on experimental colitis. In Vivo. 2012;26:381-393. [PubMed] [Cited in This Article: ] |
| 21. | Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Luo G, Peng D, Zheng J, Chen X, Wu J, Elster E, Tadaki D. The role of NO in macrophage dysfunction at early stage after burn injury. Burns. 2005;31:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861-1866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Dobbs BR, Rogers GW, Xing HY, Fraser R. Endotoxin-induced defenestration of the hepatic sinusoidal endothelium: a factor in the pathogenesis of cirrhosis? Liver. 1994;14:230-233. [PubMed] [Cited in This Article: ] |
| 25. | Svistounov D, Zykova SN, Cogger VC, Warren , A , McMahon AC, Fraser R, Le Counteur DG. Liver Sinusoidal Endothelial Cells and Regulation of Blood Lipoproteins. In: Kelishadi R. Dyslipidemia - From Prevention to Treatment. In Tech 2012; 263-279. [DOI] [Cited in This Article: ] |
| 26. | Okabayashi K, Ohtani M, Morio M, Kajihara H. Structural changes of Kupffer cells in rat liver following experimental thermal injury. Burns. 1990;16:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Brandão DF, Ramalho LN, Ramalho FS, Zucoloto S, Martinelli Ade L, Silva Ode C. Liver cirrhosis and hepatic stellate cells. Acta Cir Bras. 2006;21 Suppl 1:54-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |