Published online May 28, 2016. doi: 10.4254/wjh.v8.i15.649
Peer-review started: January 28, 2016
First decision: February 29, 2016
Revised: April 4, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: May 28, 2016
AIM: To estimate the progression of the hepatitis C virus (HCV) epidemic and measure the burden of HCV-related morbidity and mortality.
METHODS: Age- and gender-defined cohorts were used to follow the viremic population in Argentina and estimate HCV incidence, prevalence, hepatic complications, and mortality. The relative impact of two scenarios on HCV-related outcomes was assessed: (1) increased sustained virologic response (SVR); and (2) increased SVR and treatment.
RESULTS: Under scenario 1, SVR raised to 85%-95% in 2016. Compared to the base case scenario, there was a 0.3% reduction in prevalent cases and liver-related deaths by 2030. Given low treatment rates, cases of hepatocellular carcinoma and decompensated cirrhosis decreased < 1%, in contrast to the base case in 2030. Under scenario 2, the same increases in SVR were modeled, with gradual increases in the annual diagnosed and treated populations. This scenario decreased prevalent infections 45%, liver-related deaths 55%, liver cancer cases 60%, and decompensated cirrhosis 55%, as compared to the base case by 2030.
CONCLUSION: In Argentina, cases of end stage liver disease and liver-related deaths due to HCV are still growing, while its prevalence is decreasing. Increasing in SVR rates is not enough, and increasing in the number of patients diagnosed and candidates for treatment is needed to reduce the HCV disease burden. Based on this scenario, strategies to increase diagnosis and treatment uptake must be developed to reduce HCV burden in Argentina.
Core tip: This is a study evaluating potential policies to diminish hepatitis C virus (HCV) disease burden. Increasing diagnoses and treated individuals with the high current sustained virologic response rates, will diminish HCV disease burden.
- Citation: Ridruejo E, Bessone F, Daruich JR, Estes C, Gadano AC, Razavi H, Villamil FG, Silva MO. Hepatitis C virus infection in Argentina: Burden of chronic disease. World J Hepatol 2016; 8(15): 649-658
- URL: https://www.wjgnet.com/1948-5182/full/v8/i15/649.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i15.649
Chronic hepatitis C virus (HCV) liver disease is a global public health issue, with an estimated prevalence of 170 million infected people. Every year, 3000000 to 4000000 new HCV infections are diagnosed, and a mean global seroprevalence of nearly 3%[1].
In many countries, while HCV prevalence is decreasing, its morbidity and mortality is increasing[2]. Population aging results in a rise in all-cause mortality. This leads to a reduction in the total of infected patients. Progression to advanced HCV related liver disease combined with populace aging, is associated with a rising in mortality due to advanced liver disease[2,3].
In Argentina, the exact HCV prevalence is unknown. According to different studies it varies between 0.17% to 5.6%; in some areas of high endemicity it may vary between 2.2% to 7.3%[4]. Nosocomial transmission appears to be the main route of infection, and genotype 1 is most prevalent in the infected population[5,6]. Precise data for incidence and prevalence estimates are lacking in Argentina. Also, there are no data about the burden of the disease and its impact on public health. Data on the percentage of HCV patients treated and their outcomes are also scarce. It has been estimated that only 0.15% of HCV patients have been treated in the last 15 years in Argentina[7]. These results are comparable to other countries in the region. Our aim was, using a modeling method, to describe HCV-related disease progression at the national level.
A model was also used to evaluate the influence of distinct actions aimed at diminishing the burden of HCV disease (e.g., multiply the percentage of treated patients, improved cure rates and improved case identification). This model has been already validated and used in similar studies in different countries[8-11].
A systematic review of the literature was done to find studies addressing the proportion of HCV patients who had been diagnosed, received treatment and achieve sustained virologic response (SVR) in Argentina. The review included all studies published between January 1990 and July 2014.
PubMed and EMBASE databases were consulted looking for indexed articles. Non-indexed sources were identified by searching in the National Ministry of Health Website, proceedings of local medical meetings, unpublished data and data from large liver centers.
Also, an expert panel including epidemiologists, hepatologists, infectious disease specialists, public health professionals and virologists, gathered in a person to person meeting to analyze all the retrieved information.
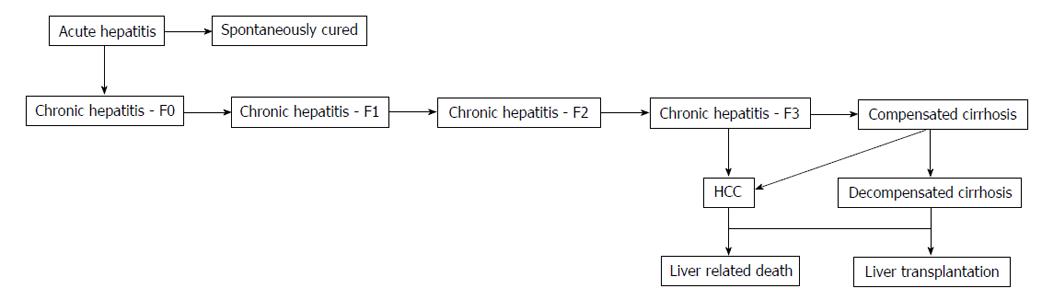
Data from countries with similar healthcare practices and/or risk factors, or expert consensus were used there was no input data available. Some of these data were included in a previous global report[2,3]. To populate a disease progression model and to assess the magnitude of the HCV-infected populace according to liver fibrosis stages (METAVIR score F0-F4), country-specific inputs from 2013-2030 were loaded in Microsoft Excel® database (Microsoft Corp., Redmond, WA) (Figure 1). Crystal Ball, an Excel add-in by Oracle, was utilized for uncertainty and sensitivity analyses. For the uncertainty model, beta-PERT distributions were utilized associated with all inputs. To analyze the incertitudes that had the biggest repercussion on in 2030 HCV prevalence, a sensitivity analysis was utilized.
Populace information were arranged by sex, five-year age groups, and year (1950-2100) and obtained from the United Nations population database[12]. Based on expert inputs, in adults (persons aged ≥ 20 years) HCV viremia prevalence in Argentina in 2013, was estimated at 1.5%. The HCV viremic rate in Argentina is 80%, as previously reported[13].
Using a 0.83% viremic prevalence, it was calculated that 342000 persons had HCV RNA detectable in 2013.
A hybrid distribution was constructed to calculate age and gender specific HCV diagnosis rates by five-year age group using notification inputs for HCV infection for persons aged 0 to 59 years[14], and transplant inputs classified by age and gender for persons aged ≥ 60 years[15]. The notified and transplanted people were weighted to the national estimate for total prevalence and aged to the year 2013, accounting for mortality and cured patients.
To estimate HCV genotype distribution, data from over 200 treated patients was used[16]. Genotype 1 (G1) subtypes distribution was calculated using data from another study[5]. The genotype distribution applied in the model was G1/other = 63%, G2 = 25%, G3 = 11%, G4 = 1%.
As outlined in a previous work, annual patients progress through each disease state were include in the model using age and gender specific transition probabilities[2,3].
Changes in historical HCV incidence were estimated according to expert opinion. Changes in historical HCV incidence were estimated according to expert opinion. After an estimated peak incidence in 1989, it has markedly decrease with the introduction of antiHCV screening in blood donors. In Argentina, it was estimated that 1850 new infections were diagnosed in 2013.
It was estimated that 350 and 200 patients receive treatment in 2014 and 2015, respectively, based on expert consensus and IMS data for pegylated-interferon (IFN) units sold in Argentina[17]. A multiplier was used to account for under-reporting in IMS data. The Argentinean genotype distribution was used to estimate the average number of weeks of treatment per patient with 85% compliance/persistence.
In 2013, 74 of 329 (22.4%) patients receiving a liver transplant were related to HCV end stage liver disease. Data from the national organ registry for the years 1999 to 2013 showed that the percentage of liver transplant in HCV patients was 22.0% before adoption of model for end stage liver disease (MELD) based allocation and 22.4% after MELD implementation[15,16].
Database from the Pan American Health Organization allow us to estimate the diagnosed population based upon data for HCV positive blood donors[7]. The annual number of confirmed cases was balanced to account for diagnosis in other settings. It was assumed that 118800 persons were previously diagnosed and 6560 new cases were confirmed in 2010. The Berkeley Human Mortality database was used to estimate mortality rate by year, age group and gender[18] (Table 1).
| Historical | Year | 2013 (Est.) | |
| HCV infected cases | 427890 (132720-829480) | 2013 | 428260 |
| AntiHCV prevalence | 1.0% (0.3%-2.0%) | 1.0% | |
| Total viremic cases | 342310 (106170-663580) | 2013 | 342310 |
| Viremic prevalence | 0.8% (0.3%-1.6%) | 0.8% | |
| Viremic rate | 80.0% | 80.0% | |
| HCV diagnosed (viremic) | 112270 | 2010 | 117250 |
| Viremic diagnosis rate | 32.8% | 34.2% | |
| Annual newly diagnosed | 4920 | 2010 | 4920 |
| New infections | 1950 | ||
| New infection rate (per 100K) | 4.7 | ||
| Treated | |||
| Number treated | 650 | ||
| Annual treatment rate | 0.2% | ||
| Risk factors | |||
| Number of active IDU with HCV | 31950 | ||
| Percent active IDU | 9.3% | ||
| Previous blood transfusion | 48420 | ||
| Percent previous blood transfusion | 14.1% |
Using estimates of 65000 active injection drug users (IDU) and a 54.6% HCV prevalence in Argentina, it was calculated that in 2001, 9.3% of the HCV population were IDU[19,20].
Using a standard mortality ratio (SMR) of 10.0 for persons between 15 and 44 years old, a raised mortality was estimated among active IDU[21-26].
It was estimated that 20.8% of the HCV patients were related blood transfusions in 2005, according to data from a national study[6]. In this subgroup of patients, a SMR of 1.5 was applied for all age groups[27].
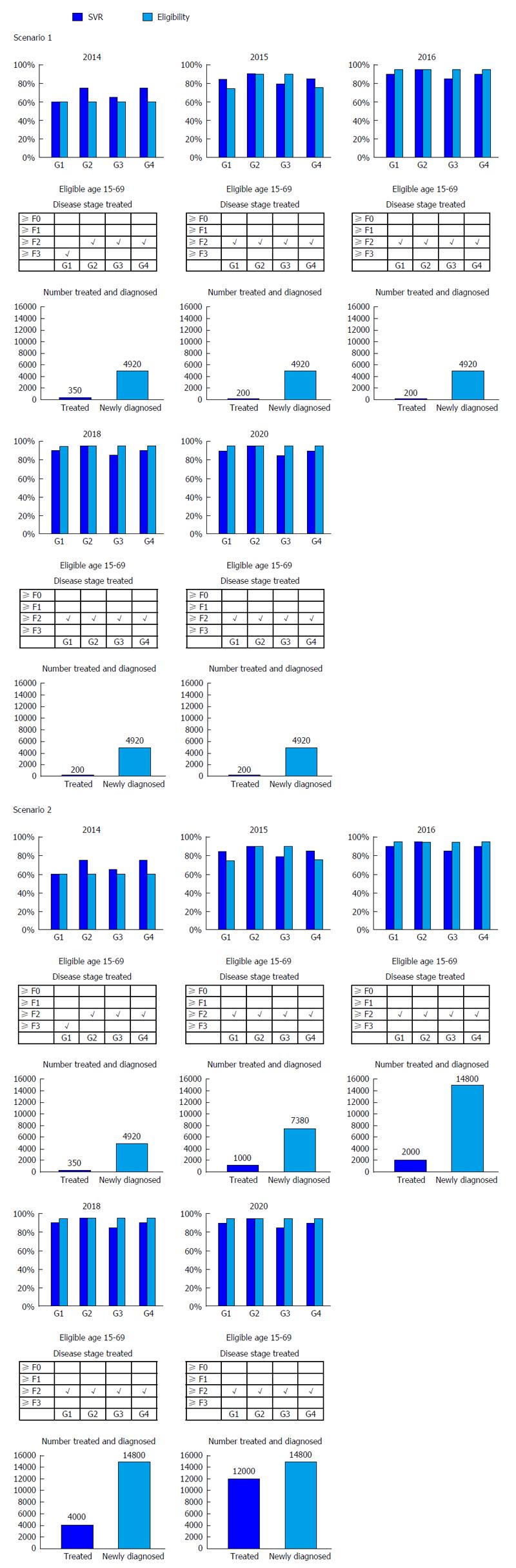
Base scenario: Patients aged 15-69 years were considered for treatment and 60% of potential patients in Argentina were considered candidates for antiHCV therapy. It was considered that median SVR rates were 60% (G1), 75% (G2/4), and 65% (G3). Treated populations of 350 patients in 2014 and 200 patients annually during 2015-2030 were modeled, was and were restricted to patients with fibrosis stages ≥ F3 (G1) and ≥ F2 (G2/3/4).
It was considered that until 2016 patients with severe liver disease such as decompensated cirrhosis or eligible for transplantation, or those with hepatocellular carcinoma (HCC), were not candidates for treatment.
Scenario 1: Increased efficacy: It was assumed that by 2016, treatment eligibility raised to 95% for all genotypes and SVR rates steadily raised to 90% (G1/4), 95% (G2), and 85% (G3). The number of patients treated and newly diagnosed every year remained constant, while treatment was extended to fibrosis stages ≥ F2 in all genotypes (Figure 2).
Scenario 2: Increased efficacy and treatment: SVR, treatment eligibility, and fibrosis restriction increases were the same as in scenario 1. The number of patients newly diagnosed every year progressively escalated to 14770 in 2016, while the number of patients treated every year progressively escalated to 12000 by 2020 (Figure 3).
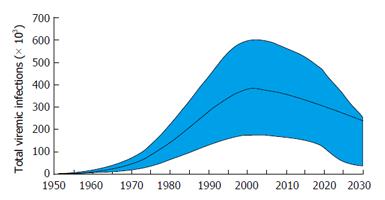
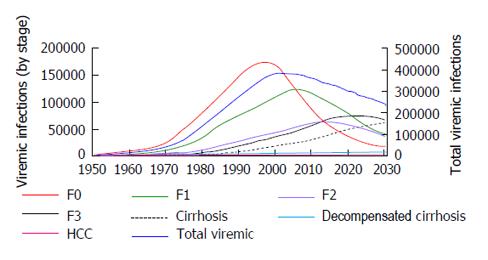
According to the model, the HCV prevalence in Argentina peaked in 2002 at 376000 viremic individuals. In 2013, there were an estimated 342000 (95%CI: 146000-517000) infected individuals, a 10% decline from 2002. In the base scenario, viremic cases are estimated at 241000 in 2030, a decline of 30% from 2014 (Figure 4). The incidence of HCV in Argentina peaked in 1989 with an estimated 21340 new infections, and declined by 90% in 2013 with an estimated 1850 cases new infections.
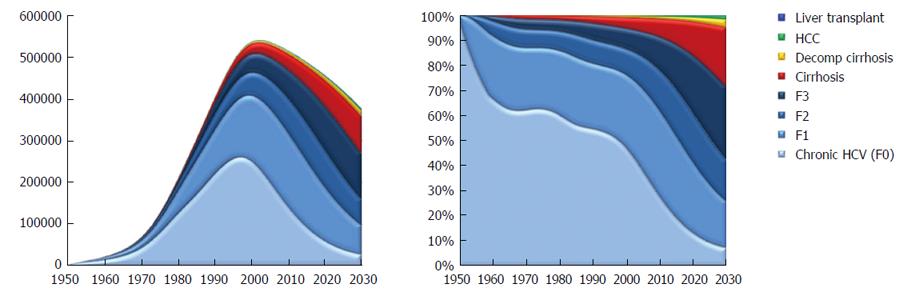
There were 42910 compensated cirrhotic patients in 2013 and it was calculated that there will be 69600 by 2030. Also by 2030 there will be 2500 new cases of HCC 7830 patients will develop decompensated cirrhosis. By 2030, 2890 patients will die from HCV related liver disease in contrast to 1520 patients who died in 2013. The proportion of viremic patients who have compensated cirrhosis or decompensated cirrhosis or HCC will increase to 34% in 2030, as compared with 14% in 2013 (Figures 5 and 6).
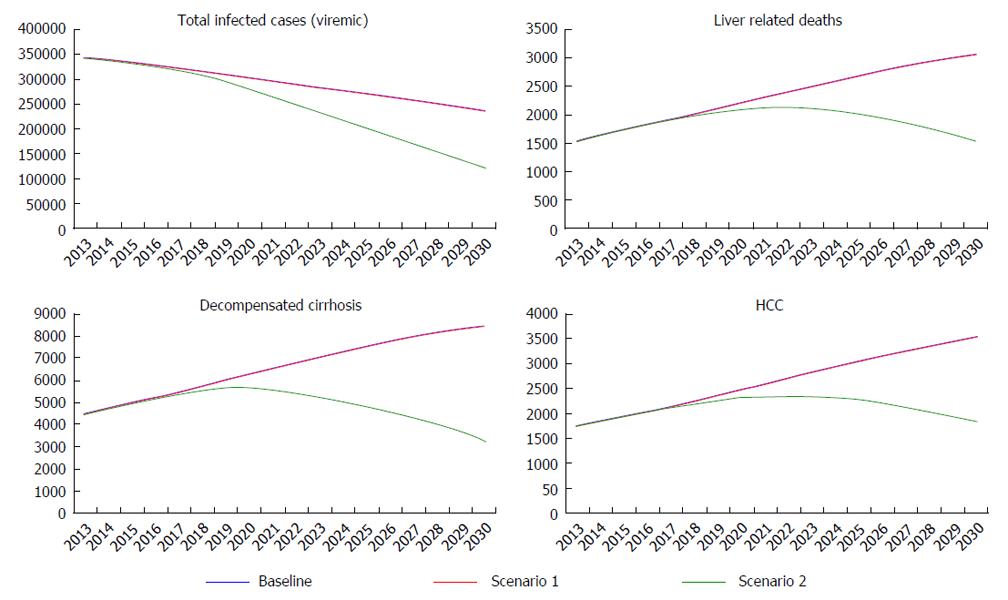
New HCV treatment strategies imply an increase in SVR rates. Based on recent results SVR rates will increase to at least 90% (G1/4), 95% (G2), and 85% (G3) by 2016. In the same period, treatment eligibility will increase to 95% for all genotypes. According to the model, increasing treatment efficacy but keeping the same low number of treated patients (scenario 1) will result in 660 fewer viremic patients in 2030, a 0.3% reduction as compared to the base case.
Compared with the base case, by 2030 it was estimated a 0.3% decrease in the number of HCC cases (2490 cases), a 0.3% decrease in liver related deaths (2880 cases), a 0.2% decrease in decompensated and 0.3% in compensated cirrhosis new cases (7800 and 69380 cases, respectively) (Figure 7).
Increased treatment efficacy alone seems to have little impact in decreasing HCV burden, so another scenario was developed with the same SVR rates but increasing numbers of patients diagnosed and treated (scenario 2).
If the number of diagnosed and treated patients is markedly increased, a 45% reduction in the number of viremic patients can be obtained by 2030, meaning 107000 fewer infected patients. A 60% reduction in HCC cases is expected, with 1000 new HCC cases diagnosed by 2030. It is expected that the number of liver related deaths will also decrease with 1260 by 2030, meaning a 55% reduction when compared to the base case. New cirrhosis cases will decrease by 55% in decompensated and by 60% in compensated cases by 2030 (3390 and 29210, respectively) (Figure 7).
Increasing access to HCV diagnosis and treatment are pending actions in Argentina and in Latin-America. It is estimated that less than 20%-30% of patients are diagnosed and only 1%-2% of those diagnosed have been treated[7]. Approval of new HCV treatments in the region is delayed compared with Europe or the United States. In the last months of 2015, three novel regimens were approved in Argentina. Upcoming IFN and ribavirin free regimens are safe and effective, offering SVR rates over 90%-95% for most genotypes. To impact the burden of disease, patients must be diagnosed and treatment availability must increase.
Our study shows important results for our country. The greatest burden of HCV-related advanced liver disease will come in the next 5 to 15 years. HCV burden will increase if no action is taken. Our model showed that the only way to significantly reduce HCV burden is to increase diagnosed and treated patients 10 times the current number of treated persons. Similar results have been reported in many countries around the world, including some in Latin-America, including Brazil and Mexico[2,3].
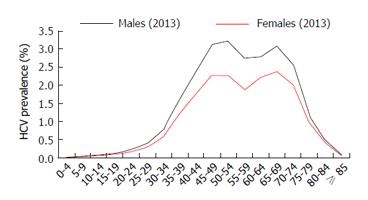
The main challenge in the region is to develop strategies to increase diagnosis. Strategies must be country specific since epidemiology and risk factors for HCV infection vary between countries. For example, the United States Centers for Disease Control and Prevention has recommended a birth-year based screening strategy: Persons born during 1945-1965 in the United States have an increased rate of HCV infection and focused screening of this cohort is an efficient use of resources[28]. But this strategy might not be effective in Argentina, since in 2013 the majority of HCV patients are estimated to be 40 to 75 years old (Figure 2), meaning that they were born between 1938 and 1973. The same was shown in Brazil where most patients were born between 1950 and 1980[29]. Country specific screening campaigns must be developed to achieve this goal.
Another pending issue is adequate access to care and treatment. This means that all people involved in HCV management must make an effort to achieve this goal. Patients need greater access to new therapies, but the main restriction is treatment cost. In resource constrained countries, treating all patients with current drug costs is unaffordable. There must be strategies to reduce HCV treatment costs and at the beginning, prioritization of treatment may be necessary. For example, the sickest patients will be treated first with the safest and more effective drugs. Then earlier stage patients will be treated later to reduce the impact of the disease.
This is the first study evaluating HCV burden in Argentina. These results might help public health authorities take action to reduce its impact. But it has to be mentioned that our results have some limitations.
First, each input may have its limitations, but to our knowledge the best data from published and unpublished studies available in Argentina were applied in our model. Second, some patients may have progressive liver disease despite achieving SVR; progression of cured patients was not evaluated in this model[30]. And finally, we did not include extrahepatic manifestations of HCV infection in the model, which may had contribute to all-cause mortality and may lead to underestimation in mortality among viremic patients[9].
In conclusion, the present analysis, with the available data, showed that HCV prevalence is decreasing in Argentina, but advanced liver disease prevalence is expected to raise as HCV infected patients get older. There is an urgent need to enhance diagnosis and treatment rates to reduce the future disease burden and its impact on Argentina’s public health.
Chronic hepatitis C virus (HCV) infection is one of the main causes of end stage liver disease, liver transplantation, hepatocellular carcinoma (HCC) and liver-related mortality in Argentina. Burden of HCV disease is unknown, and strategies to reduce it are not yet developed.
An epidemiological model has been developed to estimate HCV disease burden and to evaluate different diagnostic and therapeutic strategies that may impact in HCV natural history.
This model allows them for the first time to evaluate HCV burden in Argentina. This estimated data can help health authorities to develop a national plan to manage HCV disease. Also, it permits the authors to estimate the number of persons needing treatment to reduce HCV burden in the next 15 years.
This study shows that HCV treatment impacts in its disease burden and that a major work has to be done in improving its diagnosis and access to treatment.
HCV disease burden implies the development of liver related disease: Cirrhosis, HCC, liver failure, liver transplantation and death.
In this study the authors have used a modeling approach to describe HCV-related disease progression in Argentina. The methods are well designed and are exposed very clearly for the reader. In general, it is a good manuscript.
P- Reviewer: Ciftci S, Medina P S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1796] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 2. | Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Z, Mossong J, Schréter I, Baatarkhuu O, Acharya S, Aho I. The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm - volume 2. J Viral Hepat. 2015;22 Suppl 1:26-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Saraswat V, Norris S, de Knegt RJ, Sanchez Avila JF, Sonderup M, Zuckerman E, Arkkila P, Stedman C, Acharya S, Aho I. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. J Viral Hepat. 2015;22 Suppl 1:6-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Reggiardo MV, Tanno F, Mendizabal M, Galdame O. [Argentine consensus on hepatitis C 2013]. Acta Gastroenterol Latinoam. 2014;44:154-173. [PubMed] [Cited in This Article: ] |
| 5. | Vladimirsky S, Silvina MM, Otegui L, Altabert N, Soto S, Brajterman L, Echenique H, González J; Unidades Centinela para Hepatitis Virales. [Surveillance of viral hepatitis in Argentina: analysis of information from sentinel units 2007-2010]. Acta Gastroenterol Latinoam. 2013;43:22-30. [PubMed] [Cited in This Article: ] |
| 6. | Ridruejo E, Adrover R, Cocozzella D, Fernández N, Reggiardo MV. Efficacy, tolerability and safety in the treatment of chronic hepatitis C with combination of PEG-Interferon - Ribavirin in daily practice. Ann Hepatol. 2010;9:46-51. [PubMed] [Cited in This Article: ] |
| 7. | Kershenobich D, Razavi HA, Sánchez-Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguez-Perez F, Rosado B. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int. 2011;31 Suppl 2:18-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 9. | Myers RP, Krajden M, Bilodeau M, Kaita K, Marotta P, Peltekian K, Ramji A, Estes C, Razavi H, Sherman M. Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol. 2014;28:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Flisiak R, Halota W, Tomasiewicz K, Kostrzewska K, Razavi HA, Gower EE. Forecasting the disease burden of chronic hepatitis C virus in Poland. Eur J Gastroenterol Hepatol. 2015;27:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Willemse SB, Razavi-Shearer D, Zuure FR, Veldhuijzen IK, Croes EA, van der Meer AJ, van Santen DK, de Vree JM, de Knegt RJ, Zaaijer HL. The estimated future disease burden of hepatitis C virus in the Netherlands with different treatment paradigms. Neth J Med. 2015;73:417-431. [PubMed] [Cited in This Article: ] |
| 12. | United Nations, Department of Economic and Social Affairs. Population division (2011). World population prospects: The 2010; comprehensive tables. New York New York United Nations, 2010. [Cited in This Article: ] |
| 13. | del Pino N, Oubiña JR, Rodríguez-Frías F, Esteban JI, Buti M, Otero T, Gregori J, García-Cehic D, Camos S, Cubero M. Molecular epidemiology and putative origin of hepatitis C virus in random volunteers from Argentina. World J Gastroenterol. 2013;19:5813-5827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Personal Communication. Situación epidemiológica en Argentina. 2014;. [Cited in This Article: ] |
| 15. | Instituto Nacional Central Único Coordinador de Ablación e Implante. El Sistema Nacional de Información de Procuración y Trasplante de la República Argentina. 2014;. [Cited in This Article: ] |
| 16. | Cejas NG, Villamil FG, Lendoire JC, Tagliafichi V, Lopez A, Krogh DH, Soratti CA, Bisigniano L. Improved waiting-list outcomes in Argentina after the adoption of a model for end-stage liver disease-based liver allocation policy. Liver Transpl. 2013;19:711-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | IMS Health. IMS Health MIDAS. Data. 2013;. [Cited in This Article: ] |
| 18. | Wilmoth JR, Shkolnikov V. Human Mortality Database. Berkeley, United States: University of California. Rostock, Germany: Mack Planck Institute for Demographic Research 2013; . [Cited in This Article: ] |
| 19. | Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug Policy. 2007;18:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 949] [Cited by in F6Publishing: 936] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 21. | Engström A, Adamsson C, Allebeck P, Rydberg U. Mortality in patients with substance abuse: a follow-up in Stockholm County, 1973-1984. Int J Addict. 1991;26:91-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Frischer M, Goldberg D, Rahman M, Berney L. Mortality and survival among a cohort of drug injectors in Glasgow, 1982-1994. Addiction. 1997;92:419-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Hickman M, Carnwath Z, Madden P, Farrell M, Rooney C, Ashcroft R, Judd A, Stimson G. Drug-related mortality and fatal overdose risk: pilot cohort study of heroin users recruited from specialist drug treatment sites in London. J Urban Health. 2003;80:274-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Oppenheimer E, Tobutt C, Taylor C, Andrew T. Death and survival in a cohort of heroin addicts from London clinics: a 22-year follow-up study. Addiction. 1994;89:1299-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 215] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Perucci CA, Davoli M, Rapiti E, Abeni DD, Forastiere F. Mortality of intravenous drug users in Rome: a cohort study. Am J Public Health. 1991;81:1307-1310. [PubMed] [Cited in This Article: ] |
| 26. | Bjornaas MA, Bekken AS, Ojlert A, Haldorsen T, Jacobsen D, Rostrup M, Ekeberg O. A 20-year prospective study of mortality and causes of death among hospitalized opioid addicts in Oslo. BMC Psychiatry. 2008;8:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Kamper-Jørgensen M, Ahlgren M, Rostgaard K, Melbye M, Edgren G, Nyrén O, Reilly M, Norda R, Titlestad K, Tynell E. Survival after blood transfusion. Transfusion. 2008;48:2577-2584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32. [PubMed] [Cited in This Article: ] |
| 29. | Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hézode C, Lázaro P. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 30. | Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, Verbaan H, Stål P, Carlsson T, Norrgren H. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |