Published online Feb 27, 2023. doi: 10.4254/wjh.v15.i2.237
Peer-review started: November 22, 2022
First decision: December 10, 2022
Revised: December 14, 2022
Accepted: January 17, 2023
Article in press: January 17, 2023
Published online: February 27, 2023
Although many studies have investigated the impact of chronic hepatitis B virus (HBV) infection and nonalcoholic fatty liver disease (NAFLD) on liver disease, few have investigated the relationship between nonalcoholic steatohepatitis (NASH) defined by liver pathology and the prognosis of chronic HBV infection. Most patients were followed up for a short time. This study aimed to further ex
To study the effect of NAFLD confirmed using liver pathology on the outcomes of long-term serious adverse events [cirrhosis, hepatocellular carcinoma (HCC), and death] in patients with chronic hepatitis B (CHB) virus infection.
We enrolled patients with chronic hepatitis B virus (HBV) infection who under
Overall, 456 patients with chronic HBV infection were included in the study, of whom 152 (33.3%) had histologically confirmed NAFLD. The median follow-up time of the entire cohort was 70.5 mo. Thirty-four patients developed cirrhosis, which was diagnosed using ultrasound during the follow-up period. K-M survival analysis showed that NAFLD was not significantly associated with the risk of cirrhosis (log-rank test, P > 0.05). Patients with CHB with fibrosis at baseline were more prone to cirrhosis (log-rank test, P = 0.046). After PSM, multivariate analysis showed that diabetes mellitus, ballooning deformation (BD), and platelet (PLT) were independent risk factors for cirrhosis diagnosed using ultrasound (P < 0.05). A total of 10 patients (2.2%) developed HCC, and six of these patients were in the combined NAFLD group. K-M survival analysis showed that the cumulative risk of HCC in the NAFLD group was significantly higher (log-rank test, P < 0.05). Hepatocyte ballooning, and severe liver fibrosis were also associated with an increased risk of HCC (log-rank test, all P < 0.05). Cox multivariate analysis revealed that hepatocyte ballooning, liver fibrosis, and diabetes mellitus were independent risk factors for HCC.
There was no significant correlation between chronic HBV infection and the risk of cirrhosis in patients with NAFLD. Diabetes mellitus, BD, and PLT were independent risk factors for liver cirrhosis. Patients with chronic HBV infection and NASH have an increased risk of HCC. BD, liver fibrosis, and diabetes mellitus are independent risk factors for HCC.
Core Tip: A total of 456 patients with chronic hepatitis B virus infection were included in the study, of whom 152 (33.3%) had histologically confirmed nonalcoholic fatty liver disease (NAFLD). The median follow-up time of the entire cohort was 70.5 mo. Kaplan-Meier (K-M) survival analysis showed that NAFLD was not significantly associated with the risk of cirrhosis. Patients with chronic hepatitis B with fibrosis at baseline were more prone to cirrhosis. After PSM, multivariate analysis showed that diabetes mellitus, ballooning deformation, and platelet were independent risk factors for cirrhosis. A total of 10 patients (2.2%) developed hepatocellular carcinoma (HCC). K-M survival analysis showed that the cumulative risk of HCC in the NAFLD group was significantly higher. Cox multivariate analysis revealed that hepatocyte ballooning, liver fibrosis, and diabetes mellitus were independent risk factors for HCC.
- Citation: Tan YW, Wang JM, Zhou XB. Baseline hepatocyte ballooning is a risk factor for adverse events in patients with chronic hepatitis B complicated with nonalcoholic fatty liver disease. World J Hepatol 2023; 15(2): 237-254
- URL: https://www.wjgnet.com/1948-5182/full/v15/i2/237.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i2.237
Chronic hepatitis B (CHB) virus infection and nonalcoholic fatty liver disease (NAFLD) are important causes of liver-related complications and death. With the increasing prevalence of NAFLD, the number of patients with combined NAFLD and hepatitis B virus (HBV) infections is also on the increase. In Asia, the prevalence of NAFLD in patients with hepatitis B virus infection is approximately 14%-67%, which is not different from the data of western countries[1,2]. In recent years, there have been many studies on hepatitis B complicated with NAFLD; however, the interaction between these two diseases is still elusive.
It is understandable that in the case of combined NAFLD, the overall prognosis of these patients seems to be worse. Both NAFLD and CHB can aggravate liver injury and increase the risk of cirrhosis and liver cancer[3-7]. Recently, a cohort study evaluated the FibroScan liver transient elastography results of 459 hepatitis B e antigen (HBeAg)-negative patients over a 10-year period, and found that hepatic steatosis in patients with CHB was associated with the progression of fibrosis[8]. Based on FibroScan examination, a study of 1202 patients with CHB found that the proportion of patients with moderate to severe fibrosis among patients with severe steatosis was significantly higher than that in patients with mild or moderate steatosis (23.2% vs 12.6%)[9,10]. A retrospective cohort study of 270 patients with CHB showed that liver steatosis confirmed by biopsy was an independent risk factor for hepatocellular carcinoma (HCC) in patients with CHB[5]. Another large multicenter multi-ethnic cohort study of 1089 patients with CHB showed that liver steatosis confirmed by biopsy was not significantly associated with clinical outcomes (HCC and death).
Although many studies have investigated the impact of chronic HBV infection and NAFLD on liver disease, few have investigated the relationship between nonalcoholic steatohepatitis (NASH) defined by liver pathology and the prognosis of chronic HBV infection. Most patients were followed up for a short time. This study aimed to further explore the impact of NAFLD and the pathological changes confirmed by liver pathology in patients with chronic HBV infection.
All patients with chronic HBV infection who underwent liver biopsy at The Third Hospital of Zhenjiang Affiliated Jiangsu University from January 2005 and September 2020 were selected. Chronic HBV infection was defined as continuous positive serum hepatitis B surface antigen (HBsAg) or HBV DNA results for more than 6 mo. The inclusion criterion was a follow-up time greater than 6 mo. The exclusion criteria were a history of excessive alcohol consumption (defined as alcohol intake ≥ 20 g/day for men and ≥ 10 g/day for women)[11], history of schistosomiasis of the liver, autoimmune hepatitis, primary biliary cirrhosis, malignancy, immunodeficiency virus infection, viral hepatitis C or D, long-term use of drugs that can cause hepatic steatosis (amiodarone, sodium valproate, tamoxifen, dexamethasone, or methotrexate), and incomplete clinical data. This study was approved by the ethics committee of The Third People’s Hospital Affiliated to Zhenjiang, Jiangsu University. It was registered in the Chinese Clinical Trial Registry (No: chictr2200060304).
The demographic data of patients (sex, age, height, and weight) were collected along with their clinical history (diabetes mellitus, hypertension, drug use history, drinking history); antiviral treatment; blood routine, biochemistry, and serological examination of hepatitis B pathogen. Other data collected were tumor index results during liver biopsy, including total bilirubin, albumin, prealbumin (PB), alanine aminotransferase (ALT), aspartate aminotransferase, γ-glutamyl transferase, alkaline phosphatase (ALP), fasting blood glucose (GLU), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein, platelets (PLT), HBsAg, HBeAg, HBV DNA level, alpha fetoprotein (AFP), other types of viral hepatitis indicators, human immunodeficiency virus antibody, and autoantibody test results. Body mass index (BMI) was calculated using each patient’s height and weight with the following formula: BMI = weight (kg)/height (m)2.
The calculation results were graded according to the Asian standard[12], in which overweight and obesity were defined as BMI ≥ 23 kg/m2 and ≥ 25 kg/m2, respectively. The detection limit of HBV-DNA was 500 IU/mL.
All liver specimens were evaluated by experienced pathologists and scored according to the nonalcoholic steatohepatitis clinical research network[13] for hepatic steatosis (0-3), lobular inflammation (0-2), portal inflammation (0-3), and ballooning degeneration (0-1). The degree of fibrosis was divided into F0-4 stages according to the METAVIR evaluation system, and the F4 stage was defined as cirrhosis[14]. NAFLD occurs when more than 5% of hepatocytes with steatosis are present in a specimen. The activity score (NAS) of nonalcoholic fatty liver disease was calculated according to the scores of steatosis, lobular inflammation, and ballooning degeneration. NAS ≥ 5 indicates the presence of NASH[13].
The start time of follow-up was the date when the patient underwent liver biopsy. The follow-up endpoint was the date of the last follow-up or the date of the occurrence of clinical outcomes (cirrhosis, HCC, or death). Follow-up of the entire cohort ended in August 2021. The electronic medical record was consulted to obtain the date of the last follow-up, test results (blood routine, HBV pathogen serology, liver function, blood lipids, AFP), and imaging results. The treatment of patients with an interval of more than 6 mo between the last follow-up date and the research deadline (telephone follow-up, regular follow-up) is recommended, and the clinical outcome of those who do not wish to follow up is determined according to the last follow-up record. If there was an out-of-hospital follow-up, the out-of-hospital examination results were obtained, and if there was a death, the time and cause of death were obtained. Those who did not have contact information or could not be contacted were regarded as being lost to follow-up.
The diagnosis of cirrhosis was made by experienced sonographers according to the ultrasound diagnostic criteria of cirrhosis, and computed tomography and magnetic resonance imaging were applied when necessary.
The diagnosis of HCC was based on histological or imaging findings, and the latter was positive lesions detected by at least two imaging techniques (ultrasound, computed tomography, or magnetic resonance imaging), or the use of imaging technology combined with AFP > 400 ng/mL.
The data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as mean ± SD or median (interquartile range), and categorical variables are expressed as percentages. T-test was applied when continuous variables were normally distributed, and the Mann–Whitney U test was used when they were non-normally distributed. Categorical variables were analyzed using the chi-squared test. Kaplan-Meier (K-M) survival analysis was used to evaluate clinical events, and Cox proportional hazards regression was applied for univariate and multivariate analyses. This study also used 1:1 propensity score matching (PSM) to match the NAFLD and non-NAFLD groups, and the caliper value was set to 0.01. All tests were two-tailed, and statistical significance was set at P < 0.05.
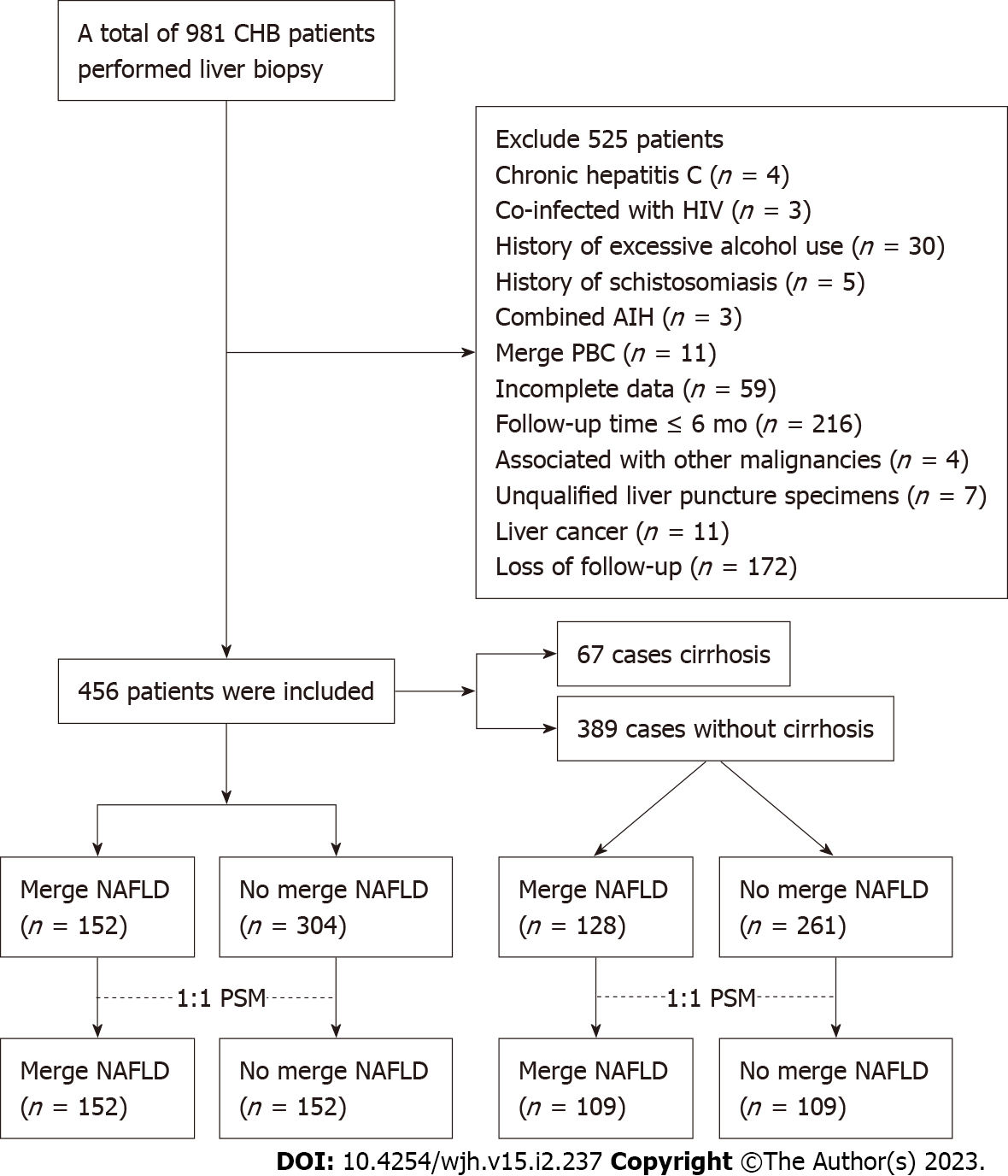
A total of 981 patients with chronic HBV infection underwent liver biopsy at The Third People’s Hospital of Zhenjiang between January 2005 and September 2020. After screening based on the inclusion and exclusion criteria, 456 patients were included in the final study, 67 of whom had histologically confirmed cirrhosis at baseline. Figure 1 shows the specific process.
Basic information of the general population: The total number of study patients was 456; of these patients, 152 (33.3%) had histologically confirmed NAFLD. The median follow-up time of the entire cohort was 70.5 mo. The average age of the population was 41 years and 45.1% were female. Regarding BMI, 43% had a normal BMI, 27.9% were overweight (BMI ≥ 23 kg/m2, < 25 kg/m2), and 29.2% were obese (BMI > 25 kg/m2). There were 42 patients with diabetes and 38 with hypertension, accounting for 9.2% and 8.3% of all patients, respectively. Most of the patients (72.4%) received antiviral therapy. At baseline, 358 patients were HBV DNA positive, with a median detection value of 3.63 × 104 (104.56) IU/mL, while in 98 (21.5%) patients the HBV DNA level was below the detection limit. Among all HBV-infected patients, 66% were HBeAg-negative. Table 1 shows the basic information of the total population.
| Parameters | Results |
| Age (yr) | 41.08 ± 10.41 |
| Sex female | 160 (45.1) |
| BMI (kg/m2) | |
| < 23 | 196 (43.0) |
| 23-25 | 127 (27.9) |
| ≥ 25 | 133 (29.2) |
| Diabetes | 42 (9.2) |
| Hypertension | 38 (8.3) |
| NAFLD | 152 (33.3) |
| Duration of follow-up (mo) | 70.5 (29-133) |
| Antiviral therapy | 330 (72.4) |
| HBV DNA (+), log10 (IU/mL) | 4.56 (3-6.92) |
| HBV DNA (-)1 | 98 (21.5) |
| HBeAg (-) | 301 (66.0) |
Comparison of baseline data of chronic HBV infection with and without NAFLD: There were 152 patients with chronic HBV infection complicated by NAFLD and 304 patients without NAFLD. Table 2 shows the demographic and main clinical indicators of the two groups. The proportion of female patients in the NAFLD group was higher than that in the NAFLD group (P < 0.05), and the median follow-up time was longer than that in the NAFLD group (73 vs 63 mo, P < 0.05). Compared with the non-NAFLD group, the NAFLD group had a higher prevalence of diabetes and higher BMI (P < 0.001), and its LDL, TG, PB, ALT levels were also significantly higher (P < 0.05). However, there were no differences in age, prevalence of hypertension, proportion of liver cirrhosis, HBV DNA, and other indicators (P > 0.05).
| Parameters | NAFLD | No NAFLD | P value |
| n = 152 | n = 304 | ||
| Age (yr) | 41.87 ± 9.44 | 40.69 ± 10.85 | 0.24 |
| < 40 | 67 (44.1) | 141 (46.4) | 0.64 |
| 40-60 | 77 (50.7) | 148 (48.7) | |
| ≥ 60 | 8 (5.3) | 15 (4.9) | |
| Sex female | 41 (27) | 119 (39.1) | 0.01 |
| BMI (kg/m2) | < 0.001 | ||
| < 23 | 31 (20.4) | 165 (54.3) | |
| 23-25 | 49 (32.2) | 78 (25.7) | |
| ≥ 25 | 72 (47.4) | 61 (20.1) | |
| Diabetes | 27 (17.8) | 15 (4.9) | < 0.001 |
| Hypertension | 18 (11.8) | 20 (6.6) | 0.07 |
| Cirrhosis | 24 (15.8) | 43 (14.1) | 0.64 |
| Duration of follow-up (mo) | 71 (27-118) | 73 (32-114) | 0.08 |
| HBV DNA (IU/mL) | 0.08 | ||
| < 500 IU/mL | 29 (19.1) | 69 (22.7) | |
| < 4 log10 | 21 (13.8) | 60 (19.7) | |
| ≥ 4 log10 | 102 (67.1) | 175 (57.6) | |
| HBeAg (-) | 91 (59.9) | 210 (69.1) | 0.05 |
| TBil (µmol/L) | 15.69 ± 9.06 | 15.91 ± 9.1 | 0.8 |
| ALB (g/L) | 44.22 ± 3.42 | 43.62 ± 3.81 | 0.1 |
| PB (mg/L) | 241.53 ± 67.19 | 224.74 ± 73.69 | 0.02 |
| ALT (U/L) | 85 (32-275) | 77 (26-263) | 0.002 |
| ≤ 40 | 67 (44.1) | 172 (56.6) | 0.012 |
| > 40 | 85 (55.9) | 132 (43.4) | |
| AST (U/L) | 76 (27-248) | 83 (26.5-247) | 0.28 |
| ALP (U/L) | 84.7 ± 26.3 | 83.92 ± 31.68 | 0.8 |
| GGT (U/L) | 40.88 ± 36.95 | 36.31 ± 38.17 | 0.23 |
| GLU (mmol/L) | 5.48 ± 1.07 | 5.34 ± 1.02 | 0.2 |
| TC (mmol/L) | 4.32 ± 0.88 | 4.17 ± 0.71 | 0.08 |
| TG (mmol/L) | 1.54 ± 1.04 | 1.23 ± 0.5 | 0.001 |
| LDL (mmol/L) | 2.7 ± 0.68 | 2.56 ± 0.7 | 0.049 |
| HDL (mmol/L) | 1.32 ± 0.4 | 1.35 ± 0.31 | 0.27 |
| PLT (× 109/L) | 167.05 ± 54.18 | 159.13 ± 56.02 | 0.15 |
| AFP (ng/mL) | 3.25 (2.15-5.83) | 2.93 (2.03-5.81) | 0.33 |
Comparison of clinical characteristics of patients with chronic HBV infection without cirrhosis at baseline with NAFLD and those without NAFLD: Taking patients without cirrhosis at the time of liver biopsy as the research object, the demographic and main clinical indicators of the NAFLD and non-NAFLD groups were compared (Table 3). There were significant differences in sex, BMI, prevalence of diabetes, follow-up duration, HBV DNA, ALT, TC, TG, LDL, and other indicators between the two groups (all P < 0.05). However, there was no difference in the antiviral status and other indicators (P > 0.05). The prevalence of diabetes, follow-up duration, and ALT levels in the two groups were 1:1 PSM. There were 109 patients in the NAFLD and non-NAFLD groups. After PSM, there were no significant differences in sex, diabetes prevalence, follow-up duration, HBV DNA, ALT, and other indicators between the two groups (P > 0.05); however, there were differences in BMI, PA, and TG (P < 0.05). Moreover, the NAFLD group was divided into the NASH (21 cases) and non-NASH (88 cases) groups.
| Parameters | P values before PSM | PSM | P value | |
| NAFLD | No NAFLD | |||
| (n = 109) | (n = 109) | |||
| Age (yr) | 0.58 | 0.63 | ||
| < 40 | 52 (47.7) | 47 (43.1) | ||
| 40-60 | 51 (46.8) | 58 (53.2) | ||
| ≥ 60 | 6 (5.5) | 4 (3.7) | ||
| Sex female | < 0.05 | 29 (26.6) | 37 (33.9) | 0.24 |
| BMI (kg/m2) | < 0.05 | < 0.05 | ||
| < 23 | 21 (19.3) | 58 (53.2) | ||
| 23-25 | 35 (32.1) | 30 (27.5) | ||
| ≥ 25 | 53 (48.6) | 21 (19.3) | ||
| Diabetes | < 0.05 | 4 (3.7) | 4 (3.7) | 1 |
| Hypertension | 0.07 | 12 (11) | 6 (5.5) | 0.14 |
| Duration of follow-up (mo) | < 0.05 | 71 (28-119.5) | 52 (25-111) | 0.3 |
| Antiviral drugs | 0.59 | 0.3 | ||
| Entecavir | 49 (61.3) | 56 (71.8) | ||
| Tenofovir | 26 (32.5) | 19 (24.4) | ||
| Other | 5 (6.3) | 3 (3.8) | ||
| Antiviral duration | 0.48 | 0.46 | ||
| Never | 29 (26.6) | 31 (28.4) | ||
| < 5 yr | 50 (45.9) | 54 (49.5) | ||
| ≥ 5 yr | 30 (27.5) | 24 (22) | ||
| HBV DNA (IU/mL) | < 0.05 | 0.06 | ||
| < 500 IU/mL | 18 (19.3) | 24 (22) | ||
| < 4 log10 | 13 (32.1) | 21 (19.3) | ||
| ≥ 4 log10 | 78 (48.6) | 64 (58.7) | ||
| HBeAg (-) | 0.12 | 65 (59.6) | 75 (68.8) | 0.16 |
| TBil (µmol/L) | 0.94 | 15.73 ± 7.17 | 14.25 ± 5.73 | 0.09 |
| ALB (g/L) | 0.21 | 44.15 ± 3.32 | 44.29 ± 3.78 | 0.78 |
| PB (mg/L) | 0.28 | 243.24 ± 65.74 | 225.67 ± 62.19 | < 0.05 |
| ALT (U/L) | < 0.05 | 0.89 | ||
| ≤ 40 | 43 (19.3) | 44 (40.4) | ||
| > 40 | 66 (32.1) | 65 (59.6) | ||
| AST (U/L) | 0.16 | 36 (27.5-49.5) | 37 (26.5-50) | 0.95 |
| ALP (U/L) | 0.1 | 85.55 ± 26.37 | 82.82 ± 22.08 | 0.41 |
| GGT (U/L) | 0.12 | 37.44 ± 25.34 | 34.76 ± 33.13 | 0.5 |
| GLU (mmol/L) | 0.06 | 5.25 ± 0.68 | 5.41 ± 0.89 | 0.15 |
| TC (mmol/L) | < 0.05 | 4.37 ± 0.88 | 4.22 ± 0.64 | 0.16 |
| TG (mmol/L) | < 0.05 | 1.47 ± 0.75 | 1.22 ± 0.51 | < 0.05 |
| LDL (mmol/L) | < 0.05 | 2.77 ± 0.65 | 2.62 ± 0.61 | 0.09 |
| HDL (mmol/L) | 0.38 | 1.31 ± 0.36 | 1.32 ± 0.3 | 0.82 |
| PLT (× 109/L) | 0.12 | 174.29 ± 51.81 | 162.83 ± 47.49 | 0.09 |
| AFP (ng/mL) | 0.29 | 3.1 (2.1-5.3) | 2.9 (2.1-5.5) | 0.74 |
| NASH | 15 (13.7) | |||
Comparison of the pathological characteristics of chronic HBV infection with and without NAFLD: Among patients with NAFLD, 58.6% had mild hepatic steatosis, 31.6% had moderate hepatic steatosis, and 9.9% had severe hepatic steatosis. Differences were observed between the NAFLD group and non-NAFLD group in the degree of liver fibrosis, portal inflammation, and NAS score (P < 0.05). There was no difference in lobular inflammation or ballooning deformation (BD) between the two groups (P > 0.05) (Table 4).
| Pathological features | Total | NAFLD | No NAFLD | P value |
| (n = 456) | (n = 152) | (n = 304) | ||
| Fibrosis | 0.045 | |||
| F0 | 109 (23.9) | 30 (19.7) | 79 (26) | |
| F1 | 129 (28.3) | 36 (23.7) | 93 (30.6) | |
| F2 | 93 (20.4) | 41 (27) | 52 (17.1) | |
| F3 | 58 (12.7) | 21 (13.8) | 37 (12.2) | |
| F4 | 67 (14.7) | 24 (15.8) | 43 (14.1) | |
| Steatosis | ||||
| 0 | 304 (66.7) | 304 (100) | ||
| 1 | 89 (19.5) | 89 (58.6) | ||
| 2 | 48 (10.5) | 48 (31.6) | ||
| 3 | 15 (3.3) | 15 (9.9) | ||
| Portal tract inflammation | < 0.001 | |||
| 0 | 35 (7.7) | 8 (5.3) | 27 (8.9) | |
| 1 | 275 (60.3) | 79 (52) | 196 (64.5) | |
| 2 | 117 (25.7) | 50 (32.9) | 67 (22) | |
| 3 | 29 (6.3) | 15 (9.9) | 14 (4.6) | |
| Lobular inflammation | 0.13 | |||
| 0 | 91 (20) | 28 (18.4) | 63 (20.7) | |
| 1 | 315 (69.1) | 101 (66.4) | 214 (70.4) | |
| 2 | 50 (11) | 23 (15.1) | 27 (8.9) | |
| Ballooning degeneration | 0.004 | |||
| 0 | 153 (33.6) | 58 (38.2) | 165 (54.3) | |
| 1 | 195 (42.8) | 61 (40.1) | 96 (31.6) | |
| 2 | 108 (23.7) | 33 (21.7) | 43 (14.1) | |
| NASH | 32 (21.1) | 32 (21.1) | ||
| NAS score | 1.7 ± 1.2 | 2.7 ± 1.2 | 1.2 ± 0.8 | < 0.001 |
There was no significant difference in the proportion of patients receiving antiviral treatment, types of antiviral drugs, and duration of antiviral treatment between the NAFLD group and non-NAFLD group (P > 0.05). At the last follow-up, 302 (91.2%) patients who received antiviral therapy were HBV DNA-negative, including 102 (78.9%) in the NAFLD group and 200 (93%) in the non-NAFLD group. There was no significant difference in the proportion of HBV DNA negativity between the two groups (P > 0.05). A total of 275 (83.1%) patients had normal ALT levels: 90 (77.6%) in the NAFLD group and 185 (56%) in the NAFLD group. There was no significant difference in the proportion of normal ALT levels between the two groups (P = 0.05) (Table 5).
| Parameters | NAFLD | No NAFLD | P value |
| (n = 152) | (n = 304) | ||
| Antiviral | 116 (76.3) | 215 (70.7) | 0.21 |
| Antiviral drugs | 0.5 | ||
| Entecavir | 73 (62.9) | 131 (60.9) | |
| Tenofovir | 35 (30.2) | 68 (31.6) | |
| Others | 8 (6.9) | 16 (7.4) | |
| Antivirus duration | 0.31 | ||
| < 5 yr | 70 (46.1) | 129 (42.4) | |
| ≥ 5 yr | 46 (30.3) | 86 (28.3) |
Occurrence of cirrhosis: Patients without cirrhosis at the time of liver biopsy were selected as research participants, and the risk of progression to cirrhosis was observed. During the follow-up period, 34 patients developed liver cirrhosis diagnosed by ultrasound, with a median follow-up time of 72 (30-134) mo, including 10 (7.8%) in the NAFLD group and 24 (9.2%) in the NAFLD group. This study was conducted during the follow-up period.
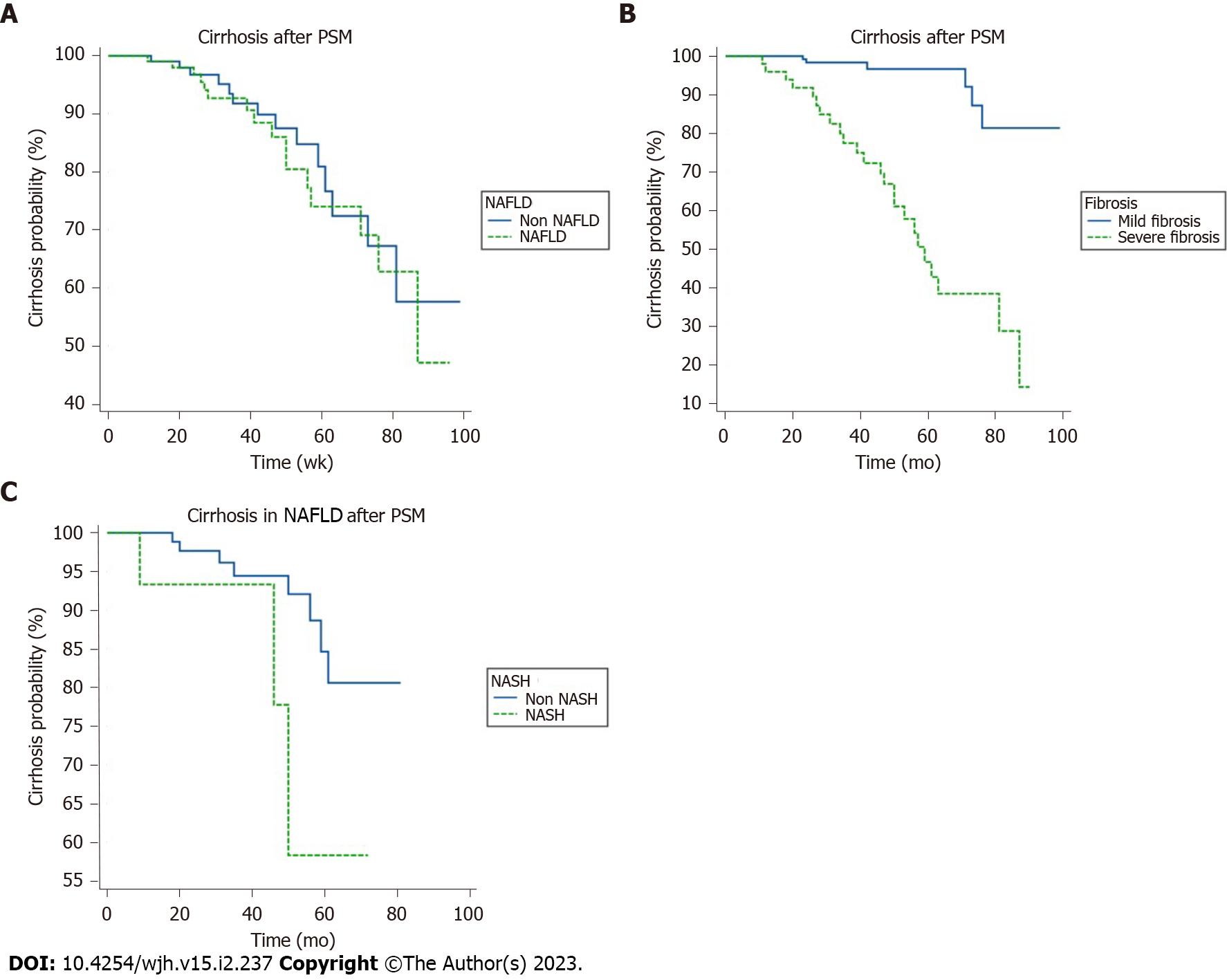
K-M survival analysis of NAFLD and the risk of cirrhosis: The results of the K-M survival analysis showed that there was no significant increase in the risk of liver cirrhosis diagnosed using ultrasound in the combined NAFLD group before and after PSM (log-rank, P = 0.69). The results of the K-M survival analysis after PSM are shown in Figure 2A. F0, 1, 2 was regarded as mild fibrosis, while F3, 4 was regarded as severe fibrosis. Patients with fibrosis after PSM had an increased risk of cirrhosis diagnosed using ultrasound (log rank, P < 0.05). The results of K-M survival analysis after matching are shown in Figure 2B. Figure before PSM. In the NAFLD group, three out of 21 cases in the NASH group and seven out of 88 patients in the non-NASH group developed cirrhosis. The results of K-M survival analysis after PSM are shown in Figure 2C. There was no statistical difference between the two groups (P = 0.17).
Univariate and multivariate Cox regression analysis of cirrhosis: Cox regression univariate analysis showed that age, antiviral duration, lobular inflammation, BD, liver fibrosis, ALP, LDL, and PLT were related to cirrhosis (P < 0.05). Multivariate analysis showed that age, ballooning degeneration, liver fibrosis, and PLT were independent risk factors for cirrhosis diagnosed using ultrasound (all P < 0.05) (Table 6).
| Parameters | Univariate | P value | Multivariate | P value |
| HR (95%CI) | HR (95%CI) | |||
| Clinical factors | ||||
| Sex | 1.36 (0.63-2.92) | 0.43 | ||
| Age (yr) | 1.08 (1.04-1.12) | < 0.001 | 1.06 (1.02-1.10) | 0.003 |
| BMI (kg/m2) | 0.96 (0.64-1.45) | 0.86 | ||
| Diabetes | 2.57 (0.78-8.52) | 0.12 | ||
| Hypertension | 2.71 (0.95-7.76) | 0.06 | ||
| Duration of antiviral (≥ 5 yr/never or < 5 yr) | 1.65 (1.07-2.54) | 0.02 | 1.32 (0.84-2.06) | 0.23 |
| Pathological factors | ||||
| NAFLD | 1.12 (0.53-2.37) | 0.76 | ||
| NASH | 1.38 (0.33-5.86) | 0.66 | ||
| Lobular inflammation | 2.35 (1.19-4.66) | 0.01 | 0.77 (0.33-1.80) | 0.54 |
| Portal tract inflammation | 1.40 (0.90-2.20) | 0.14 | ||
| Ballooning degeneration | 3.34 (1.65-6.75) | 0.001 | 2.57 (1.05-6.28) | 0.04 |
| Fibrosis | 1.49 (1.08-2.06) | 0.02 | 1.39 (1.04-1.87) | 0.028 |
| Laboratory examination | ||||
| TBil (µmol/L) | 0.99 (0.95-1.04) | 0.8 | ||
| ALB (g/L) | 0.94 (0.85-1.04) | 0.25 | ||
| PB (mg/L) | 1.00 (0.997-1.00) | 0.83 | ||
| ALT (> 40/≤ 40U/L) | 1.00 (0.996-1.00) | 0.64 | ||
| AST (U/L) | 1.00 (0.99-1.01) | 0.54 | ||
| ALP (U/L) | 1.01 (1.00-1.02) | 0.008 | 1.01 (1.00-1.02) | 0.08 |
| GGT (U/L) | 1.01 (1.00-1.01) | 0.13 | ||
| GLU (mmol/L) | 1.31 (0.96-1.78) | 0.09 | ||
| TG (mmol/L) | 0.57 (0.28-1.18) | 0.13 | ||
| TC (mmol/L) | 0.95 (0.61-1.49) | 0.83 | ||
| HDL (mmol/L) | 1.71 (0.65-4.49) | 0.28 | ||
| LDL (mmol/L) | 0.46 (0.27-0.79) | 0.005 | 0.76 (0.45-1.29) | 0.31 |
| PLT (× 109/L) | 0.99 (0.98-0.99) | 0.001 | 0.98 (0.97-0.99) | 0.001 |
| AFP (ng/mL) | 0.99 (0.96-1.03) | 0.63 | ||
| HBeAg (-) | 0.91 (0.43-1.90) | 0.79 | ||
| HBV DNA (≥ 4 log IU/mL) | 0.82 (0.55-1.23) | 0.34 |
Univariate and multivariate Cox regression analysis of cirrhosis after PSM: Cox regression univariate analysis showed that age, hypertension, diabetes mellitus, lobular inflammation, portal inflammation, liver fibrosis, ALT, and PLT were related to cirrhosis diagnosed using ultrasound (P < 0.1). Multivariate analysis showed that diabetes mellitus, BD, and PLT were independent risk factors for liver cirrhosis diagnosed using ultrasound (P < 0.05) (Table 7).
| Parameters | Univariate | P value | Multivariate | P value |
| HR (95%CI) | HR (95%CI) | |||
| Age (yr) | 1.06 (1.01-1.11) | 0.02 | 1.05 (0.99-1.11) | 0.11 |
| Hypertension | 3.26 (0.89-11.90) | 0.07 | 0.59 (0.12-2.97) | 0.52 |
| Diabetes | 6.74 (0.77-58.81) | 0.08 | 12.21 (1.1-134.4) | 0.04 |
| Lobular inflammation | 2.63 (1.02-6.82) | 0.046 | 1.20 (0.26-5.57) | 0.82 |
| Ballooning degeneration | 4.33 (1.01-5.15) | 0.006 | 1.80 (0.86-4.48) | 0.02 |
| Portal tract inflammation | 2.37 (1.18-4.75) | 0.02 | 72.61 (2.16-2436) | 0.48 |
| Fibrosis | 1.57 (0.96-2.56) | 0.07 | 1.03 (0.48-2.25) | 0.93 |
| ALT(U/L) | 1.00 (1.00-1.01) | 0.06 | 1.00 (0.99-1.01) | 0.26 |
| PLT (× 109/L) | 0.98 (0.96-0.99) | < 0.001 | 0.98 (0.96-0.99) | 0.001 |
Characteristics of patients with HCC: During the follow-up period, 10 patients (2.2%) developed HCC (Table 7), and two died (one died due to HCC and the cause of death in the other case was not related to liver disease). The median time interval between liver biopsy and HCC diagnosis was 100.5 mo (68.5-128.0). HCC occurred in six patients (3.3%) with NAFLD and four patients (1.6%) without NAFLD. Seven patients developed cirrhosis at the time of liver biopsy, and one of the other three developed cirrhosis before HCC.
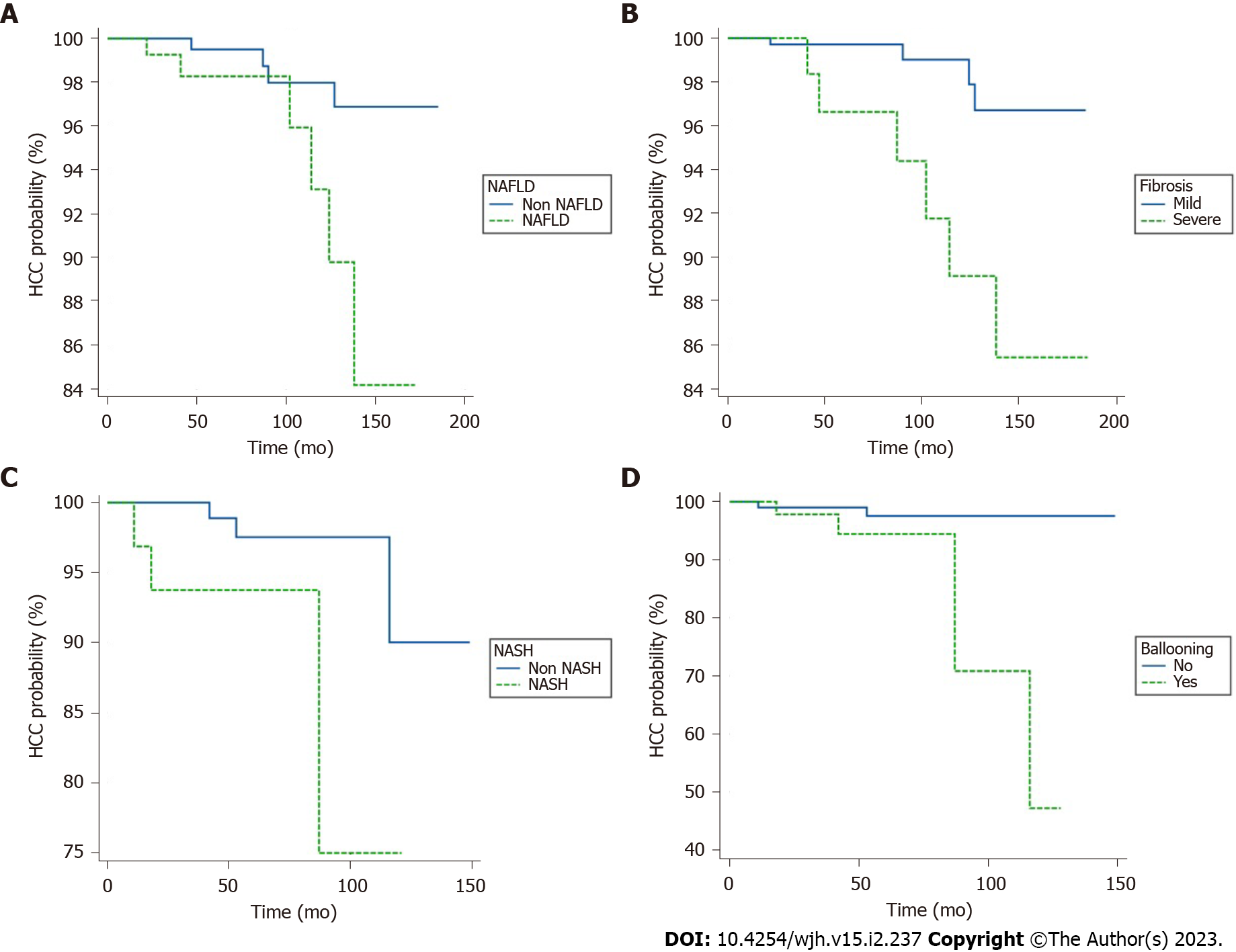
K-M survival analysis of HCC risk: Patients with baseline cirrhosis were included in the K-M survival analysis of HCC risk. It was found that the cumulative risk of HCC in the NAFLD group was significantly higher than that in the non-NAFLD group (log rank, P = 0.02) (Figure 3A). At the same time, the risk of HCC in patients with severe liver fibrosis (F3-4) was also significantly increased (log rank, P = 0.005) (Figure 3B). When the NAFLD group was divided into the NASH group with 32 patients (3 HCCs) and the non-NASH group with 120 patients (3 HCCs), the risk of HCC in the NASH group was increased (log rank, P = 0.03) (Figure 3C), and the risk of HCC in patients with hepatic ballooning was significantly increased (log rank, P = 0.01) (Figure 3D). There was no significant difference in the risk of HCC among patients with steatosis, lobulitis, and portal inflammation (log rank, P > 0.05) (figures ignored).
Univariate and multivariate Cox regression analysis of HCC: Cox regression univariate analysis showed that diabetes mellitus, NAFLD, NASH, lobular inflammation, BD, liver fibrosis, GLU, TC, TG, and PLT were correlated with HCC (all P < 0.05). Multivariate analysis of factors with P < 0.10 in the Cox regression univariate analysis showed that ballooning, liver fibrosis, and diabetes were independent risk factors for HCC (Table 8).
| Parameters | Univariate | P value | Multivariate | P value |
| HR (95%CI) | HR (95%CI) | |||
| Clinical factors | ||||
| Sex | 40.03 (0.17-9369.3) | 0.19 | ||
| Age (yr) | 1.06 (0.99-1.13) | 0.054 | 0.29 (0.03-2.50) | 0.26 |
| BMI (kg/m2) | 0.97 (0.78-1.21) | 0.8 | ||
| Diabetes | 14.36 (4.01-51.47) | < 0.001 | 34.8 (2.27-534.1) | 0.01 |
| Hypertension | 1.99 (0.25-15.71) | 0.52 | ||
| Antivirus duration (≥ 5 yr, never or < 5 yr) | 1.32 (0.38-4.56) | 0.66 | ||
| Pathological factors | ||||
| NAFLD (steatosis ≥ 5%) | 4.29 (1.20-15.41) | 0.025 | 2.28 (0.44-11.69) | 0.33 |
| NASH | 4.36 (1.35-24.80) | 0.002 | 0.53 (0.01-1.84) | 0.39 |
| Lobular inflammation | 7.2 (2.16-23.91) | 0.001 | 5.16 (0.53-49.96) | 0.16 |
| Portal tract inflammation | 2.02 (0.98-4.15) | 0.056 | 0.49 (0.31-1.85) | 0.29 |
| Ballooning degeneration | 8.69 (1.57-29.64) | 0.008 | 5.16 (0.83-19.96) | 0.03 |
| Fibrosis stage | 14.25 (13.68-55.14) | < 0.001 | 8.37 (1.39-50.44) | 0.02 |
| Laboratory examination | ||||
| TBil (µmol/L) | 1.02 (0.97-1.09) | 0.37 | ||
| PB (mg/L) | 0.998 (0.99-1.01) | 0.64 | ||
| LB (g/L) | 0.97 (0.82-1.14) | 0.69 | ||
| ALT (> 40/≤ 40 U/L) | 0.999 (0.99-1.01) | 0.71 | ||
| AST (U/L) | 0.999 (0.98-1.01) | 0.89 | ||
| ALP (U/L) | 1.01 (0.99-1.03) | 0.42 | ||
| GGT (U/L) | 0.99 (0.96-1.02) | 0.46 | ||
| GLU (mmol/L) | 1.45 (1.08-1.94) | 0.01 | 0.64 (0.37-1.12) | 0.12 |
| TG (mmol/L) | 0.18 (0.04-0.92) | 0.04 | 0.085 (0.004-1.7) | 0.11 |
| TC (mmol/L) | 0.36 (0.15-0.85) | 0.02 | 0.39 (0.15-1.01) | 0.053 |
| HDL (mmol/L) | 0.39 (0.04-3.67) | 0.41 | ||
| LDL (mmol/L) | 0.47 (0.18-1.26) | 0.13 | ||
| PLT (× 109/L) | 0.97 (0.96-0.99) | 0.004 | 0.99 (0.97-1.01) | 0.26 |
| AFP (ng/mL) | 0.99 (0.91-1.07) | 0.77 | ||
| HBeAg (-) (%) | 1.43 (0.40-5.08) | 0.58 | ||
| HBV DNA (≥ 4 log IU/mL) | 1.49 (0.39-5.77) | 0.56 | ||
According to the WHO hepatitis report in 2017, 96% of the 1.3 million deaths caused by viral hepatitis worldwide in 2015 were caused by HBV and hepatitis C virus (HCV). In China, 70 million patients with HBV, accounting for 27% of the global population of patients with HBV[14]; furthermore, 68% (95%CI: 60-74) of patients with liver cirrhosis in China are infected with HBV[15]. With the increasing prevalence of obesity and metabolic syndrome, NAFLD has become the most common cause of chronic liver disease worldwide[14]. Therefore, these two liver diseases are often observed, and with the increasing prevalence of NAFLD, the number of patients with combined NAFLD and HBV infections is also on the increase. Studies have reported that the prevalence of NAFLD, confirmed by biopsy in patients with CHB, ranges from 14% to 52%[5,16-22]. The prevalence of NAFLD in this study was 33.3%, which was also within this range.
NAFLD is associated with metabolic syndrome, and this was also reflected in our study. Compared with those without NAFLD, chronic HBV-infected patients with NAFLD have a higher prevalence of diabetes and BMI, and their low-density lipoprotein, triglyceride, and apolipoprotein B levels were also significantly higher. With the aggravation of hepatic steatosis, the proportion of overweight and obese patients and the average BMI gradually increased. Studies have shown that metabolic syndrome can delay the serum clearance of HBeAg, increase the risk of liver fibrosis and cirrhosis, and thus enhance the development of HCC[8,23,24]. Considering that NAFLD is the main hepatic manifestation of obesity and metabolism-related diseases, chronic HBV infection overlapping with NAFLD may further increase the risk of cirrhosis and HCC. NAFLD is not associated with an increased risk of cirrhosis in patients with chronic HBV infection. After propensity matching for follow-up duration, diabetes mellitus, and ALT, there was no difference in diabetes mellitus and most variables between the two groups, while multivariate analysis still showed that diabetes mellitus was an independent risk factor for the occurrence of liver cirrhosis diagnosed using ultrasound.
In addition, NAFLD, obesity, and hyperlipidemia have been found to be associated with accelerated clearance of HBsAg and lower HBV DNA in many clinical studies[9,15,25]. In our study, it was not found that combined NAFLD was associated with decreased HBV DNA levels, both at baseline and at the last follow-up. However, in the cohort of patients without cirrhosis, we found that the proportion of high-level HBV DNA in the combined NAFLD group was lower than that in the non NAFLD group, although the difference between the two groups was not statistically significant after matching, and the comparison was not stratified by antiviral treatment, age, etc. However, NAFLD and CHB can jointly aggravate liver injury, and increase the risk of liver cirrhosis and liver cancer[3-7,26].
In this study, 72.4% of patients received antiviral therapy, mainly nucleoside analogs; moreover, the 2017 European Association for the Study of the Liver Guidelines proposed long-term inhibition of HBV DNA as the primary endpoint of chronic hepatitis B treatment[27]. Previously, with the development and clinical application of NAs antiviral drugs, HBV replication was effectively controlled[28-31]. In our study, 91.2% of patients with CHB who received antiviral therapy achieved HBV DNA negativity. However, no significant difference was observed in HBV DNA negativity between the NAFLD and non-NAFLD groups. In addition, 83.1% of the patients had normal ALT levels, and there was no significant difference in the normal ALT levels between the two groups. There are conflicting results regarding whether NAFLD affects the efficacy of antiviral therapy in patients with CHB. A recent meta-analysis showed that the efficacy of antiviral therapy decreases in CHB patients with hepatic steatosis[32]. There were significant differences in virological and biochemical reactions between the subgroups with and that without NAFLD, which may be due to the decreased bioavailability of antiviral drugs caused by fat changes, resulting in a significant reduction in the contact area between hepatocytes and antiviral drugs[32]. However, some studies have failed to find an association between hepatic steatosis and the efficacy of antiviral therapy[20,32-35]. Although the impact of hepatic steatosis on antiviral therapy in these patients remains controversial, it is still recommended to monitor the onset or progression of NAFLD during antiviral therapy to prevent potential negative effects.
In recent years, the proportion of patients with cirrhosis caused by NAFLD has increased rapidly. In particular, NASH, defined as the simultaneous presence of steatosis and inflammatory injury in the liver, with or without liver fibrosis[36,37], is an independent risk factor for the development of cirrhosis[38]. Several retrospective studies with long-term follow-up have found that the unadjusted cumulative probability of liver-related events was significantly higher in patients with NASH than in those without NASH[39,40]. A comprehensive analysis of six studies showed that in 41% of patients with NASH fibrosis progressed, with an average annual growth rate of 0.09%[43]; moreover, the progression of fibrosis can lead to the development of cirrhosis and cause other liver-related diseases, such as HCC. The annual incidence of HCC in patients with NASH is 5.29/1000 person years[36,41]. These findings further prove that the risk of HCC in patients with CHB infection complicated by NASH is significantly increased. In our study, NASH did not increase the risk of cirrhosis, but increased the risk of HCC. Furthermore, we observed that the key factor for NASH was ballooning. Ballooning and fibrosis are pre-PSM cirrhosis and HCC risk factors, whereas post-PSM ballooning is still a risk factor for cirrhosis. A previous large-scale cohort study included 1089 cases of NAFLD confirmed by biopsy in North American and European multiethnic CHB populations. After a median follow-up of 10 years, there was no obvious correlation between CHB combined with NAFLD and the risk of liver-related adverse events (cirrhosis or liver cancer), while CHB combined with NASH still led to a higher risk of liver-related adverse events, but was only significant in the population with advanced liver fibrosis[4]. This large-scale study also pointed out that ballooning degeneration and inflammation were important liver-related adverse events, but steatosis was not related to clinical events[4]. This result supports the conclusion of the present study that the combination of NASH will increase the risk of adverse events in patients with chronic HBV infection. Histological determinants of NASH, such as ballooning and lobular inflammation, are important for liver-related adverse events (cirrhosis and HCC).
First, liver fibrosis and subsequent cirrhosis are generally considered to be the prelude to HCC, which is closely related to the occurrence of HCC[26]. Second, diabetes mellitus, blood glucose, and cholesterol, which are components of the metabolic syndrome, are also closely related to the occurrence of HCC. Previous studies have proposed that obesity, diabetes, and metabolic syndrome are independent risk factors for liver fibrosis, cirrhosis, and HCC in patients with chronic hepatitis B, suggesting that metabolic factors and chronic hepatitis B have a synergistic effect in the pathogenesis of liver cancer[23,24,42,43], and extreme obesity and diabetes increase the risk of developing HBV-related HCC by 12.8-fold[45]. A prospective study in Taiwan showed that a high BMI at baseline was associated with the incidence of cirrhosis and HCC[43]; however, the participants were all male patients with chronic HBV infection. In this study, the baseline BMI was not an independent risk factor for HCC occurrence; however, it may be related to different populations, diets, living habits, and grading standards for BMI. Moreover, this may be due to the complex relationship between BMI and HCC. HCC or cirrhosis as a prelude to HCC has an impact on the nutritional status of patients; moreover, nutritional deficiency and sarcopenia can reduce BMI. The impact of a high baseline BMI on the overall prognosis of patients with HCC has also been controversial: some studies have indicated that patients with a high BMI have a worse prognosis, other studies suggest that they may have a better prognosis, and some studies have indicated that there was no significant correlation between the two[44-46].
This study has some limitations. First, the sample size of this single-center retrospective study was reduced after the application of PSM. Because of the influence of the small sample size, we could not match all factors; hence, larger samples and more rigorous prospective studies are needed for further verification. Second, the conclusion of the study is that CHB combined with NAFLD has little effect on the final outcome of liver cirrhosis, which is inconsistent with the results of mainstream studies, and could be due to our short follow-up duration. The study cohort needs to be observed for a longer time, and the conclusion may change if this is done. Third, in the study, identifying adverse events after the diagnosis of liver cirrhosis relied on ultrasound, and ultrasound itself is subject to the subjective understanding of the ultrasonologists; hence, the diagnostic error could be large.
In summary, we conducted a long-term follow-up of a cohort of CHB patients with NAFLD based on a diagnosis of liver pathology, and observed that the PSM of baseline influencing factors revealed that the risk of cirrhosis diagnosed using ultrasound was not significantly higher in the group with NAFLD than in the group without NAFLD. Before PSM, age, BD, liver fibrosis, and PLT were independent risk factors for cirrhosis. After PSM, only BD and PLT were found to be independent risk factors for liver cirrhosis diagnosed using ultrasound. The cumulative risk of HCC was significantly higher in patients with NAFLD or NASH. BD, liver fibrosis, and diabetes mellitus were independent risk factors for HCC.
Chronic hepatitis B (CHB) virus infection and nonalcoholic fatty liver disease (NAFLD) are important causes of liver-related complications and death. With the increasing prevalence of NAFLD, the number of patients with combined NAFLD and hepatitis B virus (HBV) infection is also on the increase.
This study aimed to further explore the impact of NAFLD and the pathological changes confirmed by liver pathology in patients with chronic HBV infection.
To study the effect of NAFLD confirmed using liver pathology on the outcomes of long-term serious adverse events in patients with CHB virus infection.
Among 456 cases of chronic HBV infection, 152 were confirmed by liver histology to have NAFLD, and 304 were simple chronic HBV infection. The incidence of serious clinical events at the follow-up endpoint was compared by Kaplan-Meier (K-M) survival analysis at baseline using propensity score matching balance parameters.
After a median follow-up of 70.5 mo, there were 34 cases of ultrasound-diagnosed cirrhosis and 10 cases of HCC. K-M survival analysis showed no significant difference in the occurrence of CHB complicated with NAFLD cirrhosis, and the cumulative incidence of HCC in the NAFLD group was higher than that in the non-NAFLD group (log-rank test, P < 0.05). Hepatocyte ballooning and severe liver fibrosis were also associated with an increased risk of HCC (log-rank test, all P < 0.05).
Baseline hepatocyte ballooning is a risk factor for adverse events in patients with CHB complicated with NAFLD.
Larger samples and more rigorous prospective studies are needed for further verification. The study cohort needs to be observed for a longer time, and the conclusion may change if this is done, in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil; Yang M, United States S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 671] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 3. | Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, Tanwandee T. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. 2017;37:542-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, Janssen HLA, Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, To KF, Wong VW. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 128] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 6. | Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, Barsheshet A, Shlomai A. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Mak LY, Seto WK, Hui RW, Fung J, Wong DK, Lai CL, Yuen MF. Fibrosis evolution in chronic hepatitis B e antigen-negative patients across a 10-year interval. J Viral Hepat. 2019;26:818-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, Wong DK, Lai CL, Yuen MF. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case-control study. J Viral Hepat. 2018;25:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, Leung JK, Chim AM, Kong AP, Lui GC, Chan HL, Chu WC. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin Gastroenterol Hepatol. 2021;19:2161-2171.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 10. | Farrell GC, Chitturi S, Lau GK, Sollano JD; Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6807] [Cited by in F6Publishing: 7387] [Article Influence: 388.8] [Reference Citation Analysis (5)] |
| 12. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 13. | Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJ, de Martel C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:724-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Zheng RD, Xu CR, Jiang L, Dou AX, Zhou K, Lu LG. Predictors of hepatic steatosis in HBeAg-negative chronic hepatitis B patients and their diagnostic values in hepatic fibrosis. Int J Med Sci. 2010;7:272-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, Kim KI, Kim SH, Rim KS, Hwang SG. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Thomopoulos KC, Arvaniti V, Tsamantas AC, Dimitropoulou D, Gogos CA, Siagris D, Theocharis GJ, Labropoulou-Karatza C. Prevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol. 2006;18:233-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Peng D, Han Y, Ding H, Wei L. Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol. 2008;23:1082-1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol. 2007;41:513-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, Farrell GC. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2008;23:1419-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Wong VW, Wong GL, Yu J, Choi PC, Chan AW, Chan HY, Chu ES, Cheng AS, Chim AM, Chan FK, Sung JJ, Chan HL. Interaction of adipokines and hepatitis B virus on histological liver injury in the Chinese. Am J Gastroenterol. 2010;105:132-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Hsiang JC, Wong GL, Chan HL, Chan AW, Chim AM, Wong VW. Metabolic syndrome delays HBeAg seroclearance in Chinese patients with hepatitis B. Aliment Pharmacol Ther. 2014;40:716-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, Chan HY, Tse CH, Wong VW. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, You SL, Su J, Iloeje UH, Chen CJ; REVEAL-HBV Study Group. Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes (Lond). 2013;37:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Lin S, Jiang D, Li M, Chen Y, Li J, Fan J. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int. 2020;40:496-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3100] [Article Influence: 442.9] [Reference Citation Analysis (0)] |
| 28. | Zhang Z, Zhou Y, Yang J, Hu K, Huang Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: a meta analysis. BMC Cancer. 2019;19:511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Razavi-Shearer D, Razavi H. Global prevalence of hepatitis B virus infection and prevention of mother-to-child transmission - Authors' reply. Lancet Gastroenterol Hepatol. 2018;3:599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Locarnini S, Hatzakis A, Chen DS, Lok A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J Hepatol. 2015;62:S76-S86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 31. | Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect Dis. 2008;8:167-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Zhu Y, Yang Q, Lv F, Yu Y. The Effect of Hepatosteatosis on Response to Antiviral Treatment in Patients with Chronic Hepatitis B: A Meta-Analysis. Gastroenterol Res Pract. 2017;2017:1096406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Ceylan B, Arslan F, Batırel A, Fincancı M, Yardımcı C, Fersan E, Paşaoğlu E, Yılmaz M, Mert A. Impact of fatty liver on hepatitis B virus replication and virologic response to tenofovir and entecavir. Turk J Gastroenterol. 2016;27:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Zhu LY, Wang YG, Wei LQ, Zhou J, Dai WJ, Zhang XY. The effects of the insulin resistance index on the virologic response to entecavir in patients with HBeAg-positive chronic hepatitis B and nonalcoholic fatty liver disease. Drug Des Devel Ther. 2016;10:2739-2744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Dogan Z, Filik L, Ergül B, Sarikaya M. Comparison of first-year results of tenofovir and entecavir treatments of nucleos(t)ide-naive chronic hepatitis B patients with hepatosteatosis. Saudi J Gastroenterol. 2015;21:396-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Nagral A, Sarma MS, Matthai J, Kukkle PL, Devarbhavi H, Sinha S, Alam S, Bavdekar A, Dhiman RK, Eapen CE, Goyal V, Mohan N, Kandadai RM, Sathiyasekaran M, Poddar U, Sibal A, Sankaranarayanan S, Srivastava A, Thapa BR, Wadia PM, Yachha SK, Dhawan A. Corrigendum to "Wilson's Disease: Clinical Practice Guidelines of the Indian National Association for the Study of Liver (INASL), The Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition (ISPGHAN) and the Movement Disorders Society of India (MDSI)" [J Clin Exp Hepatol 9 (2019) 74-98]. J Clin Exp Hepatol. 2020;10:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 37. | Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 38. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 39. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 40. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1701] [Cited by in F6Publishing: 1920] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 41. | Lonardo A, Byrne CD, Caldwell SH, Cortez-Pinto H, Targher G. Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:1388-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, Chen CJ. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26:5576-5582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 44. | Hashimoto M, Tashiro H, Kobayashi T, Kuroda S, Hamaoka M, Ohdan H. Influence of higher BMI for hepatitis B- and C-related hepatocellular carcinomas. Langenbecks Arch Surg. 2017;402:745-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Mathur AK, Ghaferi AA, Sell K, Sonnenday CJ, Englesbe MJ, Welling TH. Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg. 2010;14:849-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Mathur AK, Ghaferi AA, Osborne NH, Pawlik TM, Campbell DA, Englesbe MJ, Welling TH. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |