Published online Mar 16, 2012. doi: 10.4253/wjge.v4.i3.75
Revised: December 1, 2011
Accepted: March 2, 2012
Published online: March 16, 2012
Endoscopic submucosal dissection (ESD) is an advanced therapeutic endoscopic technique, which allowsresection of larger superficial tumors in the esophagus, stomach, and colon. Precise diagnosis of the boundary between tumor and the non-tumorous surrounding portion is especially important before starting ESD, because too much resection can potentially take more time and can induce a higher complication rate, while too little resection can result in a non-curative resection. The boundary diagnosis is often difficult for early gastric cancer, mainly because of the underlying condition of chronic gastritis. Due to recent developments in endoscopy, including magnified endoscopy and narrow band endoscopy, the boundary diagnosis is becoming easy and more accurate.We have also applied magnified endoscopy combined with narrow band imaging to fresh specimens immediately after resection using thetiling method and XY stage.
- Citation: Ochiai Y, Arai S, Nakao M, Shono T, Kita H. Diagnosis of boundary in early gastric cancer. World J Gastrointest Endosc 2012; 4(3): 75-79
- URL: https://www.wjgnet.com/1948-5190/full/v4/i3/75.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i3.75
Duringendoscopic mucosal resection (EMR) of early gastric cancer, large lesions were previously segmentally resected due to limitation of size, which led to insufficient pathological evaluation. With the recent development of endoscopic submucosal dissection (ESD), this limitation has been overcome and en bloc resection is now possible, and the importance of diagnosing the range of lateral advancement has increased. In this report, diagnosis of the boundary in the era of ESD is assessed.
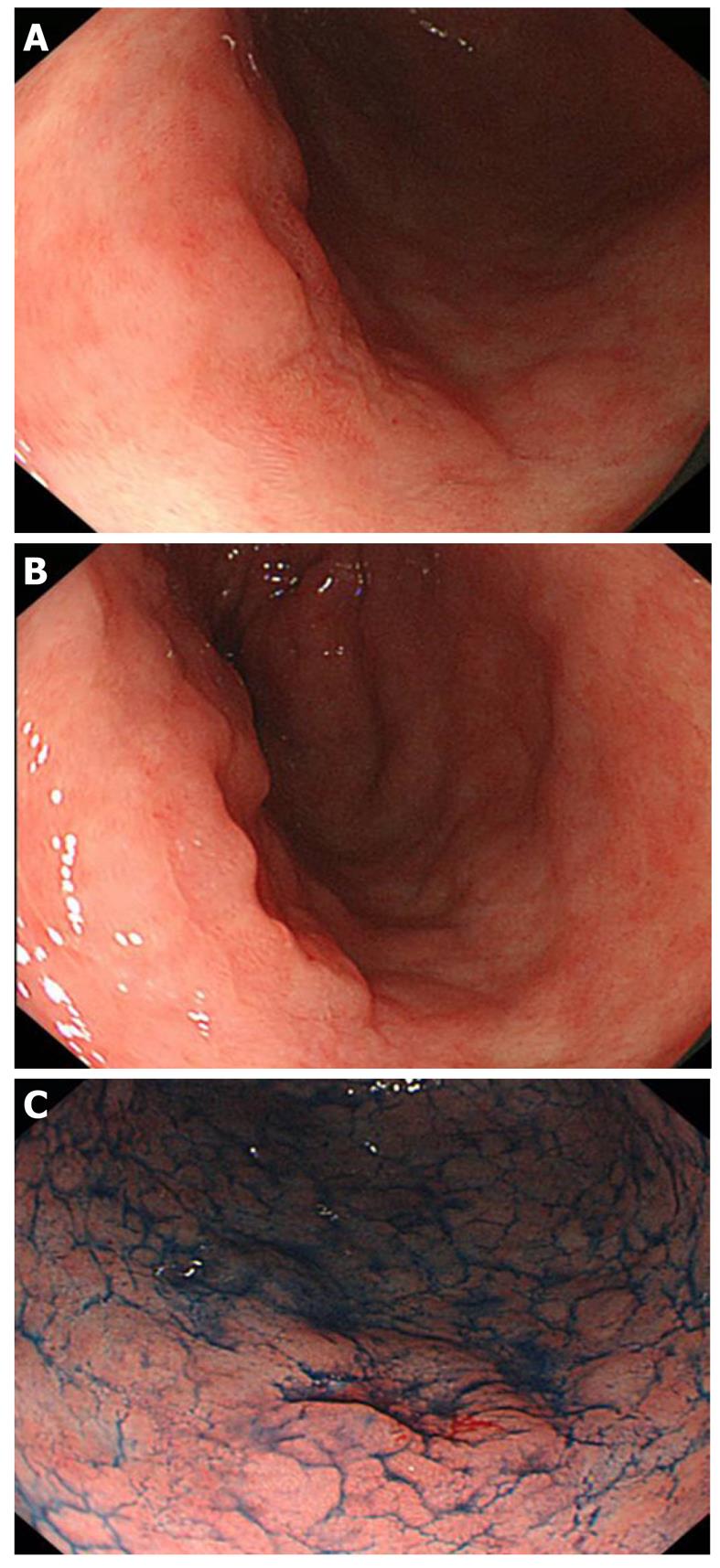
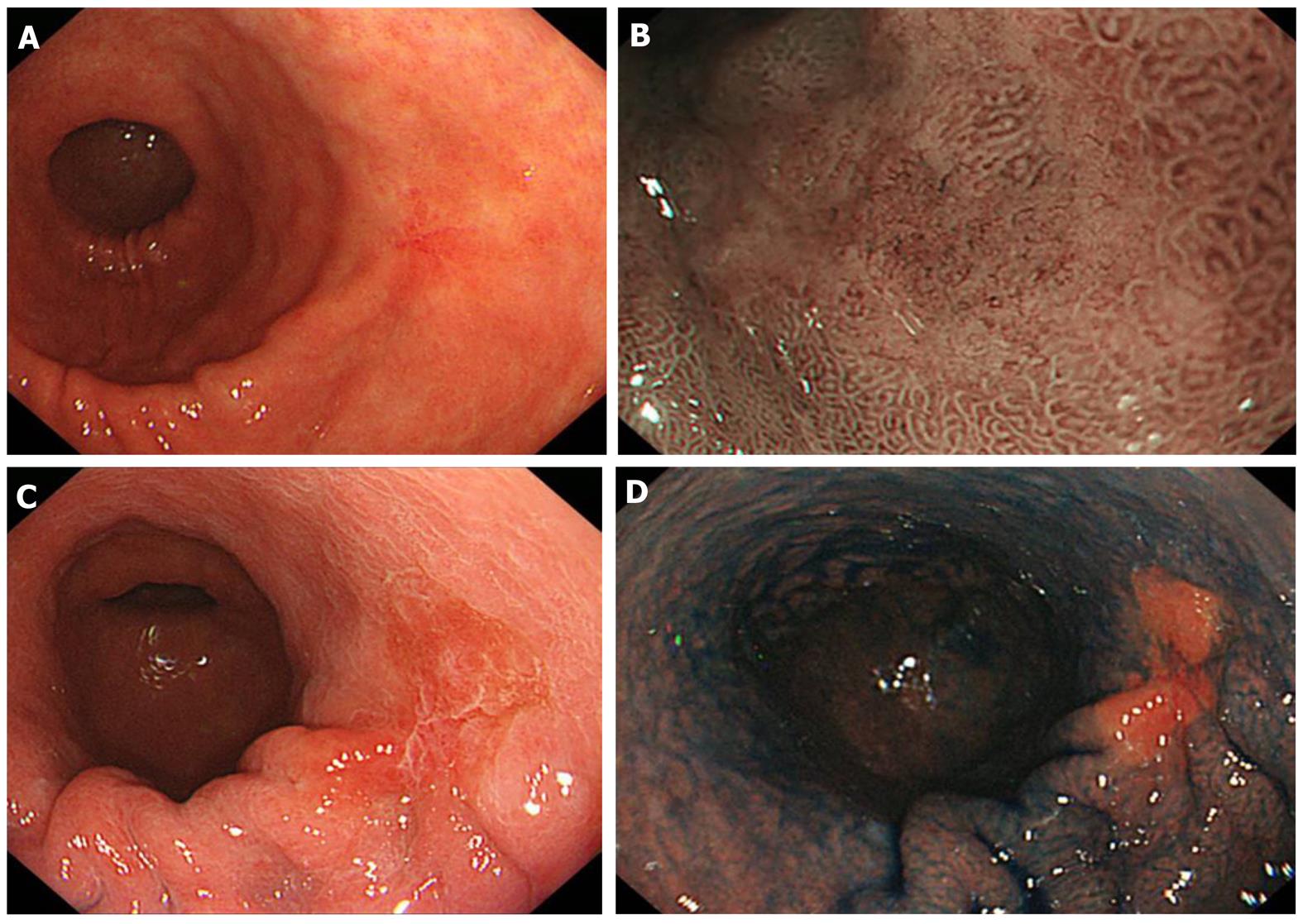
The advent of new techniques makes boundary diagnosis much easier (discussed below). Nevertheless, the conventional endoscopic method using white light is still important and requires adequate steps in order to reach a more accurate diagnosis. The procedure begins with identifying lesions by removing mucus. Since gastric mucus is viscous, pretreatment with pronase is used to degrade the mucus, and molecules are cleaved into short fragments and are easily washed out by flushing with water. It is also important to perform this procedure atraumaticallyas lesions are hemorrhagic in many cases. To determine the boundary, the mucosa is carefully observed, paying attention to the following points: (1) surface morphology, such as protrusion and concavity; (2) changes in color, such as reddening and paleness; and (3) differences from the background mucosa. For observation of the surface morphology, small differences in the height can be observed by slightly deaerating, and it is useful to adjust the air volume and observe the region from several directions (Figures 1A-B and 2A).
Chromoendoscopy is the collective name for the test methods in which the digestive tract mucosa is closely observed following the spraying of a dye. There are various methods depending of the mechanismand include a contrast method in which unevenness is emphasized using indigo carmine, a staining method in which biological tissue is stained with toluidine blue, a dye reaction method utilizing specific reactions of dyes, such as Congo red, and a fluorescence method in which a fluorescence-sensitive dye, such as acridine orange, is injected into the mucosa and fluorescence is observed. Here, the frequently used indigo carmine and acetic acid methods are outlined.
Indigo carmine is a blue/dark blue liquid. It is retained around concave and protruding regions and emphasizes unevenness, facilitating clear observation of the surface morphology of lesions. Observation from several directions and adjustment of the air volume are useful, but these should be performed after sufficient observation because differences in color become unclear after spraying the dye (Figure 1C).
In the acetic acid method, superficial mucus shows a reversible reaction, whitish turbidity, when sprayed with 1.5% acetic acid. When the mucus volume in tumor regions is smaller than that in non-tumorous regions, the boundary may be clearly visualized based on the color change. In addition, whitish turbidity emphasizes the mucosal surface, in which the boundary may be clarified by combining magnifying narrow band imaging (NBI) described below or indigo carmine spraying because the mucus is fixed[1-4] (Figure 2C-D).
NBI. The central wavelength is optimized to 415 and 540 nm as light is strongly absorbed by blood and strongly reflected/scattered at the mucosa, respectively, and the spectrum width is narrowed to enhance micro blood vessels and micro patterns on the mucosal surface. Compared to other image-enhancing techniques, this is superior for obtaining specifically clear images in the short-wavelength spectrum. The usefulness of NBI and FICE described below for diagnosing the boundary of lesions is increased by combining with magnifying endoscopic observation[5-9] (Figure 2B).
Flexible spectral imaging color enhancement (FICE). In this technique, a specific observation wavelength is set based on conventional endoscopic images to enhance and process images for visualization. FICE has flexibility: information collected under white light can be analyzed in various spectral combinations, and relatively bright images can be observed depending on the wavelength setting, showing differentcharacteristics tothose of NBI[10-12].
Using a magnifying electronic endoscope, up to about 100-times-magnified images are optically displayed on a 14-inch monitor, and micro mucosal patterns and micro blood vessels can be observed in detail. The diameter was initially large because the endoscope is equipped with a zoom lens at the tip, but recent technical progress has reduced the diameter close to that of a conventional scope. Combining other techniques with magnifying endoscopy enables theidentification of the boundary of lesions with an unclear margin of advancement undetectable using a single method.
Although determination of the boundary by biopsy alone should be avoided, the boundary is still unclear even after sufficient observation in some lesions, and biopsy of the surrounding region may be useful in such cases.
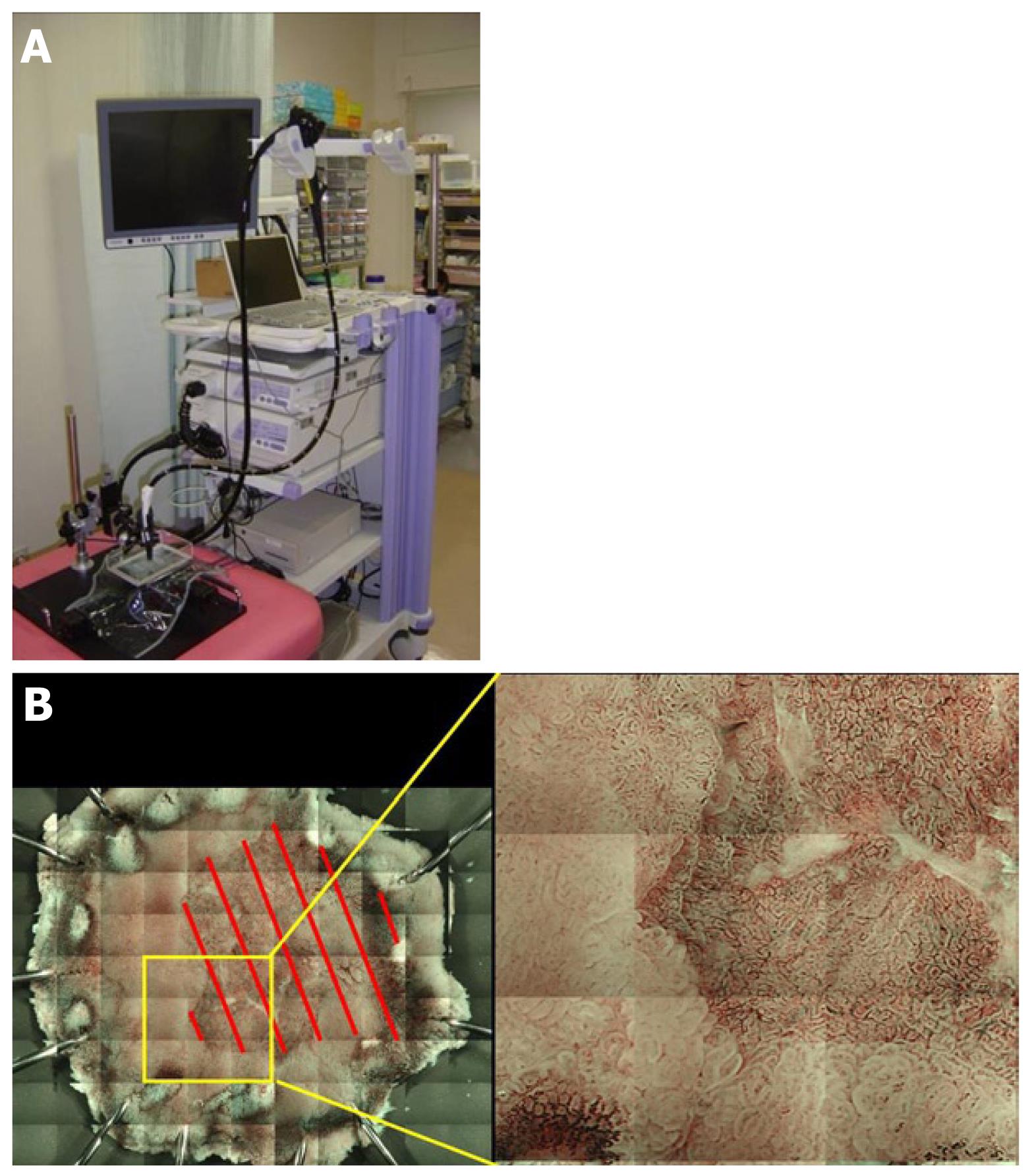
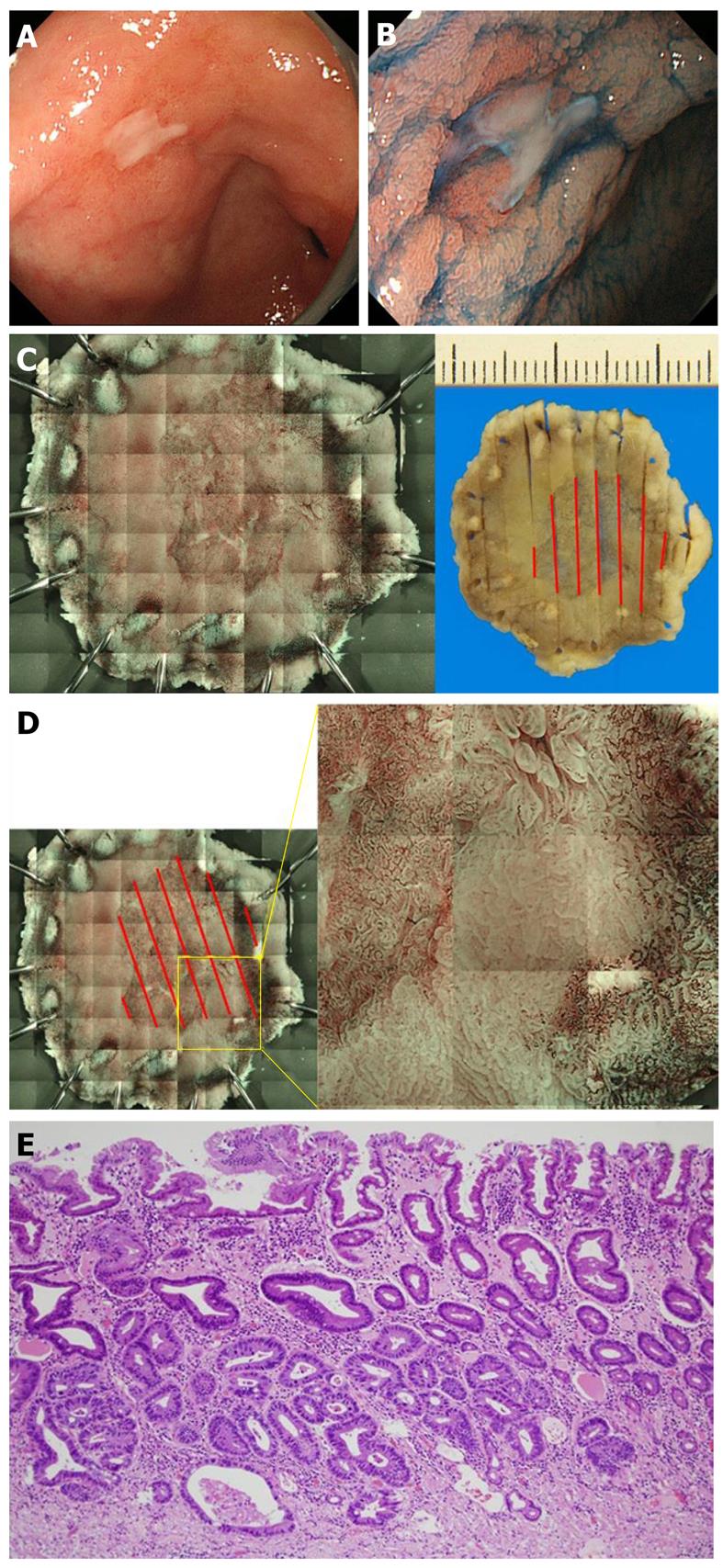
It is important to confirm whether the preoperative diagnosis of the range is correct by analyzing endoscopically resected specimens. When the diagnosis is incorrect, the correct range can be identified by investigating the reason for the failure. In addition, clarification of the conditions leading to an incorrect diagnosis of the range facilitates careful investigation in combination with other methods. We developed a method to prepare a composite of magnified images by tiling in which the mucosal surface of the excised specimen can be closely observed. In this unique method, a magnifying endoscope was fixed, the entire specimen was magnified, and images were segmentally acquired and arranged in a tile pattern to prepare a composite. Unlike the current observation under a stereoscopic microscope, resected lesions can be observed and imaged in a condition very close to that of endoscopic observation in the body, and magnified images of the entire lesion can be acquired under conventional light as well as NBI. We investigated whether an accurate range could be identified using this method (Table 1).
| Conventional (white light) | Image-enhancing | Magnifying | Microscopic | Tomographic |
| Digital Contrast method Delineation-enhanced method | Optical | Optical | Endoscopic ultrasonography | |
| Optical-digital Autofluorescence Narrow band light Infrared light | Digital | Confocal | Optical coherence tomography | |
| Chromoendoscopy Absorbed dye Contrast dye |
Seventeen lesions of differentiated gastric adenocarcinoma were imaged employing this method following ESD at our department in April-September 2010. GIF-H260Z (Olympus) was attached to a device with a modified electric XY stage, and the whole excised lesion which was immersed in water was segmentally imaged at the highest magnification employing NBI (Figure 3A). The acquired images were pasted together into a composite by tiling using a computer program (Figure 3B). After the range of the lesion was assumed by drawing a demarcation line based on the mucosal surface structure and vascular atypia, the range was compared with mapping in the pathological preparation referring to the marking to confirm the accuracy of the assumed range. The accuracy was evaluated by 3-step grading as follows: the demarcation line was perceived, O; perceived, although partially unclear, Δ; completely unclear, ×.
The lesions were tumors with a diameter of 8-43 mm (mean 19 mm). The histologic type was Tub1 in 11 lesions, Tub1+Tub2 in 3, Pap > Tub1 in 1, and Tub2 > por2 in 1. The macroscopic type was 0-IIc in 9 lesions, 0-IIIa+LLc in 2, 0-Iia in 2, 0-IIb in 2, 0-I in 1, and 0-I+IIa+IIc in 1. The accuracy of the assumed range was O in 14, Δ in 3, and × in 0.
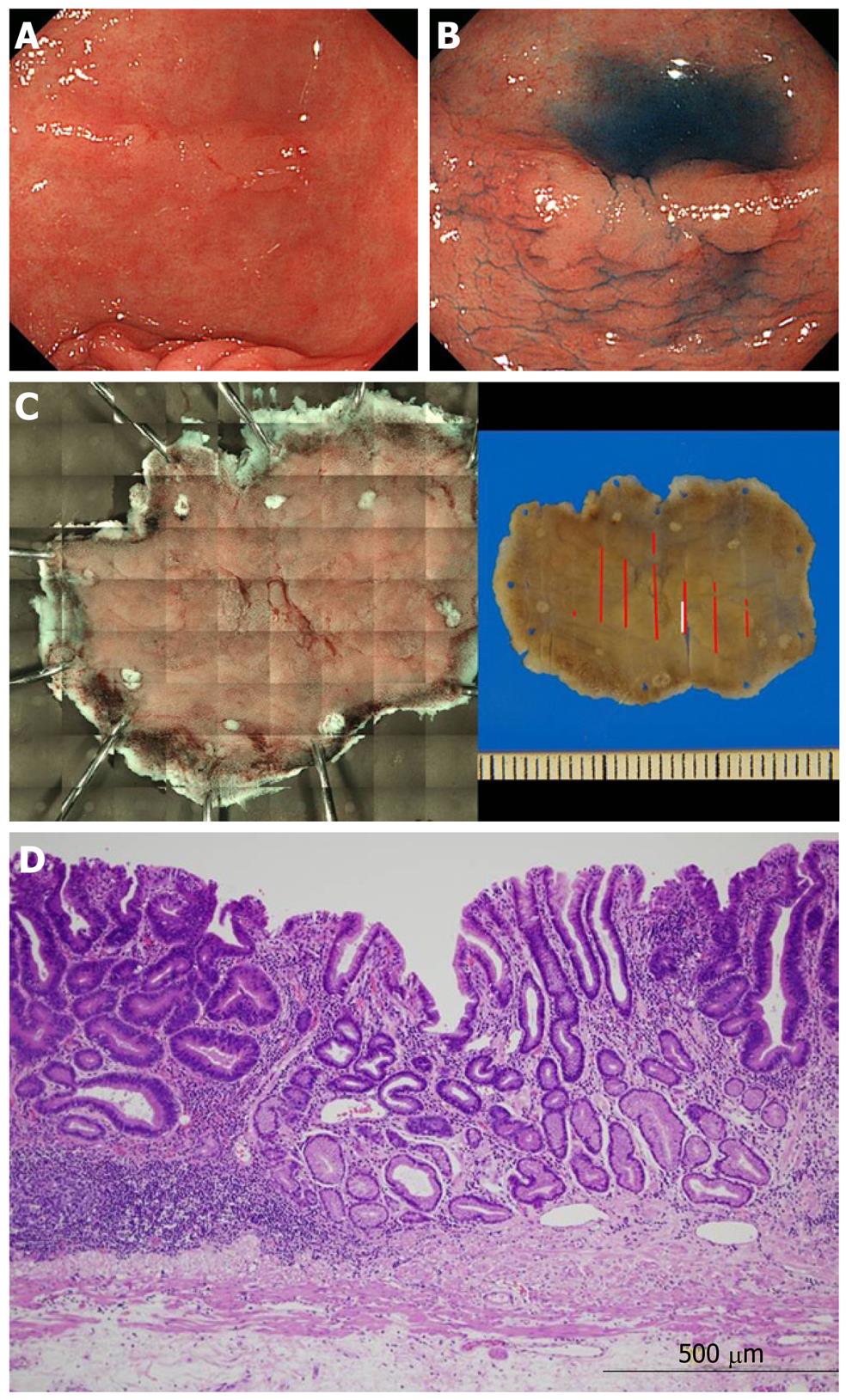
An example of the range perceived is shown in Figure 4. Indigo carmine emphasized an approximately 15-mm protruding lesion on the lesser curvature side of the stomach antrum. When the specimen excised by ESD was compared with the photograph prepared by the tiling method, the range of the perceived lesion was consistent with the histopathological range. The range was partially unclear in 3 cases. The details of these are presented in Table 2. All were concave lesions with a relatively small tumor diameter. One of these lesions is shown in Figure 5. This was a 10-mm concave lesion in the anterior wall of the stomachantrum, and the concave surface was perceived using indigo carmine.
| Tumor size (mm) | Histological type | Macroscopic type | |
| Case 1 | 8 × 4 | tub1> tub2 | 0-IIc |
| Case 2 | 16 × 12 | tub1 | 0-IIc |
| Case 3 | 15 × 7 | tub1 | 0-IIc |
When the photograph of the specimen resected by ESD was prepared employing the tiling method, the assumed range was mostly consistent with the histopathologicalrange,however, a partially unclear region was present. When the region difficult to perceive endoscopicallywas investigated in the pathological preparation, the cancerous gland duct was present only in the deep layer of the lamina propria mucosae, and the superficial layer was covered with non-tumorous epithelium, suggesting that the lesion was difficult to perceive because of poor atypia. The lesions were divided into those with a tumor diameter smaller and greater than the median (17 mm) and compared using Fisher’s exact test. The P-value was 0.08, showing no significant difference, but there may have been a tendency for significance.
In conclusion, the tiling method was capable of stably preparing clear, magnified NBI images of entire resected lesions with a small to relatively large size, and enabled sufficient evaluation of the demarcation lines, leading to accurate identification of the range in most cases. The identification of the range may be difficult in cases with cancerous gland ducts unexposed to the superficial layer. With the spread of ESD, its indication has expanded, but stump-positive cases have also increased, although the number of these cases was small. It is necessary to acquire conventional observation skills and combine various methods to make accurate diagnoses of the boundary.
Peer reviewer: Mauro Manno, MD, Nuovo Ospedale Civile S. Agostino-Estense, Gastroenterology and Digestive Endoscopy Unit, Via Giardini, 1355, 41126 Baggiovara di Modena (Mo), Italy
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
| 1. | Iizuka T, Kikuchi D, Hoteya S, Yahagi N. The acetic acid + indigocarmine method in the delineation of gastric cancer. J Gastroenterol Hepatol. 2008;23:1358-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Kawahara Y, Takenaka R, Okada H, Kawano S, Inoue M, Tsuzuki T, Tanioka D, Hori K, Yamamoto K. Novel chromoendoscopic method using an acetic acid-indigocarmine mixture for diagnostic accuracy in delineating the margin of early gastric cancers. Dig Endosc. 2009;21:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Toyoda H, Rubio C, Befrits R, Hamamoto N, Adachi Y, Jaramillo E. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointest Endosc. 2004;59:15-21. [PubMed] [Cited in This Article: ] |
| 4. | Yagi K, Aruga Y, Nakamura A, Sekine A, Umezu H. The study of dynamic chemical magnifying endoscopy in gastric neoplasia. Gastrointest Endosc. 2005;62:963-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 6. | Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Sakaki N. [Magnifying endoscopic observation on the effect of a proton pump inhibitor on the healing process of gastric ulcer]. Nihon Rinsho. 1992;50:86-93. [PubMed] [Cited in This Article: ] |
| 9. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [PubMed] [Cited in This Article: ] |
| 10. | Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Evaluation and validation of computed virtual chromoendoscopy in early gastric cancer. Gastrointest Endosc. 2009;69:1052-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Osawa H, Yoshizawa M, Yamamoto H, Kita H, Satoh K, Ohnishi H, Nakano H, Wada M, Arashiro M, Tsukui M. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc. 2008;67:226-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008;40:775-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (1)] |