Published online Dec 16, 2011. doi: 10.4253/wjge.v3.i12.241
Revised: August 21, 2011
Accepted: August 28, 2011
Published online: December 16, 2011
Ampullary adenoma is a pre-cancerous lesion arising from the duodenal papilla that is often asymptomatic. It is important to distinguish whether the adenoma is sporadic or arises in the setting of familial adenomatous polyposis as this has important implications with respect to management and surveillance. Multiple modalities are available for staging of these lesions to help guide the most appropriate therapy. Those that are used most commonly include computed tomography, endoscopic ultrasound, and endoscopic retrograde cholangiopancreatography. In recent years, endoscopy has become the primary modality for therapeutic management of the majority of ampullary adenomas. Surgery remains the standard curative procedure for confirmed or suspected adenocarcinoma. This review will provide the framework for the diagnosis and management of ampullary adenomas from the perspective of the practicing gastroenterologist.
- Citation: Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc 2011; 3(12): 241-247
- URL: https://www.wjgnet.com/1948-5190/full/v3/i12/241.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i12.241
Ampullary adenomas are glandular dysplastic lesions that arise in and around the duodenal papilla. Adenomatous tissue has been found in up to 90% of resection specimens of ampullary adenocarcinoma, suggesting that these lesions have pre-malignant potential[1-6]. Autopsy series have estimated the prevalence of ampullary adenoma to be 0.04% to 0.12%[7,8]. They may occur sporadically or in the setting of familial adenomatous polyposis (FAP). Patients with FAP almost invariably develop duodenal adenomas and have a risk for ampullary carcinoma that is 124-fold greater than the general population[3,9,10]. In fact, ampullary carcinoma is the most common malignancy and leading cause of death in FAP patients who have previously undergone colectomy[11-16]. Consequently, surveillance upper endoscopy is an important aspect of management for these patients. Ampullary adenomas are more frequently being recognized because of the increased availability of endoscopy for evaluation of gastrointestinal-related symptoms as well as surveillance programs for patients with FAP. Multiple modalities are now available for diagnosing and staging these lesions. Therefore, a good understanding of the diagnostic and therapeutic options available is essential for making an informed management decision.
Historically, ampullary adenomas were removed by radical surgery. Endoscopic advances in recent years have shifted the paradigm of treatment toward attempted endoscopic resection prior to consideration of surgery because endoscopy is less invasive and has lower morbidity. Nevertheless, the complications associated with endoscopic removal of ampullary adenomas are high compared to other endoscopic therapies, making it imperative that it be performed in experienced hands. In patients with ampullary adenocarcinoma, surgery remains the standard curative therapy, but endoscopy can provide adequate palliation in cases where the patient is deemed not to be a surgical candidate. This review will discuss the clinical manifestations, diagnosis, and management of ampullary adenomas, with particular focus on the endoscopic management of these lesions.
Ampullary adenomas are often asymptomatic and incidentally discovered on endoscopy. Patients may present with symptoms related to obstruction of the biliary or pancreatic duct. These symptoms may include jaundice from biliary obstruction, which in rare instances progresses to cholangitis[17,18]. Acute recurrent pancreatitis may result from pancreatic duct obstruction[19]. Other non-specific symptoms may include nausea, vomiting, abdominal pain, and weight loss. Significant weight loss in a patient with an ampullary lesion should alert the clinician to the possibility of a more invasive process.
The diagnosis of ampullary adenoma is based on endoscopic appearance and histology. In order for endoscopic evaluation of the lesion to be complete, a side-viewing endoscope is necessary. Endoscopic features suggesting that these lesions are benign include regular margins, no ulceration, soft consistency, and no spontaneous bleeding[20,21]. Confirmation of adenoma is necessary with biopsy of the suspect lesion. The accuracy of forceps biopsy has been questioned due to several factors. Intra-observer variability exists between pathologists in interpreting the histologic specimen, making it particularly important to have the specimen reviewed by an experienced pathologist prior to deciding to undergo therapeutic intervention. In addition, forceps biopsy may not take a representative sample of the lesion and may miss foci of adenocarcinoma within adenomatous tissue. Bellizzi et al[22] recently reported a diagnostic agreement of only 64% when comparing biopsy samples to the eventual resected specimen. Forceps biopsy has been associated with accuracy rates of 62% to 85% in other series[23-27]. Therefore, final histologic assessment should be based on the resected specimen.
Once adenoma is confirmed by biopsy, further evaluation is necessary to help dictate management decisions. Modalities that may be used include trans-abdominal ultrasound (US), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), endoscopic US (EUS), and intraductal US (IDUS).
Both trans-abdominal US and CT do not adequately visualize the ampullary area for staging of adenomatous lesions. Their primary role is to identify biliary and pancreatic ductal dilation. In cases of ampullary adenocarcinoma, CT can also provide valuable information by identifying locoregional lymphadenopathy and distant metastatic lesions.
EUS can provide information regarding the depth of the ampullary lesion as well as locoregional lymph node status. Multiple studies have shown that EUS is superior to CT, MRI, and transabdominal US in local peri-ampullary tumor staging[28-30]. IDUS is a newer imaging modality that was originally developed to visualize arterial structures in various pancreaticobiliary diseases. IDUS has higher resolution than EUS because of the use of high frequency waves (20-30 MHz) compared with EUS (7.5-10 MHz). Several studies have reported increased accuracy in staging of ampullary neoplasms with IDUS as compared to EUS[31-34]. Nevertheless, IDUS is not routinely performed as part of the ampullary adenoma staging, mainly due its lack of availability at many centers.
MRCP is typically reserved for patients with bile duct abnormalities previously identified on CT or US that need further clarification prior to more invasive investigative studies. ERCP is performed to visualize the extent of the ampullary lesion into the biliary or pancreatic duct as well as to perform decompression if there is evidence of obstruction. Given the sensitivity of other modalities now available for initial staging, ERCP with both biliary and pancreatic duct evaluation is usually performed immediately preceding possible endoscopic therapeutic intervention in the same session[35]. The use of cholangiopancreatoscopy at the time of ERCP to evaluate for intraductal spread of the adenoma has also been described[36].
An important distinction when considering the appropriate management for newly diagnosed ampullary adenoma is whether the adenoma is sporadic or arises in the setting of FAP. Patients with FAP often have multiple duodenal polyps. Spigelman et al[11] devised a classification system for duodenal polyps in the upper gastrointestinal tract in the setting of FAP (Table 1). Severity of polyposis is assessed by assigning a score (1-3) in each of four categories. Spigelman stage is then determined by the sum of the four categories (Stage 0: Score 0, Stage I: Score 1-4, Stage II: Score 5-6, Stage III: Score 7-8, Stage IV: Score 9-12). Traditionally, patients with Spigelman stage 0-III are followed with close endoscopic surveillance programs, while those with stage IV undergo more aggressive therapy. In FAP, endoscopic resection has not been shown to decrease the need for eventual pancreaticoduodenectomy, as the malignancy risk is related to the extent of polyposis within the duodenum and not just the ampullary lesion[20,37]. Interestingly, studies have found that the progression in Spigelman classification categories over time has more to do with the increase in size and number of polyps as opposed to changes in histology[38].
| Score | |||
| 1 | 2 | 3 | |
| No. of polyps | 1-4 | 5-20 | > 20 |
| Size (mm) | 1-4 | 5-10 | >10 |
| Histology | Tubulous | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
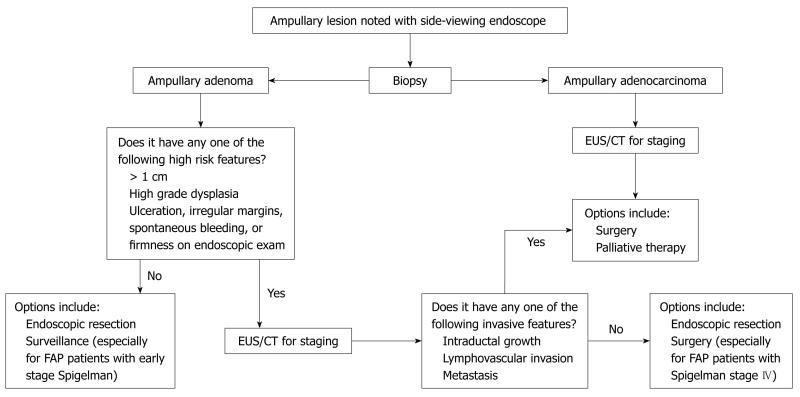
A suggested algorithm for the management of newly diagnosed ampullary adenoma is shown in Figure 1. Given the heterogeneity of the lesions and patient population, it is difficult to set out guidelines that would encompass all possible scenarios, so each case must be taken on an individual basis. Advances in endoscopic therapy have allowed clinicians to be more aggressive in endoscopic resection of adenomas and there have even been case reports of focal ampullary adenocarcinomas removed endoscopically[21,39-43]. Most clinicians would agree that patients with known ampullary adenocarcinoma should be offered surgery if they are deemed appropriate surgical candidates. On the other hand, management of high grade dysplasia (HGD) is a controversial topic. A retrospective review of 23 patients who had endoscopic resection for what turned out to be HGD or focal T1 ampullary adenocarcinoma found that none of these patients had residual tumor on follow-up endoscopy or surgically resected specimen[44]. Therefore, the authors concluded that endoscopic resection is appropriate management for ampullary adenomas with HGD. Other investigators have advocated endoscopic resection for HGD if the tumor is only extraductal, and in situations where intraductal growth is less than 1 cm[45]. Proponents of radical surgery for HGD point to several studies that underlie the fact that diagnostic yield for picking up foci of adenocarcinoma and lymphovascular invasion pre-operatively is sub-optimal[46,47].
Endoscopic removal of ampullary adenomas remains non-standardized and highly variable, which reflects the relatively small number of formal investigations into this topic. Furthermore, there is no uniform agreement on the terminology used to describe various resection modalities. The terms papillectomy and ampullectomy are frequently used interchangeably but some authors restrict the use of “papillectomy” for endoscopic resection and “ampullectomy” for surgical resection[48]. The following is a discussion of the most commonly used endoscopic resection techniques based on a review of the literature and our experience.
Submucosal injection prior to papillectomy may be performed similar to the technique used when performing endoscopic mucosal resection for colorectal polyps. The failure of a lesion to manifest a “lift sign” is associated with malignancy and is considered a contraindication to attempts at complete endoscopic removal[49,50]. It is speculated that injection of epinephrine may also decrease the risk of bleeding during resection. Most commonly injected fluids include saline and epinephrine, although methylene blue and viscous material such as hydroxypropyl methylcellulose and sodium hyaluronate have also been used[3,43,49-54]. Successful endoscopic resection of adenomas has also been described without the use of submucosal injection[40,55,56]. In fact, we generally avoid submucosal injection at our institution for two main reasons. One is the concern that injection may distort the ampullary anatomy due to the “anchoring” effect from the bile and pancreatic duct running through the lesion, creating a central depression at the site of the ampullary opening. Second, injection may create a “dome” effect and make effective snare placement for en bloc resection more difficult.
Endoscopic papillectomy is performed by the use of endoscopic snares and electrocautery. Standard or “braided” polypectomy snares are typically used, although fine wire snares specifically designed for ampullary resection are available[3,50,57]. If the lesion can be completely ensnared, en bloc resection with electrocautery may be performed. This has the advantage of shortened procedure time, reduced use of electrocautery, and providing complete tissue specimen for pathologic examination. Some authors have described the use of an electrosurgical needle knife to make an incision circumferentially around the lesion to facilitate snare capture[3]. Piecemeal resection is sometimes necessary for lesions larger than 2 cm or in cases where visible tissue is left in place with en bloc technique. The type of current and power settings used for ampullary resection are variable. Many authors describe the use of blended current, whereas others utilize pure-cutting current[58-60]. Few have also described the use of pure coagulation current[50].
The role of ablative therapies [argon plasma coagulation (APC), laser, bipolar electrocautery] is mainly to destroy any remaining tissue that may be left following snare resection of a specimen. APC is most frequently used for this purpose. The main disadvantage in using this technique is tissue that is ablated cannot be retrieved for pathology review. In fact, some clinicians avoid the use of APC altogether primarily for this reason[35]. Catalano et al[58] reported their results from 103 papillary resections and found no difference in overall rate of success or recurrence in patients who did and did not have APC.
Pancreatic or biliary sphincterotomy is often performed following papillectomy, with the goal of improving pancreaticobiliary drainage. One of the known complications of papillectomy is pancreatitis. Placement of a pancreatic duct stent following ampullary adenoma resection has been found to reduce the incidence of post-ERCP pancreatitis based on a meta-analysis of five prospective series[61]. Recently, a randomized control trial also showed a decrease in the rate of pancreatitis in patients who received a pancreatic duct stent[62]. Some authors perform sphincterotomy and placement of pancreatic duct stent prior to resection[21,50], although we favor post-resection stent placement in an attempt to maximize the opportunity for en bloc resection. Placement of a biliary stent to reduce the risk of post-procedural cholangitis is infrequently performed, and mainly done if there is concern for incomplete biliary drainage despite biliary sphincterotomy[3,50,63]. In our institution, we place a pancreatic duct stent in every patient undergoing endoscopic papillectomy as the data available strongly support its use. We reserve the use of a biliary stent only for patients that are believed to have slow drainage after biliary sphincterotomy.
A systematic review by Han et al[48] reported the success rates for endoscopic removal of ampullary adenomas to range from 46% to 92%, and recurrence rates to range from 0% to 33%. Most recently, a large retrospective series which included 102 patients diagnosed with ampullary adenoma that underwent endoscopic resection showed a success rate of 84%[64]. Factors affecting success in this study were smaller lesion size (< 2 cm) and the absence of dilated ducts.
Even in experienced hands, complications arising after endoscopic papillectomy are high compared to other endoscopic procedures. They include pancreatitis, perforation, bleeding, cholangitis, and papillary stenosis. In their review, Han et al[48] found a morbidity rate of 23% (range 10%-58%) and a mortality rate of 0.4% (range 0%-7%). Bleeding and pancreatitis were the most common complications. Each occurred in up to 25% of cases in one small study, although the remainder of the studies showed bleeding rates of 0% to 21% and pancreatitis rates of 0% to 15%[48].
There is no consensus regarding the most appropriate surveillance interval following endoscopic resection of ampullary adenomas. Initial surveillance endoscopy is generally performed at 1 mo to 6 mo following resection. Following the initial surveillance endoscopy, the clinician may decide to follow with endoscopy every 3 mo to 12 mo for the next 2 years, and then less frequent intervals thereafter[3,50,52,58,63,65-67]. A side-viewing endoscope should be used for surveillance purposes. One recent study suggests improved rates of detection of duodenal polyps with the use of chromoendoscopy in FAP patients[68]. Patients with sporadic ampullary adenomas are at increased risk for colon polyps and should be offered screening colonoscopy.
Surgery had been the traditional approach for removal of ampullary adenoma before the advances related to endoscopic therapy in the last 10 to 20 years. Surgery remains the standard curative therapy for confirmed or suspected ampullary adenocarcinoma, although endoscopy can provide adequate palliation in patients deemed not to be surgical candidates.
Surgical approaches may include pancreaticoduodenectomy, surgical ampullectomy, and pancreas-preserving duodenectomy. The reason for the shift towards endoscopic removal of adenoma is related to the significant morbidity and mortality associated with radical surgery. Data from multiple series for pancreaticoduodenectomy demonstrated an operative mortality of 1% to 9% and operative morbidity as high as 41%[69,70]. Less invasive surgical options such as surgical ampullectomy are available, but recurrence is a possibility when these less invasive surgical interventions are employed. Similar to endoscopy, these patients will also require follow-up endoscopy, whereas those who receive pancreaticoduodenectomy do not require further surveillance. FAP patients are unique in that they will require surveillance regardless of intervention given their propensity to develop adenomas throughout the duodenum.
Non-invasive therapy is also an option in certain cases of diagnosed ampullary adenoma. While there is no data studying the effect of non-steroidal anti-inflammatory drugs (NSAIDs) specifically on ampullary adenomas, there is literature that studies the effect of NSAIDs on duodenal and colorectal polyps in the FAP population. The most commonly studied NSAIDs have been celecoxib and sulindac. In a randomized control trial involving 49 post-colectomy FAP patients, celecoxib was found to significantly reduce duodenal polyposis when compared to placebo[71]. Another study involving 24 post-colectomy FAP patients found that sulindac reduced rectal polyp progression, but had no significant effect on duodenal polyp regression[72]. Increased erosions at the anastomotic site in the NSAID group have also been reported in at least one study[73].
Endoscopic advances in recent years have expanded the role of endoscopy in the therapeutic management of ampullary adenomas. Prior to considering therapy, clinicians should utilize the staging modalities available in order to make the most appropriate management decision for these patients. Radical surgery remains the treatment of choice for ampullary adenocarcinoma, adenomas with extensive intraductal growth, and should be strongly considered in a certain subset of FAP patients. Future studies and case experience will allow us to make more definitive guidelines with respect to appropriate treatment and surveillance for ampullary adenoma.
Peer reviewer: Stefanos Karagiannis, MD, PhD, Gastrointestinal and Liver Unit, General and Oncology Kifissia Hospital Agioi Anargiri, Kaliftaki 14564, Kifissia, Greece
S- Editor Yang XC L- Editor Webster JR E- Editor Zheng XM
| 1. | Baczako K, Büchler M, Beger HG, Kirkpatrick CJ, Haferkamp O. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol. 1985;16:305-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi K, Enjoji M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987;59:506-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 3. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Scarpa A, Capelli P, Zamboni G, Oda T, Mukai K, Bonetti F, Martignoni G, Iacono C, Serio G, Hirohashi S. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163-1172. [PubMed] [Cited in This Article: ] |
| 5. | Park SH, Kim YI, Park YH, Kim SW, Kim KW, Kim YT, Kim WH. Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of Vater. World J Surg. 2000;24:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, Miwa A. Adenoma and tiny carcinoma in adenoma of the papilla of Vater--p53 and PCNA. Hepatogastroenterology. 1999;46:1959-1962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | BAKER HL, CALDWELL DW. Lesions of the ampulla of Vater. Surgery. 1947;21:523-531. [PubMed] [Cited in This Article: ] |
| 9. | Yao T, Ida M, Ohsato K, Watanabe H, Omae T. Duodenal lesions in familial polyposis of the colon. Gastroenterology. 1977;73:1086-1092. [PubMed] [Cited in This Article: ] |
| 10. | Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980-1982. [PubMed] [Cited in This Article: ] |
| 11. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 446] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990;33:639-642. [PubMed] [Cited in This Article: ] |
| 13. | Beckwith PS, van Heerden JA, Dozois RR. Prognosis of symptomatic duodenal adenomas in familial adenomatous polyposis. Arch Surg. 1991;126:825-827; discussion 825-827;. [PubMed] [Cited in This Article: ] |
| 14. | Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann Surg. 1993;217:101-108. [PubMed] [Cited in This Article: ] |
| 15. | Belchetz LA, Berk T, Bapat BV, Cohen Z, Gallinger S. Changing causes of mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:384-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Bertoni G, Sassatelli R, Nigrisoli E, Bedogni G. Endoscopic snare papillectomy in patients with familial adenomatous polyposis and ampullary adenoma. Endoscopy. 1997;29:685-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Sand JA, Nordback IH. Transduodenal excision of benign adenoma of the papilla of Vater. Eur J Surg. 1995;161:269-272. [PubMed] [Cited in This Article: ] |
| 18. | Sobol S, Cooperman AM. Villous adenoma of the ampulla of Vater. An unusual cause of biliary colic and obstructive jaundice. Gastroenterology. 1978;75:107-109. [PubMed] [Cited in This Article: ] |
| 19. | Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol. 1990;12:200-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Baron TH. Ampullary adenoma. Curr Treat Options Gastroenterol. 2008;11:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Eswaran SL, Sanders M, Bernadino KP, Ansari A, Lawrence C, Stefan A, Mattia A, Howell DA. Success and complications of endoscopic removal of giant duodenal and ampullary polyps: a comparative series. Gastrointest Endosc. 2006;64:925-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Roggin KK, Yeh JJ, Ferrone CR, Riedel E, Gerdes H, Klimstra DS, Jaques DP, Brennan MF. Limitations of ampullectomy in the treatment of nonfamilial ampullary neoplasms. Ann Surg Oncol. 2005;12:971-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Blackman E, Nash SV. Diagnosis of duodenal and ampullary epithelial neoplasms by endoscopic biopsy: a clinicopathologic and immunohistochemical study. Hum Pathol. 1985;16:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Menzel J, Poremba C, Dietl KH, Böcker W, Domschke W. Tumors of the papilla of Vater--inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol. 1999;10:1227-1231. [PubMed] [Cited in This Article: ] |
| 26. | Elek G, Gyôri S, Tóth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 27. | Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, Hochwald SN. Contemporary results with ampullectomy for 29 "benign" neoplasms of the ampulla. J Am Coll Surg. 2008;206:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Chen CH, Tseng LJ, Yang CC, Yeh YH. Preoperative evaluation of periampullary tumors by endoscopic sonography, transabdominal sonography, and computed tomography. J Clin Ultrasound. 2001;29:313-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Chen CH, Tseng LJ, Yang CC, Yeh YH, Mo LR. The accuracy of endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, computed tomography, and transabdominal ultrasound in the detection and staging of primary ampullary tumors. Hepatogastroenterology. 2001;48:1750-1753. [PubMed] [Cited in This Article: ] |
| 31. | Itoh A, Goto H, Naitoh Y, Hirooka Y, Furukawa T, Hayakawa T. Intraductal ultrasonography in diagnosing tumor extension of cancer of the papilla of Vater. Gastrointest Endosc. 1997;45:251-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Menzel J, Hoepffner N, Sulkowski U, Reimer P, Heinecke A, Poremba C, Domschke W. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT--a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 35. | Hopper AD, Bourke MJ, Williams SJ, Swan MP. Giant laterally spreading tumors of the papilla: endoscopic features, resection technique, and outcome (with videos). Gastrointest Endosc. 2010;71:967-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Judah JR, Draganov PV. Intraductal biliary and pancreatic endoscopy: an expanding scope of possibility. World J Gastroenterol. 2008;14:3129-3136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Björk J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, Martinsson T, Nordling M, Hultcrantz R. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, Vasen HF. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 236] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 39. | Jung S, Kim MH, Seo DW, Lee SK. Endoscopic snare papillectomy of adenocarcinoma of the major duodenal papilla. Gastrointest Endosc. 2001;54:622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Ito K, Fujita N, Noda Y. Case of early ampullary cancer treated by endoscopic papillectomy. Dig Endosc. 2004;16:157-161. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Small AJ, Baron TH. Successful endoscopic resection of ampullary adenoma with intraductal extension and invasive carcinoma (with video). Gastrointest Endosc. 2006;64:148-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Neves P, Leitão M, Portela F, Pontes JM, Areia M, Brito D, Sousa HT, Souto P, Camacho E, Andrade P. Endoscopic resection of ampullary carcinoma. Endoscopy. 2006;38:101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Fukushima H, Yamamoto H, Nakano H, Nakazawa K, Sunada K, Wada S, Tamada K, Sugano K. Complete en bloc resection of a large ampullary adenoma with a focal adenocarcinoma by using endoscopic submucosal dissection (with video). Gastrointest Endosc. 2009;70:592-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Yoon SM, Kim MH, Kim MJ, Jang SJ, Lee TY, Kwon S, Oh HC, Lee SS, Seo DW, Lee SK. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66:701-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Seewald S, Omar S, Soehendra N. Endoscopic resection of tumors of the ampulla of Vater: how far up and how deep down can we go? Gastrointest Endosc. 2006;63:789-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Kim JH, Kim JH, Han JH, Yoo BM, Kim MW, Kim WH. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol. 2009;16:2547-2554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Heidecke CD, Rosenberg R, Bauer M, Werner M, Weigert N, Ulm K, Roder JD, Siewert JR. Impact of grade of dysplasia in villous adenomas of Vater's papilla. World J Surg. 2002;26:709-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006;63:292-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Kahaleh M, Shami VM, Brock A, Conaway MR, Yoshida C, Moskaluk CA, Adams RB, Tokar J, Yeaton P. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335-2339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Park SW, Song SY, Chung JB, Lee SK, Moon YM, Kang JK, Park IS. Endoscopic snare resection for tumors of the ampulla of Vater. Yonsei Med J. 2000;41:213-218. [PubMed] [Cited in This Article: ] |
| 52. | Charton JP, Deinert K, Schumacher B, Neuhaus H. Endoscopic resection for neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:245-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Conio M, Rajan E, Sorbi D, Norton I, Herman L, Filiberti R, Gostout CJ. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56:513-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest Endosc. 2003;57:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Yamao T, Isomoto H, Kohno S, Mizuta Y, Yamakawa M, Nakao K, Irie J. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 59. | Norton ID, Geller A, Petersen BT, Sorbi D, Gostout CJ. Endoscopic surveillance and ablative therapy for periampullary adenomas. Am J Gastroenterol. 2001;96:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 60. | Saurin JC, Chavaillon A, Napoléon B, Descos F, Bory R, Berger F, Ponchon T. Long-term follow-up of patients with endoscopic treatment of sporadic adenomas of the papilla of vater. Endoscopy. 2003;35:402-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 62. | Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 63. | Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 244] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Vogt M, Jakobs R, Benz C, Arnold JC, Adamek HE, Riemann JF. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis. 2000;32:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Ponchon T, Berger F, Chavaillon A, Bory R, Lambert R. Contribution of endoscopy to diagnosis and treatment of tumors of the ampulla of Vater. Cancer. 1989;64:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 68. | Dekker E, Boparai KS, Poley JW, Mathus-Vliegen EM, Offerhaus GJ, Kuipers EJ, Fockens P, Dees J. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009;41:666-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. [PubMed] [Cited in This Article: ] |
| 71. | Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 72. | Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 290] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 73. | Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641-645. [PubMed] [Cited in This Article: ] |