Published online Dec 15, 2022. doi: 10.4251/wjgo.v14.i12.2329
Peer-review started: July 10, 2022
First decision: September 26, 2022
Revised: October 16, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: December 15, 2022
Nucleus accumbens-1 (NAC-1) is highly expressed in a variety of tumors, inclu
To determine whether and how NAC-1 affects antitumor immunity in colon cancer.
NAC-1-siRNA was transfected into RKO colon cancer cells to knock down NAC expression; tumor cells with or without knockdown of NAC-1 were treated with CD8+ T cells to test their cytocidal effect. The level of the immune checkpoint programmed death receptor-1 ligand (PD-L1) in colon cancer cells with or without knockdown of NAC-1 was analyzed using Quantitative real-time poly
Knockdown of NAC-1 expression in colon cancer cells significantly enhanced the cytocidal effect of CD8+ T cells in cell culture experiments. The sensitizing effect of NAC-1 knockdown on the antitumor action of cytotoxic CD8+ T cells was recapitulated in a colon cancer xenograft animal model. Furthermore, knockdown of NAC-1 in colon cancer cells decreased the expression of PD-L1 at both the mRNA and protein levels, and this effect could be rescued by transfection of an RNAi-resistant NAC-1 expression plasmid. In a reporter gene assay, transient expression of NAC-1 in colon cancer cells increased the promoter activity of PD-L1, indicating that NAC-1 regulates PD-L1 expression at the transcriptional level. In addition, depletion of tumoral NAC-1 increased the number of CD8+ T cells but decreased the number of suppressive myeloid-derived suppressor cells and regulatory T cells.
Tumor expression of NAC-1 is a negative determinant of immunotherapy.
Core Tip: We determined whether and how nucleus accumbens-1 (NAC-1) affects antitumor immunity in colon cancer. Knockdown of NAC-1 expression in colon cancer cells significantly enhanced the cytocidal effect of CD8+ T cells. Knockdown of NAC-1 in colon cancer cells decreased the expression of progra
- Citation: Shen ZH, Luo WW, Ren XC, Wang XY, Yang JM. Expression of nucleus accumbens-1 in colon cancer negatively modulates antitumor immunity. World J Gastrointest Oncol 2022; 14(12): 2329-2339
- URL: https://www.wjgnet.com/1948-5204/full/v14/i12/2329.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i12.2329
Upregulation of nucleus accumbens-1 (NAC-1), a member of the Bric-a-brac/poxvirus and zinc finger (BTB/POZ) family, has been reported in a variety of cancers, including colorectal cancer, pancreatic cancer, and breast cancer[1]. The expression of NAC-1 is associated with cancer invasion, recurrence, proliferation and metastasis[2,3] and is related to patient survival, disease severity and prognosis[4,5]. We previously showed that NAC-1 could affect the expression of high mobility group box-1 protein (HMGB1) to promote autophagy and cisplatin resistance in ovarian cancer and NAC-1 dysfunction can promote cell senescence and block tumor cell proliferation and canceration[6,7]. In recent years, immunotherapy has achieved impressive progress in the treatment of various cancers; however, its clinical outcome remains unsatisfactory due to a variety of factors, including coinhibltory signals that can inhibit anticancer immunity. Immune checkpoint proteins such as programmed death receptor-1 (PD-1) have an important role in dampening the antitumor immune response. In this study, we found that tumoral expression of NAC-1 can negatively modulate the antitumor immune response, which is likely associated with its promotive effect on PD-1 ligand (PD-L1) transcription, suppressive myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in the tumor microenvironment. Our study suggests that targeting tumoral NAC-1 may be explored as a potential therapeutic strategy to enhance cancer immunotherapy.
The human colon cancer cell line RKO was purchased from ATCC, and the mouse colon cancer cell line MC-38 transfected with ovalbumin (OVA) into MC-38 cells was a gift from the Department of Immunology, University of Pennsylvania School of Medicine. The cell line was cultured using DMEM (HyClone, United States). Construction of the NAC-1 knockout MC-38-OVA stable transfection cell line: SuperFect® Transfection Reagent (QIAGEN) was used to transfer NAC-1 shRNA and control plasmids into MC-38-OVA mouse colon cancer cells to culture for 72 h. Puromycin (PM) (0, 1.0, 2.5, 5.0, 7.5, and 10.0 μg/mL) (GEMINI, United States) was added for treatment for 7-10 d, and the culture containing puromycin was replaced every two days. The concentration of puromycin at which all cells died at 3 d was selected as the optimal screening concentration.
Human CD8+ T cells were purchased from the Immunology Laboratory of the University of Pennsylvania. The cells were placed in a 24-well plate precoated with CD3 antibody (2 μg/mL) (Biolegend), CD8 (5 μg/mL) (Biolegend) and IL-2 (100 U/mL) (Biolegend) and activated for approximately 3 d for use. Eight-week-old OT-I mice were euthanized, and then the spleens were removed, cleavage red blood cells were lysed, and CD8 immunomagnetic beads (EasySepTM) were used to isolate CD8+ T cells from OT-I mice. The activation method is the same as above.
All procedures involving animals were reviewed and approved by the IRB of Third Xiangya Hospital, Central South University (No. 2020-S298). Wild-type C57BL/6 mice and OT-I mice were purchased from The Jackson Laboratory and the Experimental Animal Center of Xiangya Medical College, Central South University and were raised in a specific pathogen-free (SPF) laboratory. The study followed the guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Clinical specimens were collected from 20 patients with colon cancer admitted to the Third Xiangya Hospital of Central South University in Changsha, Hunan Province, from 2018.01-2018.12. Thirteen out of 20 patients were older than 60 years. Seven patients were younger than 60 years. Out of 20 patients, 11 were female, and 9 were male. The TNM stage of the patients was stage I-II for 15 patients and III-IV for 5 patients. Ten patients had a tumor of a size of less than 5 cm, while the other 10 patients had a tumor larger than 5 cm. The tumor tissues were removed, fixed, dehydrated, paraffin embedded, and sectioned for later use. All clinical specimens were collected with informed consent from the patients.
siRNA transfection: Colon cells were made into a single cell suspension and seeded in a six-well plate at 5 × 105 cells/well. After adherence, a mixture of transfection reagent and NAC-1-siRNA was prepared. The whole experimental process was carried out strictly in accordance with the liposome 3000 (Lipo-fectamine 3000, Lipo3000) transfection reagent instructions, and the experiment was repeated at least 3 times. See Table 1 for the siRNA sequence required for the experiment.
| Gene name | Primer sequence (5’→3’) | |
| Negative control | Sense | UUCUCCGAACGUGUCACGUTT |
| Anti-sence | ACGUGACACGUUCGGAGAATT | |
| siNAC-1 | Sense | GGACAUGAUGAGCAUGGAATT |
| Anti-sence | UUCCAUGCUCAUCAUGUCCTT | |
| siCD274 | Sense | GAGGAAGACCUGAAGGUUCAGCAUA |
| Anti-sence | UAUGCUGAACCUUCAGGUCUUCCUC |
Transient expression plasmid transfection: The plasmid was thoroughly mixed with SuperFect® Transfection Reagent (Roche), allowed to stand for 15 min, and replaced with fresh culture medium.
Different concentration ratios (1:1, 2:1, 5:1, 10:1) of CD8+ T cells were cocultured with RKO and RKO NAC-1-siRNA human colon cancer cells for 12 h, and the supernatant was collected for the detection of cytotoxicity, which was carried out according to the instructions of the kit (Promega).
After collecting the cells, TRIzol reagent was used to extract the total RNA of the cells, which was then reverse transcribed to synthesize cDNA, which was used as a template for real-time polymerase chain reaction (PCR). The PCR sequence required for the experiment is shown in Table 2.
| Gene name | Primer sequence (5’→3’) | |
| NAC-1 | Forward | TTCTTTGACCGGAACACGCT |
| Reverse | TGGCATTCATCTCGCTCTCC | |
| PD-L1 | Forward | CCCTAATTTGAGGGTCAGTTCCT |
| Reverse | CTCAGTCATGCAGAAAACAAATTGA | |
| GAPDH | Forward | GACAGTCAGCCGCATCTTCT |
| Reverse | TTAAAAGCAGCCCTGGTGAC |
After collecting the cells, RIPA lysis buffer was used to extract the total protein, and the protein concentration was determined. The total protein sample was loaded, SDS-PAGE electrophoresis was performed, and the membrane was transferred and blocked with 5% skimmed milk powder. Then, PD-L1 antibody (Proteintech) was added and incubated overnight at 4 °C. After washing, anti-rabbit or anti-mouse secondary antibody was added and incubated for 1 h. After washing and exposure, ImageJ software was used to analyze the gray value of the band, and the relative expression of the target protein was expressed as the ratio of the gray value of the band to the gray value of the internal reference band.
The classic PD-L1 transcriptional regulators, including STAT1/3, STAT2/5 and IRF-1, are mainly located within -400 bp of the PD-L1 promoter region[8]. Based on this, we constructed a luciferase plasmid containing the PD-L1 classic transcription factor binding segment (from -456 bp to -1 bp) and PD-L1 full promoter region to investigate whether NAC-1 can enhance the promoter region transcription of the PD-L1 segment. We transfected colon cancer cells into Renilla plasmid, luciferase plasmid, and pcDNA3.1-NACC1 transient expression plasmid or the corresponding empty load. After 48 h of culture, the cells were lysed, and the fluorescence value was measured by the dual luciferase method. The fluorescence value of Renilla was used as a reference. Then, the transcriptional activity of the corresponding segment of the PD-L1 promoter was obtained.
The experiment was divided into 6 groups: The control group, NAC-1-KD group, control + CTLs group, NAC-1-KD + CTLs group, control + CTLs + MEKi group, and NAC-1-KD + CTLs + MEKi group. Each group had 6 mice (3 males and 3 females) that were 8 wk old.
Construction of a homogenous subcutaneous tumor model: A total of 100 μL of 1 × 106 colon cancer cells was slowly injected into the right flanks of mice. The size of the mouse subcutaneous tumor was observed and measured [calculation method of tumor volume: tumor volume= (length × width2) × 0.52].
Treatment group: When the tumor volume reached 200 mm3, CD8+ T cells were injected in the tail vein at a quantity of 4 × 106 per mouse, or cobimetinib at 7.5 mg/kg was injected intraperitoneally 3 times a week. When the tumor volume reached 1800 mm3, the mice were sacrificed.
After the mice were euthanized, the tumor tissue was removed to prepare a cell suspension and stained with PE-conjugated anti-CD3, APC-conjugated anti-CD45, and BV650-conjugated anti-CD8 antibodies (BioLegend, United States). CD3- and CD45-positive cells were detected by an LSRII flow cytometer (BD), and then CD8+ positive cells were chosen. The cells were stained with APC-Gr-1 and FITC-conjugated anti-CD11b antibodies, and the proportion of MDSCs was detected by flow cytometry. Then, the samples were treated with predigested solution (1 × HBSS + 5 mmol/L EDTA + 1 mmol/L DTT) and with digestive solution (1 ×, containing type IV collagenase (1 mg/mL, Sigma), hyaluronidase (1%, Sigma), DNase I (0.25%, Sigma) to digest the tissues. Percoll was further purified and then stained with FITC-conjugated anti-CD4 and APC-conjugated anti-CD25 antibodies (Biolegend, United States). After breaking the nucleus and staining with PE-Foxp3 antibody (Biolegend, United States), flow cytometry was used to detect the proportion of CD4+ CD25+ Foxp3+ triple-positive cells.
Tumor tissue and adjacent tissue samples from colon cancer patients were immersed in 4% formaldehyde and fixed for more than 48 h. The tissue was dehydrated in a dehydrator, embedded in paraffin, and the paraffin was sectioned to obtain a section with a thickness of 4 μm. The slices were boiled in sodium citrate repair solution (pH 6.0, Google Bio, Wuhan, Hubei Province, China) for 17 min and cooled at room temperature. Then, the sections were incubated with NAC-1, CD274, and CD8 antibodies at 4 °C overnight and incubated with the corresponding secondary antibody (Google Bio, Wuhan, China) for 15 min. Then, a DAB kit (Mixin Biotechnology Development Company, Fuzhou, Fujian Province, China) was used to develop color.
GraphPad Prism 6 was used to statistically analyze the data. A significant difference between the two groups was determined by Student’s-test, and the statistical data of 3 groups or more was determined by one-way ANOVA. NS, P > 0.05, aP < 0.05, bP < 0.01, and cP < 0.001. The correlation analysis of NAC-1, PD-L1 and CD8 expression adopted the Pearson correlation coefficient test. Among them, the correlation coefficient r = 0.0-0.2 is a very weak correlation or no correlation, r = 0.2-0.4 is a weak correlation, r = 0.4-0.6 is a medium degree correlation, r = 0.6-0.8 is a strong correlation, and r = 0.8-1.0 is a very strong degree correlation. Pearson’s significance test P < 0.05 indicates that the correlation between the two variables is statistically significant.
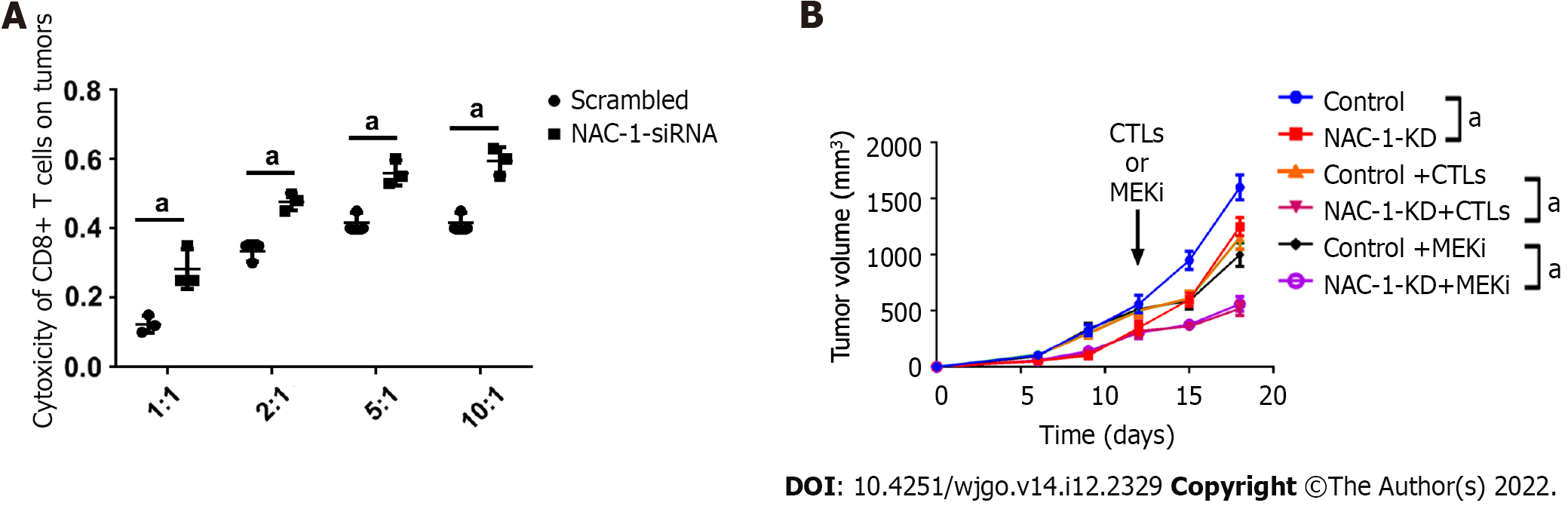
To determine whether the expression of NAC-1 in colon cancer cells affects their response to cytotoxic CD8+ T cells, we cocultured CD8+ T cells with RKO colon cancer cells with or without NAC-1 knockdown, and at the end of treatment, the killing effect of CD8+ T cells was analyzed using the CytoTox 96® assay. Figure 1A shows that knockdown of NAC-1 in cancer cells significantly enhanced the toxicity of CD8+ T cells compared with the control.
To recapitulate the effect of tumoral NAC-1 on the cytotoxicity of CD8+ T cells in vivo, we inoculated C57BL/6 homologous mice subcutaneously with MC-38-OVA colon cancer cells transfected with NAC-1-shRNA or control shRNA. CD8+ T cells isolated from the spleen of OT-I mice that can recognize OVA were then injected into the mouse subcutaneously. Figure 1B shows that the growth of tumors with depletion of NAC-1 was slower than that of NAC-1-expressing tumors in the absence or presence of CTLs; cotreatment with CTLs and cobimetinib, a small molecule inhibitor of MEK1, further inhibited tumor growth in mice.
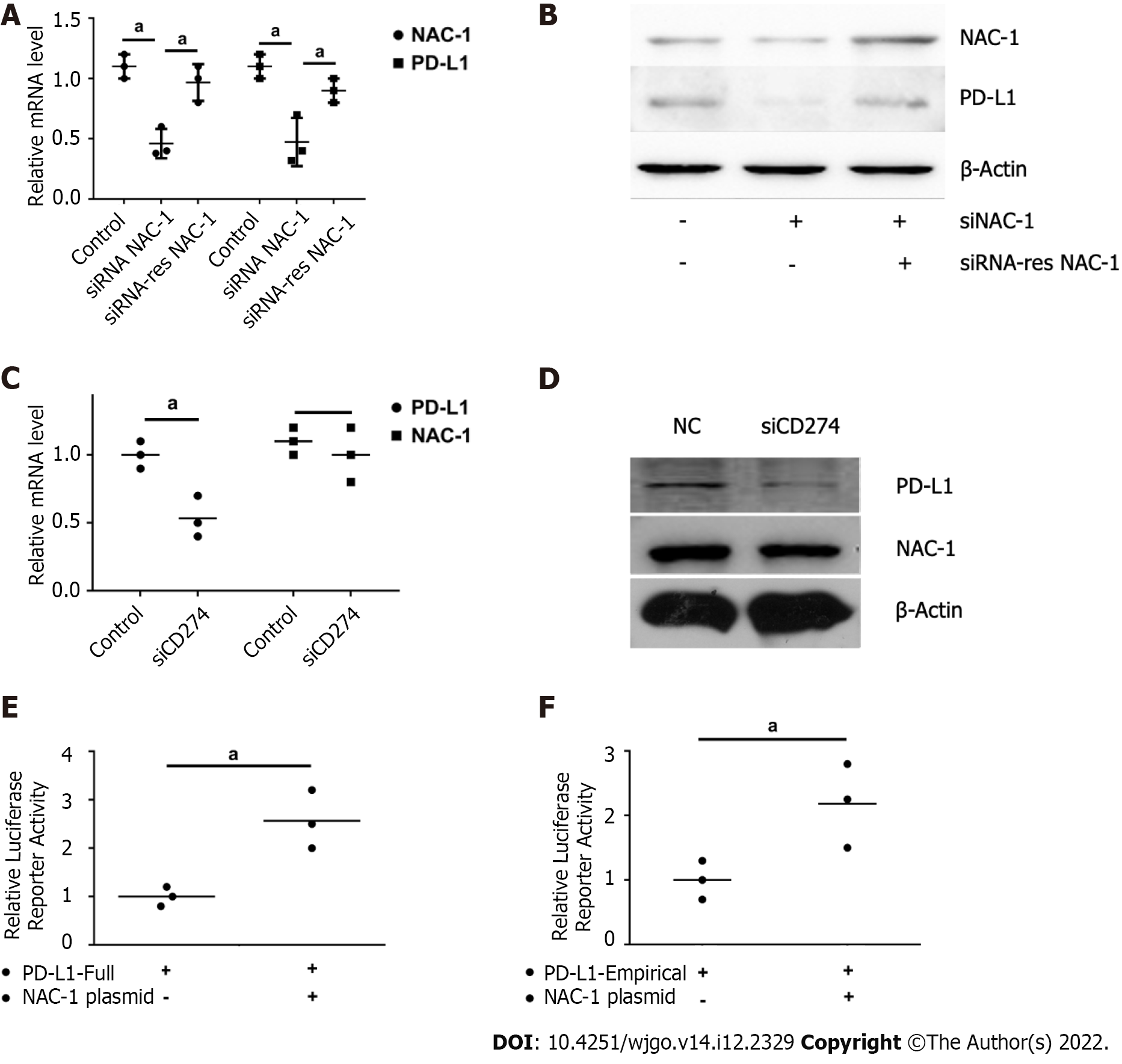
To explore how NAC-1 affects the tumor response to CTLs, we next determined the effect of NAC-1 on the expression of PD-L1, an immune checkpoint protein that inhibits the killing effect of CTLs. We found that knockdown of NAC-1 reduced the expression of PD-L1 at both the transcriptional and translational levels, as evidenced by the decreased mRNA and protein levels of PD-L1 (Figure 2A and B). To validate the effect of NAC-1 on PD-L1, we assessed whether the RNAi-resistant NAC-1 expression plasmid could rescue the effect of siRNA on PD-L1 expression. Figure 2C and D shows that transfection of RNAi-resistant cells blocked the silencing effect of NAC-1 siRNA. These results demonstrate that NAC-1 has a positive role in regulating the expression of PD-L1. To explore the mechanism by which NAC-1 regulates the expression of PD-L1, we constructed a luciferase plasmid containing the full PD-L1 promoter region and the transcription factor binding segment. We showed that transient expression of the NAC-1 plasmid in colon cancer cells increased the promoter activity of PD-L1 (Figure 2E and F), suggesting that NAC-1 promotes PD-L1 expression at the transcriptional level.
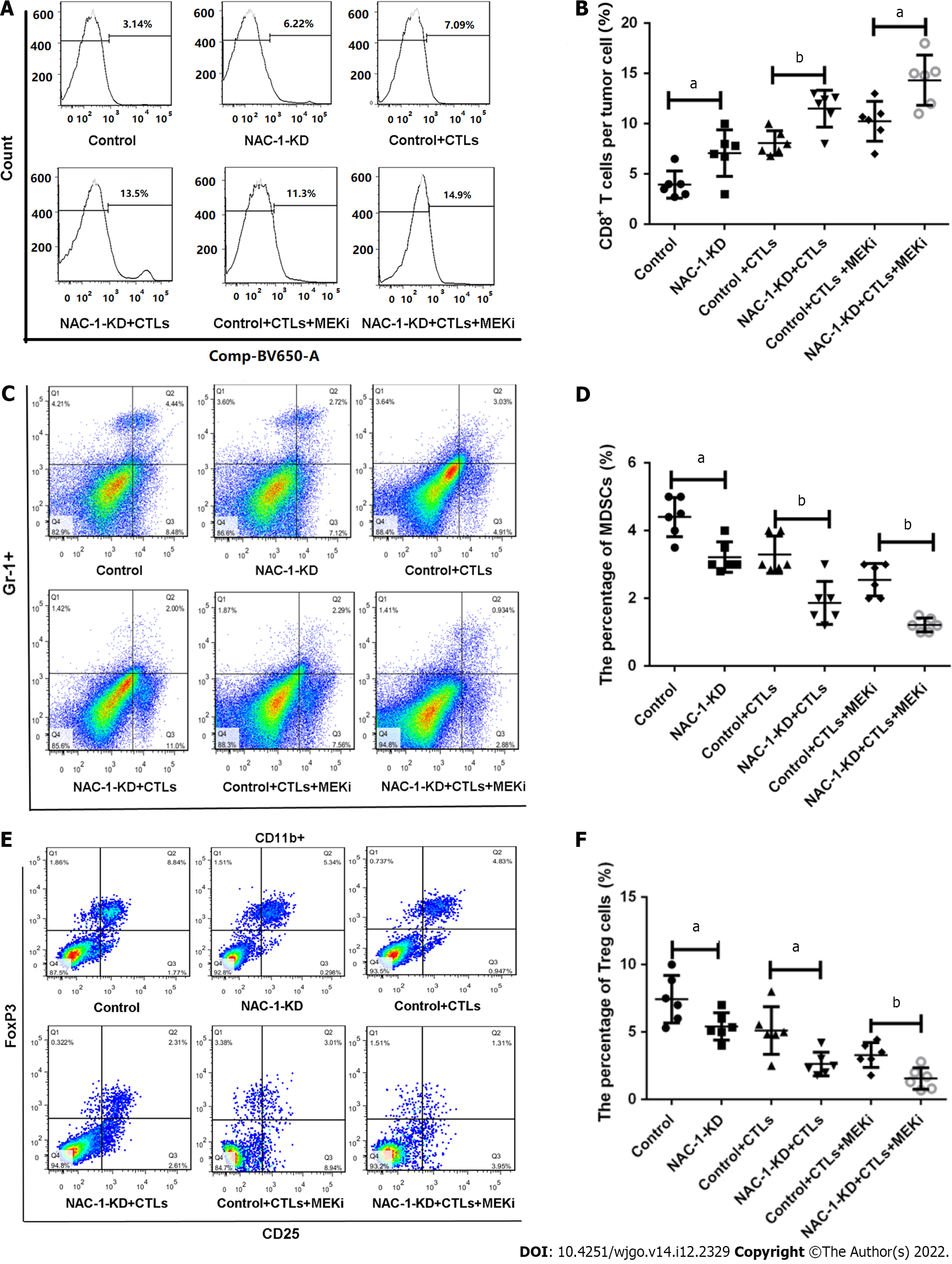
Tumoral expression of NAC-1 also affects immune cells in the tumor microenvironment (TME) of colon cancer. Using flow cytometry, we analyzed the proportions of CD8+ T cells, MDSCs, a group of heterogeneous cells derived from bone marrow that have the ability to suppress CD8+ T-cell function, and Tregs, a subpopulation of T cells that can exert a negative immunomodulatory effect by directly contacting target cells or secreting TGF-β/IL-10 in tumor tissues. Our results showed that in the tumors subjected to depletion of NAC-1, CD8+ T cells were significantly increased (Figure 3A and B), whereas MDSCs were significantly decreased, as evidenced by reductions in Gr-1+ CD11b+ cells (Figure 3C and D), when treated with CTLs and MEK inhibitor. Tregs were also reduced in NAC-depleted tumor tissues, as determined by significant decreases in CD4+ CD25+ Foxp3+ cells (Figure 3E and F). These results suggest that the expression of NAC-1 in colon cancer may suppress antitumor immunity via its effects on immune cells in the TME.
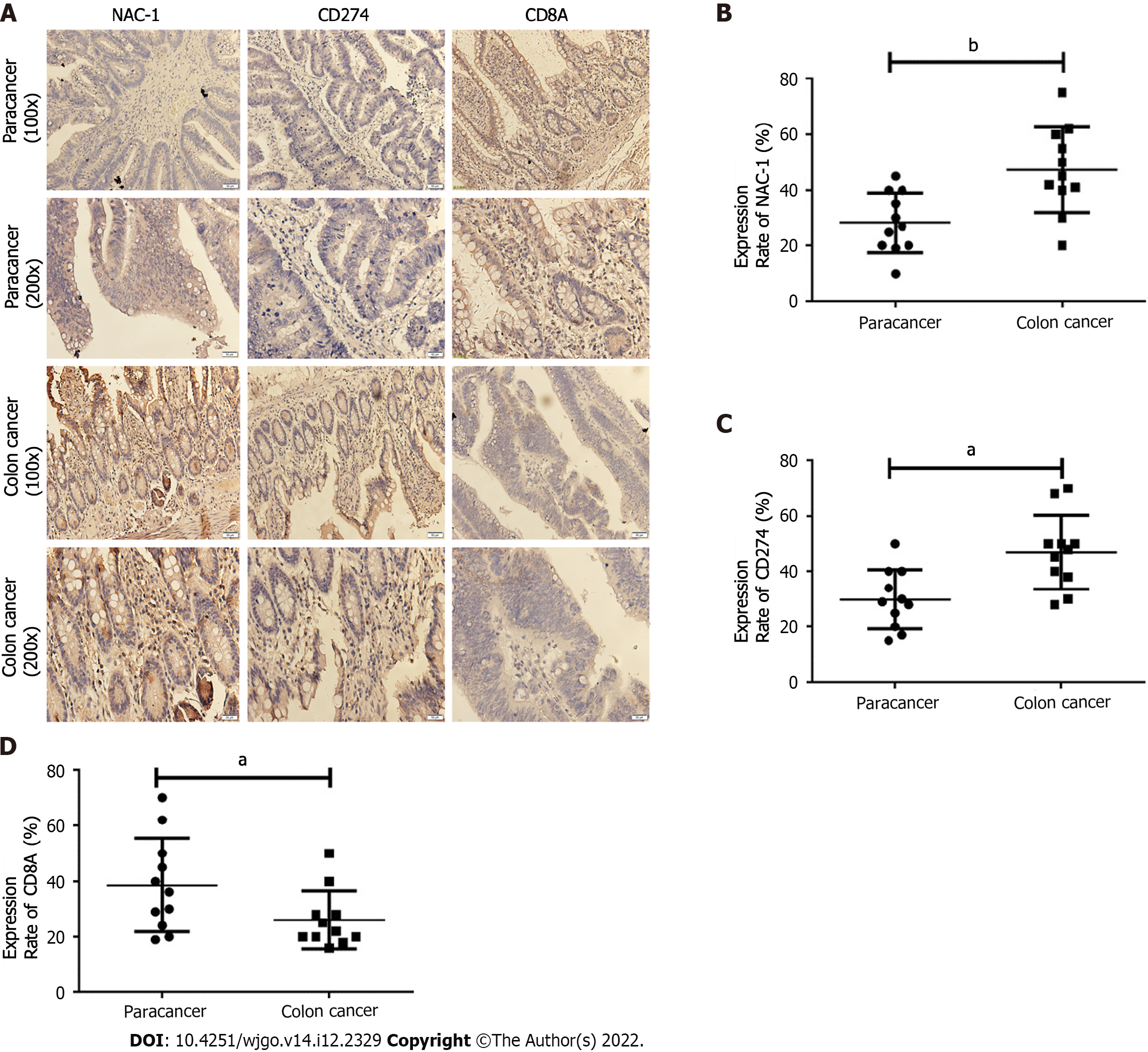
To investigate the clinical implications of tumor NAC-1 in immunotherapy, we analyzed the correlations between NAC-1, PD-L1 and CD8 T cells using paraffin embedding and serial sectioning of tumor tissues and adjacent tissues from 20 colon cancer patients. Immunohistochemical staining was performed, and Image-Pro Plus software was used to quantitatively analyze the integrated optical density (IOD) of NAC-1- and PD-L1-positive areas. The IOD of the positive area was divided by the IOD of the entire area to obtain the positive protein expression rate. Taking the average of the positive expression rates of NAC-1 and PD-L1 proteins, a 0.00-0.25 positive rate is expressed as +, a 0.25-0.50 positive rate is expressed as + + , and a positive rate above 0.50 is expressed as + + +[9]. The results showed that compared with adjacent tissues, the expression of NAC-1 and PD-L1 in colon cancer tissues was increased, and the expression of CD8 was decreased (P < 0.05) (Figure 4). These results suggest that the expression of NAC-1 in colon cancer may be further studied as a potential predictive biomarker for immunotherapy.
In this study, we showed that knockdown of NAC-1 in colon cancer cells can enhance the cytocidal activity of CD8+ T cells and reduce the expression of PD-L1. The combination of DR8+ T cells with a MEK inhibitor further increases the antitumor immune response. In a colon cancer animal model, we showed that colon cancer deficient in NAC-1 was more sensitive to CTLs. This might be associated with the decreases in PD-L1 and suppressive immune cells in the TME. We further demonstrated that NAC-1 modulates PD-L1 expression at the transcriptional level.
Nucleus accumbens-associated protein 1 (NACC1) is a potential cancer-related gene that can regulate the expression of the cell transcription factor NAC-1. The expression of NAC-1 is elevated in colon cancer and other malignant tumors. NAC-1 is a transcription factor, a member of the BTB/POZ family, and is closely related to tumor occurrence, development, proliferation, and apoptosis[10]. NAC-1 modulates autoimmunity by suppressing regulatory T cell–mediated tolerance[11].
Tumor immunity is a current research hotspot. Tumor immunotherapy that targets immune checkpoints has attracted increasing attention. Immune checkpoints are molecules that play a protective function in the body’s immune system. The activation of T lymphocytes requires activation signals regulated by costimulatory molecules. At the same time, there are coinhibitory molecules on the surface of T cells. After they bind to the corresponding ligands, they can inhibit the activation and function of T cells. These coinhibitory molecules are called immune checkpoints[12]. Various tumor cells use this feature to escape the body’s immune monitoring, thereby promoting the proliferation of tumor cells. Immune checkpoints include CTLA-4, PD-1/PD-L1, LMTK3, Fibrinogen-like protein 1 (FGL1)[13,14].
Previous research found that NAC-1 can affect the tumor microenvironment, but there is no research regarding colon cancer. We used colon cancer cell and CD8+ T-cell coculture experiments. The results suggest that NAC-1 knockdown can enhance the toxicity of CD8+ T cells to colon cancer cells and further indicate that NAC-1 may affect the body’s tumor immunity. How does NAC-1 affect the body’s tumor immunity? The immune checkpoint is a very important molecule in tumor immunity. An increasing number of immune checkpoint inhibitors are being used in clinical research, including PD-1 antibodies and PD-L1 antibodies, in the treatment of colon cancer. Targeting PD-L1 is related to a significant clinical response in many cancer patients[15]. Therefore, does NAC-1 affect immune checkpoints, especially PD-L1, which has great clinical application value? The results of the study found that NAC-1 can positively regulate the expression of PD-L1 and may increase its expression by promoting the transcription of the classic transcription factor binding segment of PD-L1.
We found at the cellular level that NAC-1 can inhibit the toxicity of CTLs to colon cancer by upregulating the immune checkpoint PD-L1. Is there a similar function in the mouse animal model? We constructed a subcutaneous tumor model with NAC-1 knockdown and found that NAC-1 can promote tumor growth and that CD8+ T cells can exert a toxic effect to kill tumor cells. NAC-1 may downregulate the number of CD8+ T cells and positively regulate the number of MDSCs and Treg cells, thereby suppressing tumor immunity. MDSCs are defined by their T cell immunosuppressive functions[15]. In addition, NAC-1 can inhibit the killing effect of CD8+ T cells and MEKi on colon cancer.
At the tissue level, we explored the relationship between NAC-1, PD-L1, and CD8 through immunohistochemical detection of tumor tissues in colon cancer patients. Compared with adjacent tissues, the expression of NAC-1 and PD-L1 in colon cancer tissues increased, and the expression of CD8 decreased. The expression of NAC-1 and PD-L1 showed a positive correlation, and the expression of NAC-1 and CD8 showed a negative correlation. This further supports the conclusion that "NAC-1 promotes the expression of PD-L1 and reduces the toxicity of CD8+ T cells and chemotherapeutic drugs to colon cancer".
In this project, we studied the impact of NAC-1 on the tumor microenvironment of colon cancer, explored its mechanism, explored its relationship with immune checkpoints, and further revealed its mechanisms of colon cancer regulation. This study provides a theoretical basis for the occurrence and development of colon cancer and tumor immunotherapy.
Nucleus accumbens-1 (NAC-1) is highly expressed in a variety of tumors, and is closely associated with tumor recurrence, metastasis, and invasion.
We determined whether and how NAC-1 affects antitumor immunity in colon cancer.
To determine whether and how NAC-1 affects tumor microenvironment in colon cancer.
NAC-1-siRNA, cytocidal test, quantitative real-time polymerase chain reaction, and Western blotting, a double luciferase reporter assay were used.
Knockdown of NAC-1 expression in colon cancer cells significantly enhanced the cytocidal effect of CD8+ T cells in cell culture experiments. The sensitizing effect of NAC-1 knockdown on the antitumor action of cytotoxic CD8+ T cells was recapitulated in a colon cancer xenograft animal model. Furth
Tumor expression of NAC-1 is a negative determinant of immunotherapy.
Our study suggests that targeting tumoral NAC-1 may be explored as a potential therapeutic strategy to enhance cancer immunotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bondre MF, Oman; Kirkik D, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman RJ, Wang TL, Shih IeM. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2006;103:18739-18744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Nakayama K, Nakayama N, Miyazaki K. Development of a novel ovarian cancer molecular target therapy against cancer-related transcriptional factor, NAC1. J Obstet Gynaecol Res. 2013;39:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Ishikawa M, Nakayama K, Yeasmin S, Katagiri A, Iida K, Nakayama N, Miyazaki K. NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int J Oncol. 2010;36:1097-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Yeasmin S, Nakayama K, Ishibashi M, Katagiri A, Iida K, Purwana IN, Nakayama N, Miyazaki K. Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin Cancer Res. 2008;14:1686-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Cheng Y, Ren X, Hori T, Huber-Keener KJ, Zhang L, Yap KL, Liu D, Shantz L, Qin ZH, Zhang S, Wang J, Wang HG, Shih IeM, Yang JM. Dysfunction of nucleus accumbens-1 activates cellular senescence and inhibits tumor cell proliferation and oncogenesis. Cancer Res. 2012;72:4262-4275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon reguLatory factor-1 is prerequisite to the constitutive expression and IFN-gamma induced upregulation of B7-H1 (CD274). FEBS Lett. 2006;580:755-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 369] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Al-Batran SE, Rafiyan MR, Atmaca A, Neumann A, Karbach J, Bender A, Weidmann E, Altmannsberger HM, Knuth A, Jäger E. Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res. 2005;65:3937-3941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Nakayama K, Rahman MT, Rahman M, Yeasmin S, Ishikawa M, Katagiri A, Iida K, Nakayama N, Miyazaki K. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol Oncol. 2010;119:469-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Yang JM, Ren Y, Kumar A, Xiong X, Das JK, Peng HY, Wang L, Ren X, Zhang Y, Ji C, Cheng Y, Zhang L, Alaniz RC, de Figueiredo P, Fang D, Zhou H, Liu X, Wang J, Song J. NAC1 modulates autoimmunity by suppressing regulatory T cell-mediated tolerance. Sci Adv. 2022;8:eabo0183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Drake CG, Lipson EJ, Brahmer JR. Breathing new Life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | Qian W, Zhao M, Wang R, Li H. Fibrinogen-like protein 1 (FGL1): the next immune checkpoint target. J Hematol Oncol. 2021;14:147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Sacher AG, Gandhi L. Biomarkers for the Clinical Use of PD-1/PD-L1 Inhibitors in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol. 2016;2:1217-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 15. | Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021;12:731798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |