Published online Aug 15, 2021. doi: 10.4251/wjgo.v13.i8.758
Peer-review started: January 22, 2021
First decision: February 24, 2021
Revised: February 27, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: August 15, 2021
Gastrointestinal (GI) cancer remains one of the most prevalent cancers in the world. The occurrence and progression of GI cancer involve multiple events. Metabolic reprogramming is one of the hallmarks of cancer and is intricately related to tumorigenesis. Many metabolic genes are involved in the occurrence and development of GI cancer. Research approaches combining tumor genomics and metabolomics are more likely to provide deeper insights into this field. In this paper, we review the roles of metabolism-associated genes, especially those involved in the regulation pathways, in the occurrence and progression of GI cancer. We provide the latest progress and future prospect into the different molecular mechanisms of metabolism-associated genes involved in the occurrence and development of GI cancer.
Core Tip: Metabolic reprogramming is one of the hallmarks of cancer and is intricately related to tumorigenesis. Many metabolic genes are involved in the occurrence and development of gastrointestinal (GI) cancer. This state-of-the-art review comprehensively describes the latest progress and prospects into the different molecular mechanisms of metabolism-associated genes involved in the occurrence and development of GI cancer.
- Citation: Miao YD, Mu LJ, Mi DH. Metabolism-associated genes in occurrence and development of gastrointestinal cancer: Latest progress and future prospect. World J Gastrointest Oncol 2021; 13(8): 758-771
- URL: https://www.wjgnet.com/1948-5204/full/v13/i8/758.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i8.758
Since the first suggestion that patients with gastric cancer (GC) have metabolic dysfunction and the metabolism study of patients with gastrointestinal (GI) tumors, nearly 80 years have passed[1-3]; however, studies on the role of metabolic processes and associated genes in the occurrence and development of GI tumors and the signal transduction pathways related to regulation have been ongoing. With the develop
The development of colorectal cancer (CRC) has long been known to involve a series of cascading events (i.e., the adenoma-carcinoma sequence): Transformation from normal colonic epithelium to adenoma intermediately and finally into adenocarcinoma[15-17]. This process involves genetic, lifestyle, and environmental risk factors[18]. The continuous accumulation of mutations in the epidermal growth factor receptor (EGFR), P53, Wnt, and transforming growth factor (TGF)-β signaling pathways results in the occurrence and development of CRC. Early APC gene mutations occur in 70% of colorectal adenomas[19]. Bell et al[20] first found that polyadenylation polymorphism in the NAT1 gene increases CRC risk[20]. Recently, the application of various “omics” techniques has opened up a new field to study GI cancer mechanisms.
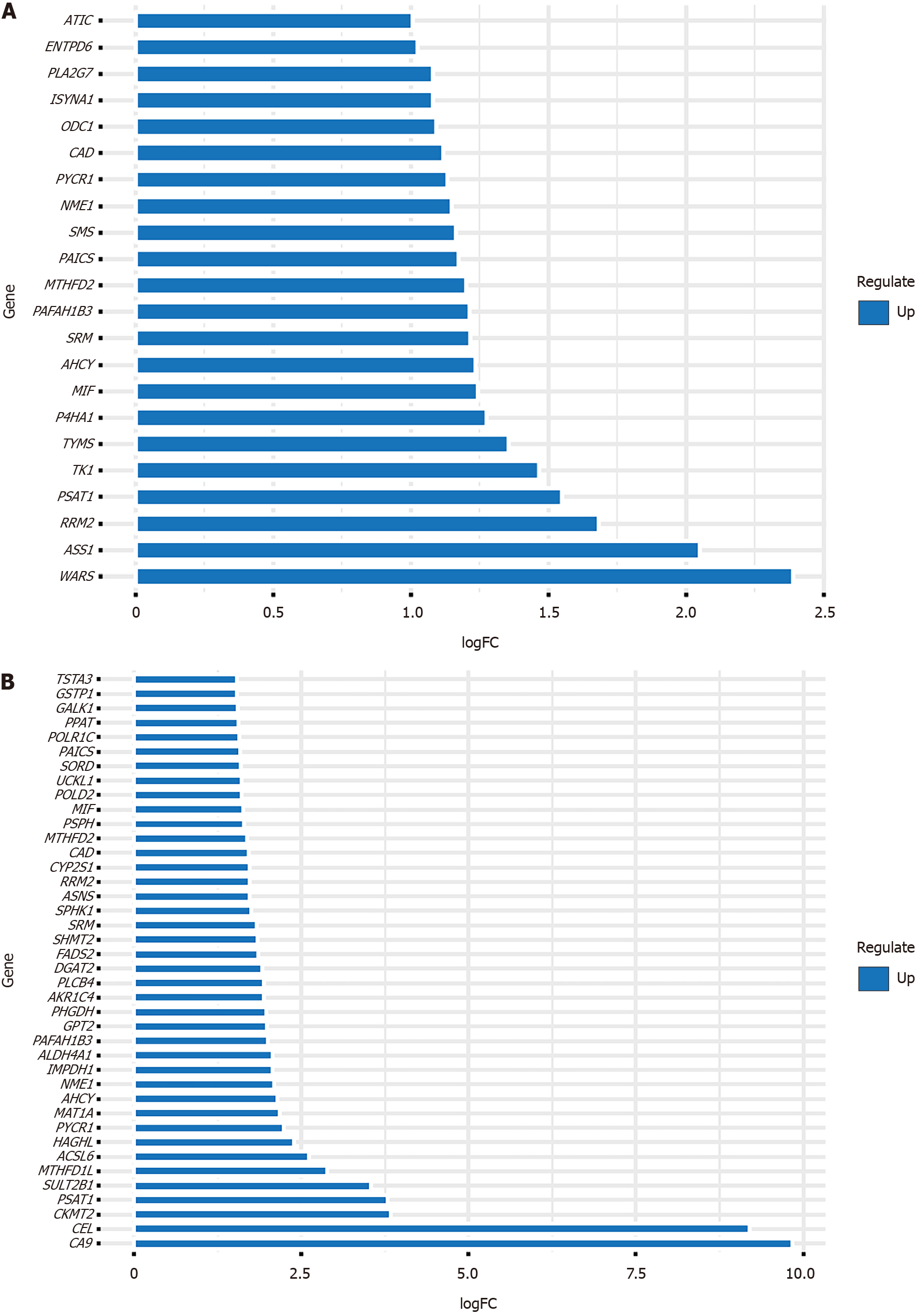
The recombination of cell metabolism represents the basic characteristics of most cancer cells. Studies in the past decades have shown that this metabolic reprogramming is an active process controlled by oncogenes and tumor suppressor genes. It provides energy for cancer cells and reduces equivalent substances and biosynthetic precursors[21]. Cancer cells use most core metabolic pathways, including glutamine, glucose, serine/glycine, amino acid, and lipid metabolism, to maintain their high cell division rate[22]. Extensive research has also shown that metabolic reprogramming is one of the hallmarks of cancer[23] and is intricately related to tumorigenesis[24,25] and cancer immune escape[26,27]. Metabolic remodeling can lead to epigenetic changes in tumors that affect cancer cell proliferation, differentiation, and therapy[7]. Metabolomics can provide biomarkers that can be used to identify early GC, thereby potentially meeting important clinical needs[28]. Research methods combining tumor genomics and metabolomics are more likely to provide deeper insights into this field. We obtained metabolism-related genes from the Molecular Signatures Database, and differentially expressed metabolism-associated genes in GC and CRC of The Cancer Genome Atlas database were obtained by bioinformatics methods. In this review, we mainly summarize the latest progress on how these differentially expressed metabolism-related genes affect the initiation and progression of GI tumors and discuss the possible mechanisms behind these changes. The up-regulated metabolism-related genes in GC [log fold change (FC) > 1 and false discovery rate (FDR) < 0.05, Table 1] and CRC (Log FC > 1.5 and FDR < 0.05, Table 2) are shown in Figure 1.
| Gene | Con mean | Treat mean | Log FC | P value | FDR |
| WARS | 6.463316413 | 33.79933632 | 2.386648392 | 5.39E-08 | 2.60E-07 |
| ASS1 | 10.23918101 | 42.338062 | 2.047854908 | 5.71E-06 | 1.81E-05 |
| RRM2 | 4.193381289 | 13.45216754 | 1.681652732 | 7.79E-13 | 9.82E-12 |
| PSAT1 | 4.984197042 | 14.58683132 | 1.549233513 | 1.15E-07 | 5.23E-07 |
| TK1 | 9.404335583 | 25.94196676 | 1.463889934 | 1.90E-08 | 9.60E-08 |
| TYMS | 5.252256309 | 13.43148698 | 1.354609806 | 8.20E-15 | 1.85E-13 |
| P4HA1 | 5.096854107 | 12.3340109 | 1.274963063 | 6.70E-16 | 3.11E-14 |
| MIF | 19.56229893 | 46.31616492 | 1.243439876 | 3.49E-07 | 1.44E-06 |
| AHCY | 14.87041149 | 34.97985067 | 1.234079561 | 2.23E-10 | 1.71E-09 |
| SRM | 15.88621779 | 36.86038375 | 1.214295404 | 2.56E-11 | 2.26E-10 |
| PAFAH1B3 | 7.126421918 | 16.5295421 | 1.213796955 | 4.78E-11 | 4.04E-10 |
| MTHFD2 | 5.447936287 | 12.51038205 | 1.199344112 | 5.74E-16 | 2.85E-14 |
| PAICS | 6.543206722 | 14.74057996 | 1.171723532 | 9.38E-17 | 6.98E-15 |
| SMS | 11.67400201 | 26.12997113 | 1.162406309 | 5.45E-15 | 1.35E-13 |
| NME1 | 6.553254278 | 14.50996199 | 1.146760323 | 5.99E-13 | 7.81E-12 |
| PYCR1 | 9.159388726 | 20.07643062 | 1.132179571 | 5.23E-06 | 1.68E-05 |
| CAD | 4.618217342 | 10.01148028 | 1.116247328 | 3.37E-17 | 3.14E-15 |
| ODC1 | 14.89150939 | 31.71876297 | 1.090846515 | 1.34E-06 | 4.97E-06 |
| ISYNA1 | 4.338447403 | 9.180413895 | 1.08138036 | 9.64E-06 | 2.92E-05 |
| PLA2G7 | 4.093119979 | 8.652040283 | 1.079839426 | 4.65E-13 | 6.29E-12 |
| ENTPD6 | 7.372506744 | 15.00708695 | 1.025416818 | 5.98E-10 | 4.20E-09 |
| ATIC | 8.653344031 | 17.36986424 | 1.005256812 | 1.25E-16 | 8.48E-15 |
| Gene | Con mean | Treat mean | Log FC | P value | FDR |
| CA9 | 0.028995 | 26.60655 | 9.841744 | 1.03E-19 | 3.91E-19 |
| CEL | 0.024311 | 14.30974 | 9.201191 | 3.23E-15 | 8.41E-15 |
| CKMT2 | 0.42259 | 6.021372 | 3.83276 | 5.80E-05 | 8.00E-05 |
| PSAT1 | 1.864971 | 25.85166 | 3.793031 | 4.00E-28 | 5.61E-27 |
| SULT2B1 | 0.785605 | 9.164399 | 3.544165 | 1.61E-27 | 1.83E-26 |
| MTHFD1L | 1.294767 | 9.556751 | 2.883827 | 2.70E-32 | 4.32E-30 |
| ACSL6 | 0.319928 | 1.964782 | 2.618552 | 2.94E-14 | 7.16E-14 |
| HAGHL | 0.505906 | 2.65869 | 2.393775 | 4.07E-29 | 8.58E-28 |
| PYCR1 | 8.324112 | 39.39321 | 2.242579 | 8.73E-29 | 1.49E-27 |
| MAT1A | 0.370363 | 1.67466 | 2.176855 | 5.78E-11 | 1.14E-10 |
| AHCY | 26.97523 | 118.8808 | 2.139808 | 3.53E-27 | 3.67E-26 |
| NME1 | 4.935536 | 21.03672 | 2.091631 | 6.46E-28 | 8.47E-27 |
| IMPDH1 | 5.830836 | 24.51912 | 2.072133 | 3.42E-27 | 3.60E-26 |
| ALDH4A1 | 1.84131 | 7.716947 | 2.067297 | 2.97E-23 | 1.57E-22 |
| PAFAH1B3 | 7.889409 | 31.58688 | 2.001336 | 5.77E-28 | 7.69E-27 |
| GPT2 | 2.992286 | 11.89982 | 1.99162 | 3.29E-27 | 3.51E-26 |
| PHGDH | 2.694106 | 10.59518 | 1.97553 | 5.21E-10 | 9.66E-10 |
| AKR1C4 | 0.277697 | 1.064175 | 1.938153 | 1.62E-27 | 1.83E-26 |
| PLCB4 | 5.394957 | 20.64288 | 1.935961 | 7.02E-08 | 1.14E-07 |
| DGAT2 | 1.148785 | 4.32325 | 1.912007 | 3.09E-25 | 2.11E-24 |
| FADS2 | 1.408937 | 5.08608 | 1.851947 | 7.10E-08 | 1.15E-07 |
| SHMT2 | 9.347261 | 33.69678 | 1.849995 | 1.28E-29 | 3.95E-28 |
| SRM | 12.16342 | 43.22303 | 1.829251 | 2.61E-25 | 1.80E-24 |
| SPHK1 | 0.955052 | 3.20702 | 1.747582 | 1.51E-14 | 3.74E-14 |
| ASNS | 3.806487 | 12.6208 | 1.729271 | 7.52E-26 | 5.73E-25 |
| RRM2 | 6.439151 | 21.27979 | 1.724542 | 6.98E-24 | 3.93E-23 |
| CYP2S1 | 18.50704 | 61.13654 | 1.723961 | 4.76E-19 | 1.75E-18 |
| CAD | 3.562422 | 11.69219 | 1.714615 | 5.30E-29 | 1.01E-27 |
| MTHFD2 | 5.47575 | 17.67988 | 1.69098 | 2.52E-26 | 2.08E-25 |
| PSPH | 2.943986 | 9.195503 | 1.643157 | 1.03E-26 | 9.54E-26 |
| MIF | 15.24002 | 47.29712 | 1.633887 | 1.32E-19 | 4.97E-19 |
| POLD2 | 12.58199 | 38.52394 | 1.614396 | 4.91E-28 | 6.66E-27 |
| UCKL1 | 6.50296 | 19.90328 | 1.613838 | 2.26E-25 | 1.58E-24 |
| SORD | 2.751114 | 8.302656 | 1.593557 | 9.78E-27 | 9.20E-26 |
| PAICS | 8.498374 | 25.43873 | 1.581768 | 7.60E-27 | 7.33E-26 |
| POLR1C | 4.549977 | 13.53895 | 1.573184 | 1.77E-29 | 4.57E-28 |
| PPAT | 1.684581 | 4.984275 | 1.564994 | 4.32E-28 | 5.95E-27 |
| GALK1 | 3.415609 | 10.02022 | 1.5527 | 5.67E-24 | 3.29E-23 |
| GSTP1 | 100.8606 | 293.7334 | 1.542144 | 1.14E-22 | 5.58E-22 |
| TSTA3 | 16.08544 | 46.68479 | 1.537197 | 1.03E-20 | 4.26E-20 |
Up to now, many metabolic genes involved in the occurrence and development of GC have been confirmed. Tsai et al[29] have reported that ASS1, a rate-limited enzyme in arginine biosynthesis, promotes GC progression by enhancing the aggressiveness caused by the accumulation of active β-catenin[29]. The most important cause of sporadic distal GC is Helicobacter pylori (H. pylori) infection[30]. During the carcino
The occurrence and progression of GC involve multiple events, including the activation or deactivation of multiple signal transduction pathways, such as PI3K/Akt signaling pathway[40], Hedgehog signaling pathway[41], EphA2-to-YAP pathway[42], Wnt/β-catenin pathway[43,44], mitogen-activated protein kinase (MAPK) signaling pathway[45], HGF/MET pathway[46], AKT1/mTOR pathway[47], etc. As mentioned in the beginning, metabolic genes are closely related to the occurrence and development of GC. For example, ASS1, a signaling pathway involved in the regulation of metabolic genes, promotes GC invasion and progression mainly through the regulation of autophagy[29]. TYMS5 is involved in the fluorouracil conversion pathway,which is associated with chemoresistance and treatment failure by 5-FU in GC[48]. Kong et al[49] reported that MIF is associated with the p53 pathway in GC[49]. PYCR1 expression was significantly correlated with PI3K/Akt axis in GC[38]. Previous research found that overexpressed RRM2 in GC cells promotes their invasiveness via the AKT/nuclear factor-kappaB (NF-κB) signaling pathway[50].
The development of CRC has long been known to involve a series of cascading events, including the metabolic process. Therefore, metabolic genes play a very crucial role in the occurrence and development of CRC. Previous reports indicate that CA9 expression was up-regulated in ulcerative colitis-associated CRC[51]. PSAT1 is overexpressed in colon tumors, promotes cell growth, and enhances chemoresistance of colon cancer cells[52]. SULT2B1, an estrogen metabolic pathway gene, was significantly highly expressed in colorectal tumor tissues and related to susceptibility to and survival of CRC[53]. Agarwal et al[54] had reported that MTHFD1L, a folate cycle enzyme, is involved in the progression of CRC[54].
Emerging evidence suggests that abnormal alternative splicing (AS) is an ordinary event in the development and progression of cancer. The AS event of ALDH4A1 was discovered in carcinogenesis and prognosis of CRC[55]. Notably, GPT2 is involved in the glycolysis activation to drive the application of glutamine as a carbon source for the abnormal tricarboxylic acid cycle in colon cancer cells. The Warburg effect supports oncogenesis by coupling pyruvate production and glutamine catabolism mediated by GPT2[56]. Mutation of the oncogene PIK3CA reprogrammed glutamine metabolism in CRC[57].
Further studies have shown that GPT2-mediated glutamine utilization enhancement is a fundamental metabolic feature of colorectal signet-ring cell carcinoma[58]. PHGDH catalyzes the first committed step to synthesize glucose-derived serine catalyzed by the phosphate serine pathway related to colon cancer[59]. FADS2 is overexpressed in CRC and promotes the proliferation of CRC cells and the growth of xenografts in vivo and in vitro by promoting the metabolism of PGE2 (a carcinogenic molecule associated with colorectal carcinogenesis)[60]. Macrophage ABHD5 promotes the growth of CRC by inhibiting the production of spermidine by SRM[61]. SphK1 overexpression and activation facilitate and enhance the development and progression of colon cancer[62] and are associated with the survival of CRC patients[63]. RRM2 is a ribonucleotide reductase small subunit, and its high expression can induce cancer and promote tumor growth and invasion. The transcription factor E2F1 regulating the transactivation of RRM2 can promote the proliferation, migration, invasion, and metastasis of CRC cells[64].
Cyp enzymes in digestive tract epithelial cells play an essential role in the oxidative metabolism of various exogenous substances containing carcinogens and endogenous compounds. Knockdown of CYP2S1, a CYP family member, promotes cell proliferation and xenograft tumor growth by enhancing the level of endogenous PGE2[65]. MTHFD2 encodes a nuclear-encoded mitochondrial bifunctional enzyme with methylenetetrahydrofolate dehydrogenase and methyltetrahydrofolate cyclohydrolase activities. Overexpression of MTHFD2 can enhance the proliferation and migration of CRC cells, promote the cell cycle, and inhibit apoptosis[66,67]. Cytokine MIF, a lymphokine involved in cell-mediated immunity, immunoregulation, and inflammation, is expressed throughout the human GI tract. MIF expression is enhanced in sporadic colorectal adenomas, and exogenous MIF promotes the tumorigenic behavior of epithelial cells in vitro. MIF also promoted intestinal tumor occurrence (primarily through angiogenesis) in ApcMin/+ mice[68]. PSPH, which belongs to a subfamily of the phosphotransferases, regulates the synthesis of serine and glycine in cells and promotes tumor growth. PSPH is overexpressed in most CRC cell lines and enhances the anticancer efficacy of 5-fluorouracil in CRC[69]. PPAT, an amino acid/nucleotide metabolism-related gene, is mutated in GC and CRC, acquires somatic mutations in MSH-H GCs and CRCs[70]. The phosphoribosylaminoimidazole carboxylase and PAICS were overexpressed in 70% of CRCs. Regardless of p53 and microsatellite status, increased PAIC expression is associated with proliferation, growth, invasion, and migration of CRC cells[71]. Glutathione S-transferase (GST) catalyzes the reaction between lipophilic and glutathione compounds with electrophilic centers, thereby neutralizing toxic compounds, exogenous substances, and oxidative stress products. Patients with wild-type GSTP1 had a significantly lower risk of TP53 mutations in CRC than patients with mutated genotypes[72]. GSTP1 is up-regulated in CRC tissue samples and facilitates the proliferation, invasion, and metastasis of CRC cells[73].
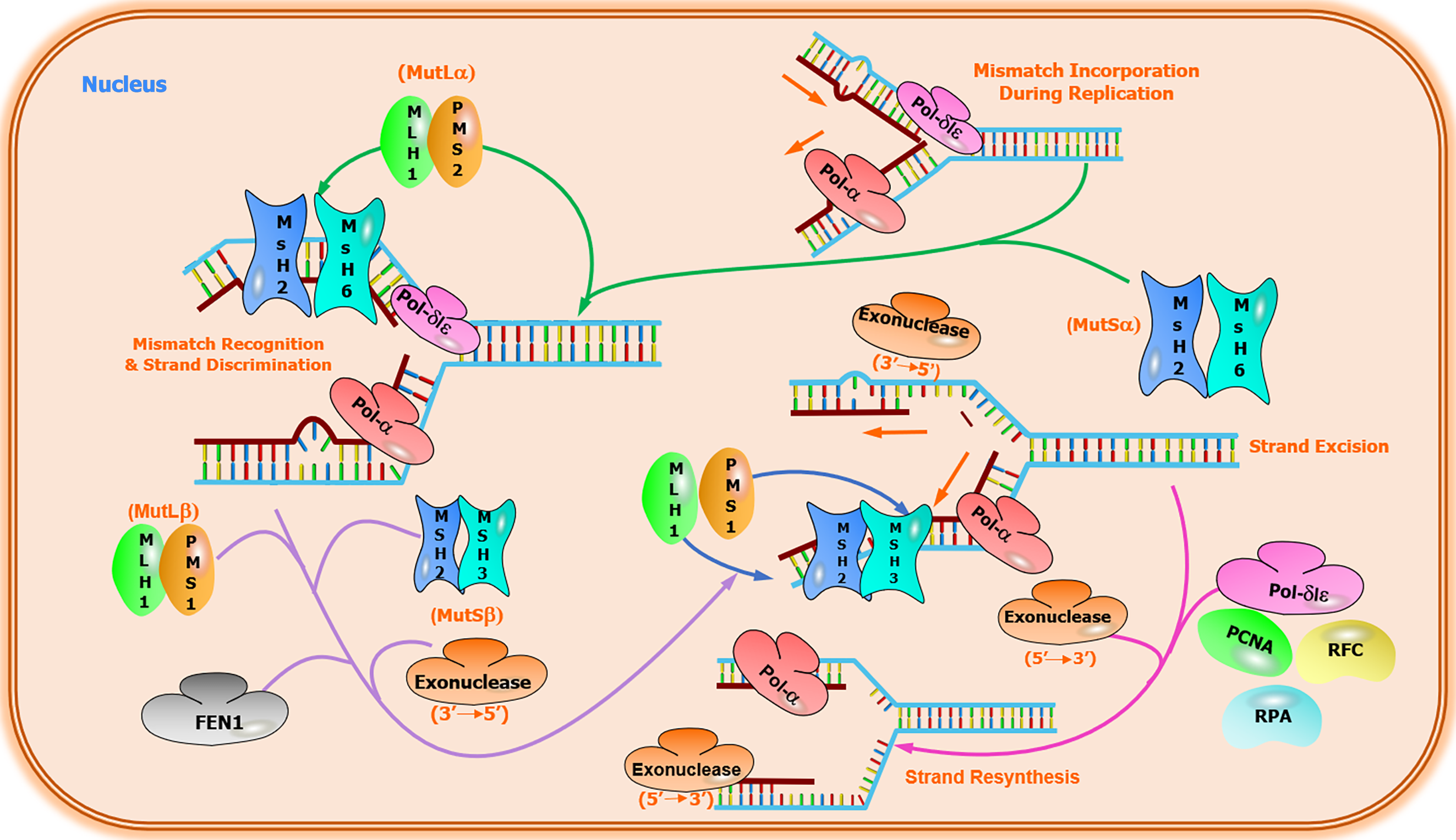
CRC is a heterogeneous disease that develops via the gradual accumulation of well-defined genetic and epigenetic alterations. CRC progression involves multiple genetic events accompanied by genomic instability and mutations [74]. The primary signal transduction pathways leading to somatic inheritance of sporadic CRC are as follows: (1) APC[75] and BRAF gene mutations cause traditional adenomas or serrated polyps, respectively[76,77]; (2) chromosomal instability (CIN) pathway[78]. CIN, observed in 65% to 70% of sporadic CRCs[79,80], is characterized by chromosome changes that include somatic copy number alterations caused by aneuploidy, insertions, amplifications, deletions, or loss of heterozygosity[81]. The Wnt pathway is activated in almost all CIN tumors, and APC mutations are found in about 80% of these tumors[82]; (3) serrated adenoma pathways[83-85]. Serrated polyps are thought to cause nearly 15% of CRCs through serrated neoplasia pathways[86]. The serrated pathway is a unique mechanism of CRC. A prominent feature of the serrated pathway is the activating V600E mutation in BRAF, a component of the MAPK pathway[87]. BRAF mutation occurs in most sessile serrated adenomas but rarely in conventional adenomas, which supports the view that the serrated pathway is an alternative pathway for CRC[88]. The MAPK pathway is located downstream of numerous growth factor receptors, including epidermal growth factor. The EGFR signaling pathway regulates proliferation, growth, and cellular differentiation in CRC cells[89]; and (4) microsatellite instability (MSI) pathway. Unlike the CIN pathway, characterized by changes in gene copy number, CRC can also develop through highly mutated pathways characterized by frequent somatic DNA base-pair mutations[81]. In sporadic CRC, mutations often occurred in the DNA mismatch repair (MMR) pathway (Figure 2). MSI is observed in nearly 15% of sporadic CRC cases. Besides, germline MMR mutations are related to Lynch syndrome, the most ordinary hereditary CRC form[90]. CRCs with MSI phenotype usually have high levels of methylation in the regulatory region of the entire genome, including CpG island methylation phenotype (CIMP)[81,91]. CIMP-hypermethylation is found in approximately 20% of CRCs, and this hypermethylation is most often associated with BRAF mutations and MLH1 hypermethylation, characteristics that describe a large proportion of MSI-H tumors[92,93].
Metabolism-related genes are also involved in several pathways that regulate the development of CRC. The mRNA level of Wnt signaling factor AXIN2 was sig
The occurrence and progression of GI cancer involve multiple events. Improving the overall survival rate of patients with GI cancer will depend on the inherent characteristics of different subtypes of GI tumors and the development of treatment strategies based on these differences. The further identification of GI tumor subtypes and the role of specific genes (including metabolism-associated genes) in the occurrence and development of GI tumors is an essential area of future research. When we clarify the impact of metabolism on GI cancer risk, we have to understand how diet, obesity, and sedentary behavior contribute to the development of GI cancer. The association of H. pylori, Clostridium pylori, and other GI microorganisms with the risk of GI cancer will lead to many studies of the influence of microbiota on GI epithelial cell transformation and cancer development. As we learn more about the pathogenesis of GI cancer and different types of GI tumors, new treatments and diagnostic approaches will emerge.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li X
| 1. | Ariel I, Pack GT, Rhoads CP. Metabolic studies in patients with cancer of the gastro-intestinal tract: VII-the influence of gastric surgery upon the chemical composition of the liver. Ann Surg. 1942;116:924-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Abels JC, Ariel IM, Rekers PF, Pack GT, Rhoads CP. Metabolic abnormalities of patients with cancer of the gastro-intestinal tract; review of recent studies. Arch Surg. 1943;46:844. [Cited in This Article: ] |
| 3. | Young NF, Abels JC, Homburger F, Collier V, Green J. Studies on carbohydrate metabolism in patients with gastric cancer. Defective hepatic glycogenesis; effects of adreno-cortical extract. J Clin Invest. 1948;27:760-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Sayaman RW, Saad M, Thorsson V, Hu D, Hendrickx W, Roelands J, Porta-Pardo E, Mokrab Y, Farshidfar F, Kirchhoff T, Sweis RF, Bathe OF, Heimann C, Campbell MJ, Stretch C, Huntsman S, Graff RE, Syed N, Radvanyi L, Shelley S, Wolf D, Marincola FM, Ceccarelli M, Galon J, Ziv E, Bedognetti D. Germline genetic contribution to the immune landscape of cancer. Immunity. 2021;54:367-386.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 6. | Etchegaray JP, Mostoslavsky R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol Cell. 2016;62:695-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nat Rev Cancer. 2016;16:694-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 8. | Phan AT, Goldrath AW, Glass CK. Metabolic and Epigenetic Coordination of T Cell and Macrophage Immunity. Immunity. 2017;46:714-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 9. | Camacho-Ordonez N, Ballestar E, Timmers HTM, Grimbacher B. What can clinical immunology learn from inborn errors of epigenetic regulators? J Allergy Clin Immunol. 2021;147:1602-1618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol. 2020;17:75-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 11. | Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19:151-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 12. | Tough DF, Rioja I, Modis LK, Prinjha RK. Epigenetic Regulation of T Cell Memory: Recalling Therapeutic Implications. Trends Immunol. 2020;41:29-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Seki T, Fujii G, Mori S, Tamaoki N, Shibuya M. Amplification of c-yes-1 proto-oncogene in a primary human gastric cancer. Jpn J Cancer Res. 1985;76:907-910. [PubMed] [Cited in This Article: ] |
| 14. | Tahara E, Yasui W, Taniyama K, Ochiai A, Yamamoto T, Nakajo S, Yamamoto M. Ha-ras oncogene product in human gastric carcinoma: correlation with invasiveness, metastasis or prognosis. Jpn J Cancer Res. 1986;77:517-522. [PubMed] [Cited in This Article: ] |
| 15. | Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451-457. [PubMed] [Cited in This Article: ] |
| 16. | Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1352] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Cooper HS, Patchefsky AS, Marks G. Adenomatous and carcinomatous changes within hyperplastic colonic epithelium. Dis Colon Rectum. 1979;22:152-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1965] [Cited by in F6Publishing: 2133] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 19. | Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3455] [Cited by in F6Publishing: 3301] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 20. | Bell DA, Stephens EA, Castranio T, Umbach DM, Watson M, Deakin M, Elder J, Hendrickse C, Duncan H, Strange RC. Polyadenylation polymorphism in the acetyltransferase 1 gene (NAT1) increases risk of colorectal cancer. Cancer Res. 1995;55:3537-3542. [PubMed] [Cited in This Article: ] |
| 21. | Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1112] [Cited by in F6Publishing: 1361] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 22. | Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3099] [Cited by in F6Publishing: 3426] [Article Influence: 428.3] [Reference Citation Analysis (0)] |
| 23. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 42945] [Article Influence: 3303.5] [Reference Citation Analysis (4)] |
| 24. | Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1998] [Cited by in F6Publishing: 2060] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 25. | Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA Jr, Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 682] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 26. | Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart LA, Oefner PJ, Andreesen R, Gottfried E, Kreutz MP. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184:1200-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 28. | Jayavelu ND, Bar NS. Metabolomic studies of human gastric cancer: review. World J Gastroenterol. 2014;20:8092-8101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Tsai CY, Chi HC, Chi LM, Yang HY, Tsai MM, Lee KF, Huang HW, Chou LF, Cheng AJ, Yang CW, Wang CS, Lin KH. Argininosuccinate synthetase 1 contributes to gastric cancer invasion and progression by modulating autophagy. FASEB J. 2018;32:2601-2614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Bornschein J, Selgrad M, Warnecke M, Kuester D, Wex T, Malfertheiner P. H. pylori infection is a key risk factor for proximal gastric cancer. Dig Dis Sci. 2010;55:3124-3131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1282] [Cited by in F6Publishing: 1367] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 32. | Kim N, Park WY, Kim JM, Park JH, Kim JS, Jung HC, Song IS. Gene expression of AGS cells stimulated with released proteins by Helicobacter pylori. J Gastroenterol Hepatol. 2008;23:643-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Shen R, Liu H, Wen J, Liu Z, Wang LE, Wang Q, Tan D, Ajani JA, Wei Q. Genetic polymorphisms in the microRNA binding-sites of the thymidylate synthase gene predict risk and survival in gastric cancer. Mol Carcinog. 2015;54:880-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Shitara K, Muro K, Ito S, Sawaki A, Tajika M, Kawai H, Yokota T, Takahari D, Shibata T, Ura T, Ito H, Hosono S, Kawase T, Watanabe M, Tajima K, Yatabe Y, Tanaka H, Matsuo K. Folate intake along with genetic polymorphisms in methylenetetrahydrofolate reductase and thymidylate synthase in patients with advanced gastric cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1311-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Tong D, Zhang J, Wang X, Li Q, Liu L, Lu A, Guo B, Yang J, Ni L, Qin H, Zhao L, Huang C. MiR-22, regulated by MeCP2, suppresses gastric cancer cell proliferation by inducing a deficiency in endogenous S-adenosylmethionine. Oncogenesis. 2020;9:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Huang N, Xu C, Deng L, Li X, Bian Z, Zhang Y, Long S, Chen Y, Zhen N, Li G, Sun F. PAICS contributes to gastric carcinogenesis and participates in DNA damage response by interacting with histone deacetylase 1/2. Cell Death Dis. 2020;11:507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Xiao S, Li S, Yuan Z, Zhou L. Pyrroline-5-carboxylate reductase 1 (PYCR1) upregulation contributes to gastric cancer progression and indicates poor survival outcome. Ann Transl Med. 2020;8:937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Cho LY, Yang JJ, Ko KP, Ma SH, Shin A, Choi BY, Kim HJ, Han DS, Song KS, Kim YS, Chang SH, Shin HR, Kang D, Yoo KY, Park SK. Gene polymorphisms in the ornithine decarboxylase-polyamine pathway modify gastric cancer risk by interaction with isoflavone concentrations. Gastric Cancer. 2015;18:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Huang Y, Zhang J, Hou L, Wang G, Liu H, Zhang R, Chen X, Zhu J. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36:194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 41. | Katoh Y, Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol Ther. 2005;4:1050-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Huang C, Yuan W, Lai C, Zhong S, Yang C, Wang R, Mao L, Chen Z. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int J Cancer. 2020;146:1937-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Liu W, Chen Y, Xie H, Guo Y, Ren D, Li Y, Jing X, Li D, Wang X, Zhao M, Zhu T, Wang Z, Wei X, Gao F, Liu S, Zhang Y, Yi F. TIPE1 suppresses invasion and migration through down-regulating Wnt/β-catenin pathway in gastric cancer. J Cell Mol Med. 2018;22:1103-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer. 2018;17:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 45. | Li C, Liu DR, Li GG, Wang HH, Li XW, Zhang W, Wu YL, Chen L. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21:6215-6228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 82] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Lordick F. Targeting the HGF/MET pathway in gastric cancer. Lancet Oncol. 2014;15:914-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z, Xu P, Sun G, Xu J, Lv J, Xu Z. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 48. | Biagioni A, Staderini F, Peri S, Versienti G, Schiavone N, Cianchi F, Papucci L, Magnelli L. 5-Fluorouracil Conversion Pathway Mutations in Gastric Cancer. Biology (Basel). 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Kong F, Deng X, Kong X, Du Y, Li L, Zhu H, Wang Y, Xie D, Guha S, Li Z, Guan M, Xie K. ZFPM2-AS1, a novel lncRNA, attenuates the p53 pathway and promotes gastric carcinogenesis by stabilizing MIF. Oncogene. 2018;37:5982-5996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Zhong Z, Cao Y, Yang S, Zhang S. Overexpression of RRM2 in gastric cancer cell promotes their invasiveness via AKT/NF-κB signaling pathway. Pharmazie. 2016;71:280-284. [PubMed] [Cited in This Article: ] |
| 51. | Nakada N, Mikami T, Horie K, Nagashio R, Sakurai Y, Sanoyama I, Yoshida T, Sada M, Kobayashi K, Sato Y, Okayasu I, Murakumo Y. Expression of CA2 and CA9 carbonic anhydrases in ulcerative colitis and ulcerative colitis-associated colorectal cancer. Pathol Int. 2020;70:523-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Vié N, Copois V, Bascoul-Mollevi C, Denis V, Bec N, Robert B, Fraslon C, Conseiller E, Molina F, Larroque C, Martineau P, Del Rio M, Gongora C. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol Cancer. 2008;7:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Li S, Xie L, Du M, Xu K, Zhu L, Chu H, Chen J, Wang M, Zhang Z, Gu D. Association study of genetic variants in estrogen metabolic pathway genes and colorectal cancer risk and survival. Arch Toxicol. 2018;92:1991-1999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Agarwal S, Behring M, Hale K, Al Diffalha S, Wang K, Manne U, Varambally S. MTHFD1L, A Folate Cycle Enzyme, Is Involved in Progression of Colorectal Cancer. Transl Oncol. 2019;12:1461-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Liu J, Li H, Shen S, Sun L, Yuan Y, Xing C. Alternative splicing events implicated in carcinogenesis and prognosis of colorectal cancer. J Cancer. 2018;9:1754-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to Coupling of the Warburg Effect with Glutamine Catabolism in Cancer Cells. Cell Rep. 2016;17:821-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 57. | Hao Y, Samuels Y, Li Q, Krokowski D, Guan BJ, Wang C, Jin Z, Dong B, Cao B, Feng X, Xiang M, Xu C, Fink S, Meropol NJ, Xu Y, Conlon RA, Markowitz S, Kinzler KW, Velculescu VE, Brunengraber H, Willis JE, LaFramboise T, Hatzoglou M, Zhang GF, Vogelstein B, Wang Z. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun. 2016;7:11971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 58. | Wang R, Xiang W, Xu Y, Han L, Li Q, Dai W, Cai G. Enhanced glutamine utilization mediated by SLC1A5 and GPT2 is an essential metabolic feature of colorectal signet ring cell carcinoma with therapeutic potential. Ann Transl Med. 2020;8:302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Guo J, Gu X, Zheng M, Zhang Y, Chen L, Li H. Azacoccone E inhibits cancer cell growth by targeting 3-phosphoglycerate dehydrogenase. Bioorg Chem. 2019;87:16-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Tian J, Lou J, Cai Y, Rao M, Lu Z, Zhu Y, Zou D, Peng X, Wang H, Zhang M, Niu S, Li Y, Zhong R, Chang J, Miao X. Risk SNP-Mediated Enhancer-Promoter Interaction Drives Colorectal Cancer through Both FADS2 and AP002754.2. Cancer Res. 2020;80:1804-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Miao H, Ou J, Peng Y, Zhang X, Chen Y, Hao L, Xie G, Wang Z, Pang X, Ruan Z, Li J, Yu L, Xue B, Shi H, Shi C, Liang H. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat Commun. 2016;7:11716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Sukocheva OA, Furuya H, Ng ML, Friedemann M, Menschikowski M, Tarasov VV, Chubarev VN, Klochkov SG, Neganova ME, Mangoni AA, Aliev G, Bishayee A. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol Ther. 2020;207:107464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 63. | Miao Y, Li Q, Wang J, Quan W, Li C, Yang Y, Mi D. Prognostic implications of metabolism-associated gene signatures in colorectal cancer. PeerJ. 2020;8:e9847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Fang Z, Gong C, Liu H, Zhang X, Mei L, Song M, Qiu L, Luo S, Zhu Z, Zhang R, Gu H, Chen X. E2F1 promote the aggressiveness of human colorectal cancer by activating the ribonucleotide reductase small subunit M2. Biochem Biophys Res Commun. 2015;464:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Yang C, Li C, Li M, Tong X, Hu X, Yang X, Yan X, He L, Wan C. CYP2S1 depletion enhances colorectal cell proliferation is associated with PGE2-mediated activation of β-catenin signaling. Exp Cell Res. 2015;331:377-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Wei Y, Liu P, Li Q, Du J, Chen Y, Wang Y, Shi H, Zhang H, Xue W, Gao Y, Li D, Feng Y, Yan J, Han J, Zhang J. The effect of MTHFD2 on the proliferation and migration of colorectal cancer cell lines. Onco Targets Ther. 2019;12:6361-6370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Ju HQ, Lu YX, Chen DL, Zuo ZX, Liu ZX, Wu QN, Mo HY, Wang ZX, Wang DS, Pu HY, Zeng ZL, Li B, Xie D, Huang P, Hung MC, Chiao PJ, Xu RH. Modulation of Redox Homeostasis by Inhibition of MTHFD2 in Colorectal Cancer: Mechanisms and Therapeutic Implications. J Natl Cancer Inst. 2019;111:584-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 68. | Wilson JM, Coletta PL, Cuthbert RJ, Scott N, MacLennan K, Hawcroft G, Leng L, Lubetsky JB, Jin KK, Lolis E, Medina F, Brieva JA, Poulsom R, Markham AF, Bucala R, Hull MA. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129:1485-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Li X, Xun Z, Yang Y. Inhibition of phosphoserine phosphatase enhances the anticancer efficacy of 5-fluorouracil in colorectal cancer. Biochem Biophys Res Commun. 2016;477:633-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Jo YS, Oh HR, Kim MS, Yoo NJ, Lee SH. Frameshift mutations of OGDH, PPAT and PCCA genes in gastric and colorectal cancers. Neoplasma. 2016;63:681-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Agarwal S, Chakravarthi BVSK, Behring M, Kim HG, Chandrashekar DS, Gupta N, Bajpai P, Elkholy A, Balasubramanya SAH, Hardy C, Diffalha SA, Varambally S, Manne U. PAICS, a Purine Nucleotide Metabolic Enzyme, is Involved in Tumor Growth and the Metastasis of Colorectal Cancer. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Ferraz JM, Zinzindohoué F, Lecomte T, Cugnenc PH, Loriot MA, Beaune P, Stücker I, Berger A, Laurent-Puig P. Impact of GSTT1, GSTM1, GSTP1 and NAT2 genotypes on KRAS2 and TP53 gene mutations in colorectal cancer. Int J Cancer. 2004;110:183-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | FeiFei W, HongHai X, YongRong Y, PingXiang W, JianHua W, XiaoHui Z, JiaoYing L, JingBo S, Kun Z, XiaoLi R, Lu Q, XiaoLiang L, ZhiQiang C, Na T, WenTing L, YanQing D, Li L. FBX8 degrades GSTP1 through ubiquitination to suppress colorectal cancer progression. Cell Death Dis. 2019;10:351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1570] [Cited by in F6Publishing: 2062] [Article Influence: 412.4] [Reference Citation Analysis (2)] |
| 75. | Balch C, Ramapuram JB, Tiwari AK. The Epigenomics of Embryonic Pathway Signaling in Colorectal Cancer. Front Pharmacol. 2017;8:267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Caputo F, Santini C, Bardasi C, Cerma K, Casadei-Gardini A, Spallanzani A, Andrikou K, Cascinu S, Gelsomino F. BRAF-Mutated Colorectal Cancer: Clinical and Molecular Insights. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 77. | Sanz-Garcia E, Argiles G, Elez E, Tabernero J. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol. 2017;28:2648-2657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 78. | Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059-2072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 79. | Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 665] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 80. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5242] [Cited by in F6Publishing: 5189] [Article Influence: 471.7] [Reference Citation Analysis (0)] |
| 81. | Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 82. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5743] [Cited by in F6Publishing: 6142] [Article Influence: 511.8] [Reference Citation Analysis (0)] |
| 83. | De Palma FDE, D'Argenio V, Pol J, Kroemer G, Maiuri MC, Salvatore F. The Molecular Hallmarks of the Serrated Pathway in Colorectal Cancer. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 84. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 85. | Murakami T, Sakamoto N, Nagahara A. Endoscopic diagnosis of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. World J Gastroenterol. 2018;24:3250-3259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (5)] |
| 86. | Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681-8686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1834] [Cited by in F6Publishing: 1803] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 87. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7459] [Cited by in F6Publishing: 7436] [Article Influence: 338.0] [Reference Citation Analysis (0)] |
| 88. | Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 571] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 89. | Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 725] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 90. | Li SKH, Martin A. Mismatch Repair and Colon Cancer: Mechanisms and Therapies Explored. Trends Mol Med. 2016;22:274-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 91. | Liu X, Zhang T, Li Y, Zhang Y, Zhang H, Wang X, Li L. The Role of Methylation in the CpG Island of the ARHI Promoter Region in Cancers. Adv Exp Med Biol. 2020;1255:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, Koh H, Simms L, Barker M, Leggett B, Levine J, Kim M, French AJ, Thibodeau SN, Jass J, Haile R, Laird PW. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1423] [Cited by in F6Publishing: 1418] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 93. | Hinoue T, Weisenberger DJ, Lange CP, Shen H, Byun HM, Van Den Berg D, Malik S, Pan F, Noushmehr H, van Dijk CM, Tollenaar RA, Laird PW. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res. 2012;22:271-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 94. | Takahashi H, Suzuki Y, Nishimura J, Haraguchi N, Ohtsuka M, Miyazaki S, Uemura M, Hata T, Takemasa I, Mizushima T, Yamamoto H, Doki Y, Mori M. Characteristics of carbonic anhydrase 9 expressing cells in human intestinal crypt base. Int J Oncol. 2016;48:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 780] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 96. | Yan K, Xu X, Wu T, Li J, Cao G, Li Y, Ji Z. Knockdown of PYCR1 inhibits proliferation, drug resistance and EMT in colorectal cancer cells by regulating STAT3-Mediated p38 MAPK and NF-κB signalling pathway. Biochem Biophys Res Commun. 2019;520:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 97. | Liu F, Liu Y, Gao H, Wang L. [Serine hydroxymethyl transferase 2 regulates the AMPK/mTOR pathway and induces autophagy to promote chemotherapy resistance in colon cancer cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35:344-350; 356. [PubMed] [Cited in This Article: ] |
| 98. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2025] [Cited by in F6Publishing: 2068] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 99. | Jin Z, Li H, Hong X, Ying G, Lu X, Zhuang L, Wu S. TRIM14 promotes colorectal cancer cell migration and invasion through the SPHK1/STAT3 pathway. Cancer Cell Int. 2018;18:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Bao Y, Li K, Guo Y, Wang Q, Li Z, Yang Y, Chen Z, Wang J, Zhao W, Zhang H, Chen J, Dong H, Shen K, Diamond AM, Yang W. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget. 2016;7:26780-26792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Toda K, Kawada K, Iwamoto M, Inamoto S, Sasazuki T, Shirasawa S, Hasegawa S, Sakai Y. Metabolic Alterations Caused by KRAS Mutations in Colorectal Cancer Contribute to Cell Adaptation to Glutamine Depletion by Upregulation of Asparagine Synthetase. Neoplasia. 2016;18:654-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 102. | Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, Settleman J, Giovannini M, Jacks T. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 450] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 103. | Yang C, Zhou Q, Li M, Tong X, Sun J, Qing Y, Sun L, Yang X, Hu X, Jiang J, Yan X, He L, Wan C. Upregulation of CYP2S1 by oxaliplatin is associated with p53 status in colorectal cancer cell lines. Sci Rep. 2016;6:33078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Nakayama M, Oshima M. Mutant p53 in colon cancer. J Mol Cell Biol. 2019;11:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 105. | Yang H, Wei Y, Yang Y, Zhang Q, Jia Y, Zang A, Ren L. TGF-β1 suppresses proliferation and promotes apoptosis in colon cancer cell by inactivating ERK pathway. Panminerva Med. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-β signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:57-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2741] [Cited by in F6Publishing: 2667] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 108. | Kolodner RD. Guarding against mutation. Nature. 2000;407:687, 689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |