Published online Mar 15, 2016. doi: 10.4251/wjgo.v8.i3.258
Peer-review started: July 8, 2015
First decision: October 13, 2015
Revised: December 7, 2015
Accepted: December 29, 2015
Article in press: January 4, 2016
Published online: March 15, 2016
Venous thromboembolism event (VTE) is a common and morbid complication in cancer patients. Patients with gastrointestinal cancers often suffer from symptomatic or incidental splanchnic vein thrombosis, impaired liver function and/or thrombocytopenia. These characteristics require a thorough risk/benefit evaluation for individual patients. Considering the risk factors for the development of VTE and bleeding events in addition to recent study results may be helpful for correct initiation of primary pharmacological prevention and treatment of cancer-associated thrombosis (CAT), preferably with low molecular weight heparins (LMWH). Whereas thromboprophylaxis is most often recommended in hospitalized surgical and non-surgical patients with malignancy, there is less agreement as to its duration. With regard to ambulatory cancer patients, the lack of robust data results in low grade recommendations against routine use of anticoagulant drugs. Anticoagulation with LMWH for the first months is the evidence-based treatment for acute CAT, but duration of secondary prevention and the drug of choice are unclear. Based on published guidelines and literature, this review will focus on prevention and treatment strategies of VTE in patients with gastrointestinal cancers.
Core tip: The risk for venous thrombosis and pulmonary embolism is clearly elevated in patients with gastrointestinal cancers. This risk is highest for patients with pancreatic, gastric or colorectal cancer and those receiving anti-cancer therapies. Available guidelines usually refer to thromboembolism in cancer patients without differentiating between types of cancer. Those patients with gastrointestinal cancers are more likely to present with additional problems such as hepatopathy-associated low platelet counts and/or prolonged prothrombin times. Furthermore, symptomatic or incidental thromboembolism of the visceral veins may occur more often. Identifying the risk factors for the development of venous thromboembolism and bleeding events may be helpful for correct initiation of primary pharmacological prevention and treatment of cancer-associated thromboembolism. Based on published guidelines and literature, this review will focus on prevention and treatment strategies of venous thromboembolism in patients with gastrointestinal cancers.
- Citation: Riess H, Habbel P, Jühling A, Sinn M, Pelzer U. Primary prevention and treatment of venous thromboembolic events in patients with gastrointestinal cancers - Review. World J Gastrointest Oncol 2016; 8(3): 258-270
- URL: https://www.wjgnet.com/1948-5204/full/v8/i3/258.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i3.258
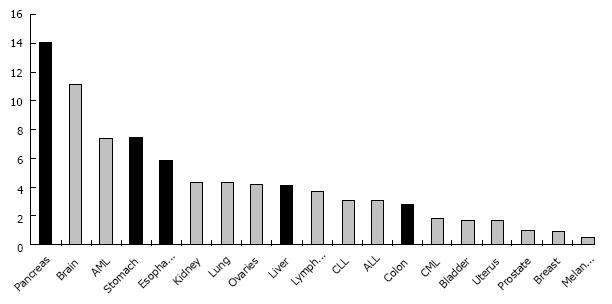
Venous thromboembolism (VTE) commonly presents as deep vein thrombosis (DVT) or pulmonary embolism (PE), occasionally as thrombosis of the hepatic, portal, or splanchnic veins. Patients with cancer are at increased risk for VTE[1-4]. In addition to prothrombogenic cancer-related factors, the risk for symptomatic VTE is modulated by patient-specific and treatment-related factors (Table 1). Among the different subtypes of malignancy, some gastrointestinal cancers have a clinically relevant VTE incidence of more than 5% in the first year after cancer diagnosis (Figure 1), such as pancreatic (16%-22%), gastric (12%-17%) and colorectal (8%-12%) cancers[2,5-8]. Symptomatic VTE is not only associated with substantial morbidity and complications in clinical management, but has been shown to have a detrimental effect on cancer survival[9-11]. Acute and follow-up complications such as intestinal necrosis and esophageal bleeding due to portal hypertension may be life threatening if intra-abdominal veins are involved[12-15].
| Cancer-related | Patient-related | Treatment-related |
| Reduced mobility | ||
| Primary cancer (e.g., pancreatic cancer > colo-rectal cancer) | Age | Operation |
| Stage (IV > III) | History of VTE | Chemotherapy |
| Histology (e.g., adeno- > squamous cell-carcinoma) | Infection/fever | Central line/ port-catheter |
| Grade (3 > 2) | Parenteral nutrition | |
| Thrombocytosis | Radiation therapy | |
| Leukocytosis | ||
| Acute phase (elevated CRP) | ||
| Elevated D-dimer |
Furthermore, anticoagulation with a vitamin K antagonist (VKA), such as warfarin, is complicated by drug interactions and variable drug absorption due to the changing nutritional status of cancer patients[16]. In fact, therapeutic anticoagulation with VKA does not prevent VTE recurrences as effectively as in non-cancer patients, and major bleeding complications are also more than twice as common[17]. Acute anticoagulation and secondary prevention with low molecular weight heparin (LMWH) has been established as an effective treatment of VTE in cancer patients[18], but daily subcutaneous application may result in local complications such as hematoma or infections, thus negatively influencing quality of life.
Most research into the primary prevention and treatment of VTE included both cancer and non-cancer patients. Recommendations for patients with malignancies are therefore based on the sparse available evidence and the guidelines differ only marginally (Table 2)[19-27]. Most clinical studies focusing on VTE in cancer patients do not differentiate between different kinds of cancer, resulting in a lack of specific data concerning VTE in gastrointestinal cancers.
| Primary prophylaxis/prevention of VTE in cancer patients | Treatment of cancer-associated VTE | ||||
| Surgical patients | In-patients (non surgical) | Out-patients | Acute/initial | Long-term/secondary prevention | |
| ASCO Guidelines 2013[15] | UFH, LMWH (Dalteparin, Enoxaparin), Fondaparinux | UFH, LMWH (Dalteparin, Enoxaparin), Fondaparinux | Not recommended routinely1 LMWH may be considered | UFH, LMWH (Dalteparin, Enoxaparin, Tinzaparin), Fondaparinux | LMWH (Dalteparin, Enoxaparin,Tinzaparin) |
| A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest risk patients. | VKA not recommended | VKA (INR 2-3) acceptable if LMWH is not available Use of NOACs is not recommended | |||
| Patients undergoing major cancer surgery should receive prophylaxis starting before surgery and continuing for at least 7 to 10 d and it should be considered an extension up to 4 wk in patients undergoing abdominal and pelvic surgery | Treatment of splanchnic or visceral vein thrombi diagnosed incidentally should be considered on a case-by-case basis, considering potential benefits and risks of anticoagulation | ||||
| ESMO Guidelines 2011[16] | LMWH (Dalteparin, Enoxaparin), UFH Fondaparinux not recommended | LMWH (Dalteparin, Enoxaparin), Fondaparinux, UFH | Not recommended routinely May be considered in high risk patients | LMWH (Enoxaparin, Dalteparin), UFH | Treatment for a total of 6 mo. Initial dose of LMWH 100% for 1 mo, thereafter 5 mo with 75%-80% of the initial dose of LMWH |
| Cancer patients undergoing elective major abdominal or pelvic surgery should receive in hospital and post-discharge prophylaxis with s.c. LMWH for up to 1 mo after surgery | |||||
| International Consensus Groupe 2013[17] | LMWH (Dalteparin, Enoxaparin, Nadroparin, Tinzaparin), Fondaparinux, UFH For 10 ± 2 d or 25-31 d (28 d) extended use (Bemiparin sodium 3500 IU per day for 28 d) | LMWH (Dalteparin, Enoxaparin, Nadroparin, Tinzaparin), Fondaparinux, UFH | Not recommended routinely To be considered/recommended: Patients with locally advanced or metastatic lung or pancreatic cancer treated with chemotherapy and having a low bleeding risk | LMWH (Dalteparin, Enoxaparin), UFH | LMWH (Dalteparin, Enoxaparin) for 3 to 6 mo |
| British Committee for Standards in Haematology 2015[18] | Patients undergoing abdominal and pelvic surgery for cancer should be considered for extended thromboprophylaxis | Patients with active or recent cancer should receive thromboprophylaxis throughout their admission unless contraindicated Patients without a history of venous thromboembolism receiving adjuvant hormonal therapies for cancer should not routinely receive thrombo-prophylaxis | Patients should be assessed for thrombosis risk and although most do not routinely require thromboprophylaxis, it should be considered for high risk patients | Initial treatment should be with LMWH for six months | Warfarin and other oral anticoagulants are acceptable alternatives if LMWH is impractical and anticoagulation is indicated Anticoagulation should be continued, taking pt status and wishes and bleeding risk into consideration. There is a rationale but little direct evidence for preferring to continue to use LMWH |
| Cancer patients with incidental pulmonary embolus or deep vein thrombosis should be therapeutically anticoagulated as for symptomatic disease | |||||
| Australian Governments National Health and Medical Research Council 2009[19] | LMWH, continue for at least 7 to 10 d following major general surgery Consider using extended thromboprophylaxis with LMWH for up to 28 d after major abdominal or pelvic surgery for cancer, especially in patients who are obese, slow to mobilise or have a past history of VTE | LMWH, UFH | - | - | |
| MAYO CLINIC VTE Prevention and Management Guidelines 2014[20] | UFH, LMWH (Enoxaparin, Dalteparin), Fondaparinux Cancer patients undergoing pelvic or abdominal surgery should receive 4 wk of LMWH | UFH, LMWH (Enoxaparin, Dalteparin), Fondaparinux | UFH, LMWH (Enoxaparin, Dalteparin), Fondaparinux VKA (INR2-3) | Anticoagulants are continued until there is no evidence of active malignant disease defined as any evidence of cancer on cross-sectional imaging or any cancer-related treatment (surgery, radiation, or chemotherapy) within the past 6 mo | |
| ASH Guidelines 2013[21] | UFH, LMWH, Fondaparinux in all patients undergoing major surgical intervention for malignant disease Prolonged prophylaxis for up to 4 wk may be considered in patients undergoing major abdominal or pelvic surgery for cancer with high-risk features such as residual malignant disease, obesity, and prior history of VTE | UFH, LMWH, Fondaparinux | Routine VTE prophylaxis in ambulatory patients receiving chemotherapy is not recommended | LMWH | LMWH Continue treatment with LMWH is preferred for at least the initial 6 mo of treatment |
| German Guidelines[22,23] | LMWH, Fondaparinux, (UFH) Patients undergoing abdominal and pelvic surgery for cancer are recommended to get extended thromboprophylaxis (28 to 35 d) | LMWH, Fondaparinux, (UFH) | LMWH, Patients should be assessed for thrombosis risk and thromboprophylaxis should be considered for high risk patients | LMWH, Fondaparinux, UFH | LMWH for 3 to 6 mo If cancer persists extended secundary prophylaxis (with LMWH, VKA, or NOAC) is usefull (till death) |
The risk for VTE is higher in cancer patients undergoing surgery without prophylaxis in comparison to non-cancer surgical patients[27,28]. Administration of LMWH or fondaparinux (FPX) in these patients significantly reduces the rate of VTE[29,30]. Dosages of LMWH in the range of 3000-5000 anti-FXa units per day are more effective than and as safe as lower doses. Patients undergoing major abdominal or pelvic surgery for malignancy remain at risk for VTE for up to five weeks after surgery. Thus, a prolonged pharmacologic thromboprophylaxis (LMWH or FPX once daily) should be considered, unless contraindicated because of high bleeding risk or active bleeding[31,32]. Prophylactic regimens begin 12-24 h pre- or 6-24 h postoperatively[29-32]. Based on these studies[31-33], and according to the different guidelines (Table 2), antithrombotic prophylaxis is recommended for a minimum of 7 d and up to 35 d postoperatively. FPX (2.5 mg/d) was found to be as effective and safe in preventing VTE after abdominal surgery as the LMWH dalteparin (5000 antiFXa units/d). A post-hoc analysis of 1407 (out of 2048) patients with cancer demonstrated a significant (39%) risk reduction of VTE[29]. Accordingly, the European (ESMO) guideline[20] recommends extended prophylaxis for all patients undergoing elective cancer surgery. In the American guidelines (ASCO, NCCN)[19,34], extended prophylaxis is only recommended in the presence of high thromboembolic risk factors such as residual or advanced cancer, aged 60 or older, obesity, previous history of VTE, duration of surgery longer than 2 h or prolonged postoperative immobilization. In patients with contraindications to pharmacological anticoagulant prevention strategies (e.g., increased risk of hemorrhage), the use of intermittent pneumatic compression devices or compression stockings is advised[19-27].
The recommendations for patients undergoing minimally invasive or laparoscopic surgery are even less evidence-based. In a recently published randomized study evaluating postoperative antithrombotic prophylaxis with LMWH for one vs four weeks in patients undergoing laparoscopic surgery for colorectal cancer, extended antithrombotic prophylaxis was safe and reduced the 3-mo risk of VTE by more than 90%, and that of proximal DVT by 50%, as compared to the one week regimen[35]. These data are consistent with the suggestion to follow the same recommendations regardless of whether a cancer patient is to undergo an open or laparoscopic surgical intervention[36,37].
Splanchnic vein thromboses (SVT), including portal vein thrombosis (PVT), mesenteric vein thrombosis, splenic vein thrombosis and the Budd Chiari syndrome are frequent events in patients with hepato-biliary-pancreatic cancers, with cancer-associated PVT responsible for 21% of all cases[14]. The risk of VTE and SVT is increased in patients with cirrhosis[38,39], and PVT is a relevant complication of hepato-biliary-pancreatic surgery[40], reported to occur in 9% of patients after liver resections[41]. In this context, thromboprophylaxis with LMWH was demonstrated to be effective and safe in a retrospective comparative cohort study of 201 patients undergoing liver resections for liver cancers, with a reduction in PVT from 10% to 2%[42]. These data suggest that prophylactic anticoagulation with LMWH - as recommended in patients undergoing major abdominal cancer surgery - is also effective in cancer patients undergoing liver resection.
Hospitalized patients with active cancer - a term not uniformly defined - were included in all published randomized clinical trials investigating the role of unfractionated heparin (UFH), LMWH or FPX for the prevention of VTE. Treatment of hospitalized patients for 6-14 d with low-dose enoxaparin (20 mg/d s.c.) was ineffective[43], whereas higher prophylactic doses of LMWH (enoxaparin 40 mg/d, or dalteparin 5000 anti FXa units/d)[43,44] or FPX (2.5 mg/d)[45] demonstrated superiority compared to placebo in the prevention of VTE with minimal or no increase in major bleeding events. Subgroup analyses failed to identify a subgroup which did not benefit from pharmacological thromboprophylaxis[46]. This was also true for a small subgroup (5%-15% of the study population) of cancer patients.
Subgroup analysis of 274 patients with active cancer from the CERTIFY study comparing UFH (3 × 5000 IU/d) with LMWH (certoparin 3000 anti FXa units/d) demonstrated a similar VTE risk (5.3% vs 4.1%) and similar rates of any (4.0 vs 3.9) or major (0.7% vs 0.5%) bleeding compared to the 2965 patients without cancer[47,48].
Based on three studies, extended prophylaxis with the anticoagulant drugs LMWH[49] or non-vitamin K oral anticoagulant drugs (NOACs)[50,51] cannot be recommended in medical patients. In the MAGELLAN trial[51], cancer patients (n = 592; 7.3%) had higher rates of VTE when prophylaxis with rivaroxaban was extended from 10 to 35 d.
Discussion is ongoing as to whether all cancer patients hospitalized for reasons such as infectious complications or complex chemotherapy regimens should generally receive medical thromboprophylaxis unless contraindicated by active bleeding or high bleeding risk[52-55]. According to the available guidelines (Table 2), routine thromboprophylaxis should at least be considered. Italian investigators assessing the risk of VTE in hospitalized medical patients confirmed cancer to be a major predisposing factor for VTE (PADUA prediction score), without differentiating between cancers[56]. However, the existence of active cancer on its own does not classify as a high VTE risk unless additional risk factors are present (Table 1).
Patients who have been recently diagnosed with cancer are at high risk for VTE[57,58]. Nowadays these patients usually receive non-surgical therapies such as radiation, chemotherapy or radiochemotherapy in an outpatient setting. Long-term central venous catheters (e.g., port or picc line catheters) are used to facilitate drug administration in many of these patients. There is an increased risk of DVT in the subclavian or jugularis interna veins after insertion of a central line, with an estimated incidence of asymptomatic catheter-related DVT of about 20%, although less than 5% develop clinical symptoms, which very rarely result in symptomatic PE or relevant post-thrombotic sequelae[59-64]. Multicenter randomized double blind placebo-controlled studies to assess the efficacy and safety of LMWH or VKA demonstrated a significant reduction in asymptomatic catheter-related thrombi, but failed to show significant benefit with regard to clinically symptomatic DVT[59]. International guidelines therefore recommend against routine prophylaxis for this indication.
The risk of VTE in cancer patients is especially high during the first weeks or months of specific therapy[4,62,65]. Several clinical trials have evaluated the role of primary prevention in outpatients with selected or unselected malignancies. An early study on patients receiving chemotherapy for advanced breast cancer found low dose warfarin to be safe and efficacious, with 85% relative reduction in VTE compared to placebo[66]. Two other randomized trials in patients with breast or lung cancers using prophylactic dosages of LMWH failed to show a benefit[67]. The PROTECHT study, however, showed a statistically significant relative risk reduction of 50% for symptomatic VTE in cancer outpatients receiving chemotherapy for different types of malignancy (including gastrointestinal cancers) in favor of prophylactic dosages of the LMWH nadroparin (Table 3)[68]. Moreover, despite a relatively low event rate of less than 3% in the placebo group, prophylactic dosages of semuloparin, an ultralow molecular weight heparin (not further developed), demonstrated a highly significant reduction (65%) of VTE in patients with metastatic or locally advanced solid cancers (1590 of 3212 with pancreatic, gastric or colorectal cancers) receiving chemotherapy[69] (Table 3), without an increase in the incidence of clinically relevant or major bleeding complications. Whereas these trials investigated the effect of prophylactic dosages of antithrombotic drugs, two randomized open-label studies in patients with advanced pancreatic cancer undergoing chemotherapy investigated primary prophylaxis with higher doses of LMWH (Table 3). The phase II FRAGEM-trial[70] examined the therapeutic dose of dalteparin (200 anti FXa units/kg per day), and the phase III CONKO-004 trial[71] used half the therapeutic dosage of the LMWH enoxaparin (1 mg/kg per day). Despite differences in study design and primary endpoint of effectiveness, both trials found this intensity of anticoagulation to be safe and reported a more than 80% relative risk reduction of thromboembolic events compared to observation (Table 3).
| Study | Cancer | n | VTE placebo | VTE LMWH | VTE U-LMWH | RR |
| PROTECHT1 | Gastorintestinal2 | 148/272 | 2.7% | 1.5% | -44% | |
| Agnelli et al[68], 2009 | Pancreas | 17/36 | 5.9% | 8.3% | 40% | |
| SAVE-ONCO | Colo-rectal | 461/464 | 2.0% | 1.1% | -45% | |
| Agnelli et al[69], 2012 | Pancreas | 128/126 | 10.9% | 2.4% | -78% | |
| Stomach | 207/204 | 1.9% | 0.5% | -75% | ||
| FRAGEM | Pancreas | 60/63 | 23% | 3.4% | -85% | |
| Maraveyas et al[70], 2012 | ||||||
| CONKO 004 | Pancreas | 152/160 | 9.9% | 1.3% | -87% | |
| Pelzer et al[71], 2015 |
Nevertheless, available international guidelines do not recommend routine prophylaxis in all ambulatory cancer patients receiving anti-cancer chemotherapy (Table 2). ACCP and ASCO guidelines[19,62] suggest considering antithrombotic prophylaxis in outpatients receiving chemotherapy with unspecified solid tumors and additional risk factors, whereas others recommend considering primary prevention, especially in patients with lung or pancreatic cancer[21] in accordance with a recent meta-analysis[72].
A number of attempts have been made to classify individual cancer outpatients receiving chemotherapy more precisely according to VTE risk. In this context, the scoring system developed by Khorana et al[73] (Table 4), which associates pancreatic and stomach cancer with a very high VTE risk, demonstrated potential for the identification of cancer outpatients at risk for VTE prior to the start of chemotherapy with simple and commonly available criteria. A prospective randomized trial investigating the benefit of primary antithrombotic prophylaxis in high risk patients according to this score is ongoing. Until results from this study become available, the initiation of pharmacological prophylaxis in high risk patients is suggested by the standardization subcommittee of the ISTH[74]. Supplementing the Khorana score with lab parameters such as D-dimer and soluble P-selectine[75] or chemotherapy components such as gemcitabine and cis- or car-boplatin[76] may further categorize patients according to their VTE risk.
| Khorana score criteria[73] | Score |
| Primary cancer | |
| With very high risk (pancreas, stomach) (high grade glioma1) | 2 |
| With high risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Platelet count prior to chemotherapy > 350000/μL | 1 |
| Hb < 10 g/dL or ESA-application | 1 |
| Leukocyte count prior to chemotherapy > 11000/μL | 1 |
| Body mass index > 35 kg/m² | 1 |
| High risk | > 3 |
| Vienna prediction score (additional parameters to Khorana score)[75] | |
| D-dimer > 1.44 μg/mL | 1 |
| Soluble P-selectin > 153.1 μg/mL | 1 |
| High risk | > 4 |
| Protecht prediction score (additional parameters to Khorana score)[76] | |
| Cisplatin or carboplatin | 1 |
| Gemcitabine | 1 |
| High risk | > 3 |
With the exception of patients suffering from pancreatic cancer, for whom primary prophylaxis with LMWH is highly warranted unless contraindicated by increased bleeding risk, insufficient evidence is available regarding other gastrointestinal cancers to recommend routine primary prevention with anticoagulant drugs. Until further evidence has accumulated, the Khorana score is helpful for classification of patients with gastrointestinal cancer according to their VTE risk and as an aid in decision-making regarding initiation of pharmacologic prophylaxis. While prophylactic dosages of LMWH may be effective in most gastrointestinal cancers, half-therapeutic dosages of LMWH should be considered for patients with advanced pancreatic cancer in the first three months of chemotherapy.
The introduction of new drugs which target angiogenesis, tumor stroma or immunity in gastrointestinal cancer medicine led to the realization that individual VTE risk may be strongly influenced by the drugs. For example, the introduction of bevacizumab for the treatment of advanced colorectal cancer not only resulted in a clinically relevant increase in bleeding, but in thromboembolic complications as well[75,77,78].
Moreover, drug-associated VTE may become an important factor limiting further cancer therapy development. At the ASCO meeting in May 2015, Hingorani et al[79] presented a phase II trial in pancreatic cancer patients treated with gemcitabine, nab-paclitaxel and recombinant pegylated hyaluronidase - an experimental drug - which had to be stopped due to high numbers of VTEs. A prophylactic dose of LMWH (40 mg enoxaparin/d) was not effective, but the use of half therapeutic doses of enoxaparin (1 mg enoxaparin/kg per day) allowed the continuation of the study.
The treatment of patients with active cancer and acute VTE with the standard treatment for non-cancer VTE patients (LMWH or FPX plus concurrent introduction of anticoagulation with VKA) resulted in a two- to three-fold increase of both re-thrombosis and bleeding[17,18]. Several randomized controlled trials compared prolonged application of LMWH to oral anticoagulation with VKA in patients with cancer-associated thrombosis (CAT) and demonstrated superior efficacy of LMWH with no increase in bleeding complications (Table 5), results confirmed by meta-analysis[18,19,80,81]. Based on these results, guidelines recommend a standard treatment of three to six months of weight-adjusted LMWH regardless of whether the patient suffers from gastrointestinal or another type of cancer (Table 2). The underlying malignancy, probably representing the most important risk factor for VTE, continues beyond six months after the initial VTE in most of these patients. Therefore, anticoagulation beyond this period may be warranted and is recommended in most guidelines (Table 2). Nevertheless, evidence for this recommendation is clearly weaker, as prospective randomized trials investigating type of anticoagulation, dosage of anticoagulant drugs or duration of prolonged anticoagulation have not been reported. Available data suggest that the pro-thrombotic risk correlates with disease progression due to increasing tumor load, decreasing performance status, progressive mobility reduction and increased use of palliative chemotherapy[82-84]. On the other hand, an increased bleeding risk, associated with progression of cancer, prolonged anticoagulation and prolonged chemotherapy needs to be considered in order to balance the benefit of preventing recurrent VTE against the risk of bleeding[83,84]. Furthermore, the impact of prolonged anticoagulation on the quality of life of cancer patients needs to be considered.
| CLOT | CATCH | VKA | Dalteparin | P | VKA | Tinzaparin | P | |
| Study-population characteristics | ||||||||
| n | 676 | 900 | ||||||
| Women | 52% | 59% | ||||||
| Median age (yr) | 63 | 59 | ||||||
| ECOG 0-1 | 63% | 77% | ||||||
| Metastasized cancer | 67% | 55% | ||||||
| Brest cancer | 17.6% | 9% | ||||||
| Colo-rectal cancer | 17.8% | 13% | ||||||
| Lung cancer | 14.8% | 12% | ||||||
| Gynecological cancer | 11.2% | 23% | ||||||
| Pancreatic cancer | 4.8% | |||||||
| Urogenital cancers | 14.2% | |||||||
| Brain cancers | 5.5% | |||||||
| Hematological cancers | 10% | 10% | ||||||
| Study outcomes | ||||||||
| VTE | 15.8% | 8.0% | 0.002 | 10.0% | 6.9% | 0.07 | ||
| DVT | 11.0% | 4.2% | 5.3 | 2.7 | 0.04 | |||
| Fatal PE | 2.1% | 1.7% | 3.8% | 3.8% | ||||
| Non-fatel PE | 2.7% | 2.7% | 0.7% | 0.4% | ||||
| Major bleeding | 4% | 6% | 0.25 | 2.7% | 2.9% | |||
| CRNM-bleeding | 16% | 11% | 0.03 | |||||
| Any bleeding | 19% | 14% | 0.09 | |||||
| 6-mo mortality | 41% | 39% | 41% | 40% | ||||
| INR < 2 | 30% | 26% | ||||||
| INR 2-3 | 46% | 47% |
Although clinical guidelines (Table 2) currently recommend considering indefinite anticoagulation in patients with advanced malignancy, they do not recommend a specific anticoagulant drug due to the limited evidence available.
Acute thrombosis of splanchnic veins - PVT in particular - is a frequent complication in patients with cancers of the hepato-biliary-pancreatic system[23,85]. PVT results in portal hypertension and impairment of liver perfusion. Spread of thrombosis to splenic or mesenteric veins can result in fatal complications such as splenic or intestinal infarction. Anticoagulation is the therapy of choice in non-cirrhotic, non-cancer patients, with successful recanalization in about half of acute PVT cases[86,87]. Anticoagulation is considered in patients with cirrhosis and PVT with a small increase in bleeding complications[86], and can safely be combined with placement of transjugular intrahepatic portosystemic shunts[87]. Unfortunately cancer patients, e.g., those with gastrointestinal cancers, were excluded from these trials[86,87].
Treatment of cancer-associated PVT is therefore based on evidence from non-cancer patients with or without cirrhosis. Despite the fact that prophylactic and therapeutic anticoagulation seems to be effective and safe for cirrhotic patients[86-88], an individual risk/benefit evaluation which takes prognosis, anticancer treatment options and acute anticoagulation-associated bleeding risk, as well as future complications with the associated consequences for surgical interventions and the risk of variceal hemorrhage into consideration is challenging.
Thrombocytopenia is a well-known effect of chemotherapy in cancer patients and higher degrees of thrombocytopenia increase the risk of hemorrhage. Anticoagulation should therefore be administered to thrombocytopenic patients after thorough evaluation of the risks and benefits. The risk of recurrent VTE is high in the month following the initial diagnosis on beginning cancer therapy[1,4]. Full therapeutic anticoagulation with LMWH is considered appropriate in these situations when the platelet count is above 50000/μL, and a reduction of anticoagulation intensity to 75% or even 50% may be reasonable later in the course of treatment. When platelets are below 50000/μL but above 20000/μL, a half-therapeutic to prophylactic dose should be considered[89].
It should be recognized that increased prothrombin times and elevated international normalized ratios (INR) as a result of hepatic dysfunction only reflect reduced synthesis of coagulation factors. In fact coagulation inhibitors are also decreased, resulting in a well-balanced but more labile equilibrium with normal thrombin generation capacity[90,91]. For this reason patients with reduced hepatic capability are not protected against VTE, despite an increased INR, but patients with cancer and hepatic dysfunction have an increased VTE risk[90,92]. Again, higher grade evidence in favor of or against full-dose anticoagulation in patients with CAT and reduced liver function due to pre-existing cirrhosis or advanced hepatic metastasis resulting in increased INR is lacking. Clinical experience suggests a cautiously balanced approach of 75% to 100% of the therapeutic LMWH anticoagulation dose for acute VTE.
VTE recurrences are to be expected in more than 5% of patients within the first months despite CAT therapy according to guidelines with LMWH (Table 5). Again, there is sparse evidence on treatment of these patients. Based on a report from Carrier et al[93], a 20% increase in LMWH dose - without adjustment to laboratory parameters - is a practical, safe and effective approach.
A number of new oral anticoagulants (NOAC) have been introduced and licensed for prevention and treatment of VTE in recent years[94]. These drugs work by direct inhibition of active coagulation factors direct oral anticoagulants (DOAC), namely factor IIa/thrombin (dabigatran) or factor Xa (apixaban, edoxaban, rivaroxaban). The term non-vitamin K oral anticoagulant was created in order to maintain the acronym NOAC. Although these drugs have been successfully tested and licensed in trials investigating NOACs in the post-operative setting of elective hip or knee replacement, drug development for other surgical patients was never initiated and the programs for medical patients were stopped early[50,51]. A placebo-controlled dose-finding phase II trial for primary prophylaxis of VTE in ambulatory cancer patients undergoing chemotherapy showed relevant activity[95], but this indication has not yet been further developed. There is therefore no evidence for the use of NOACs for primary prevention in hospitalized or ambulatory cancer patients undergoing surgery or any other kind of anticancer therapy.
The four drugs mentioned above have all been compared to VKA (warfarin) in randomized phase III trials for the treatment of DVT, PE and atrial fibrillation[96-101]. All of these trials demonstrated that NOACs are at least as effective and safe as VKA. As confirmed by meta-analysis, the study data for these NOACs showed a clinically relevant reduction in bleeding, most pronounced for intracerebral bleeding events[102]. Depending on the inclusion and exclusion criteria of the individual studies and on the definition of “patients with active cancer”, all six trials included cancer patients, a subgroup adding up to 2.5% of more than 25000 patients[102]. Meta-analysis of these patients suggests a potential role for NOACs in patients with CAT, with a similar risk/benefit relationship as demonstrated in non-cancer patients[76]. There are major drawbacks to the use of NOACs for this indication, however. First of all, standard therapy of CAT is LMWH for several months and not LMWH followed by VKA - the standard treatment arm in the VTE trials. Secondly, “patients with active cancer” included in the phase III trials demonstrate a three-month-mortality of less than 15%[103-105], whereas those included in the two pivotal CAT trials (CLOT, CATCH) recruiting cancer patients ten years apart, have a six-month-mortality of 40%[80,81] (Table 5). Obviously these patient groups differ to a great extent. Thirdly, despite having less interactions with other drugs compared to VKA, NOAC still bear a largely unclear risk of interactions with cytotoxic or targeted drugs. A CLOT- or CATCH-like head to head comparison of NOACs vs LMWH, the current standard of care, is eagerly awaited, especially as the oral application of NOACs might provide an improvement in quality of life compared to s.c. administered LMWH. Unless study data are available, NOACs are not to be considered the first choice in patients with acute CAT. As an alternative to LMWH or VKA (Table 2), anticoagulant treatment with NOACs beyond six months may be reasonable in many CAT patients, as there are no studies defining the optimal drug in this period.
The risk for VTE is clearly elevated in patients with (gastrointestinal) cancer. This risk is highest for patients with pancreatic cancer and those receiving anti-cancer therapies. VTE is the second leading cause of death in patients with cancer, and mortality is increased among patients with CAT[10,106]. Whereas available guidelines usually refer to VTE in cancer patients without differentiating between types of cancer, those with gastrointestinal cancers are more likely to present with additional problems such as hepatopathy-associated low platelet counts and/or prolonged prothrombin times. Furthermore, symptomatic or incidental VTEs of the visceral veins may occur more often. Despite limited data, prophylactic anticoagulation must be endorsed in most hospitalized patients with malignancies. In ambulatory patients undergoing chemotherapy, an assessment of individual prothrombotic and prohemorrhagic factors may help transfer the beneficial effect of pharmacologic prophylaxis demonstrated in a number of trials to those patients with the highest VTE risk, in particular those suffering from pancreatic cancer. Treatment recommendations for CAT with LMWH have been reconfirmed by recent evidence. Further progress will help clarify the risk/benefit relationship of NOACs in this field, identifying economic and quality of life aspects as well (Table 6).
| Patients with gastrointestinal cancers are among those with the highest cancer-associated VTE risk (e.g., pancreatic cancer, gastric cancer) |
| Primary prevention of VTE should be considered according to an individual risk-benefit estimation |
| Scoring systems help to identify patients at high VTE risk. These patients may benefit from prophylactic anticoagulation |
| Usual prophylactic dosages of LMWH may not be effective enough in patients with the highest risk (e.g., pancreatic cancer) |
| Gastrointestinal cancer patients with VTE should have medical anticoagulation therapy with LMWH for at least three to six months |
| In patients with gastrointestinal cancers splanchnic vein thrombosis, portal hypertension, hepatopathy-associated coagulation defects (e.g., decreased prothrombin time) and thrombocytopenia may complicate anticoagulation strategies |
The authors gratefully acknowledge the help of Mrs. Sue Travis to improve the English style.
P- Reviewer: Ferroni P, Lakatos PL, Tsikouras P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, Melton LJ. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 744] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 784] [Cited by in F6Publishing: 838] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 3. | Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339-2346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 4. | Lee AY. Epidemiology and management of venous thromboembolism in patients with cancer. Thromb Res. 2003;110:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009;27 Suppl 1:63-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Lyman GH, Eckert L, Wang Y, Wang H, Cohen A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist. 2013;18:1321-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Larsen AC, Frøkjær JB, Fisker RV, Iyer V, Mortensen PB, Yilmaz MK, Møller B, Kristensen SR, Thorlacius-Ussing O. Treatment-related frequency of venous thrombosis in lower esophageal, gastro-esophageal and gastric cancer--a clinical prospective study of outcome and prognostic factors. Thromb Res. 2015;135:802-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Petterson TM, Marks RS, Ashrani AA, Bailey KR, Heit JA. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res. 2015;135:472-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1128] [Cited by in F6Publishing: 1133] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 10. | Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902-4911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Khanna A, Reece-Smith AM, Cunnell M, Madhusudan S, Thomas A, Bowrey DJ, Parsons SL. Venous thromboembolism in patients receiving perioperative chemotherapy for esophagogastric cancer. Dis Esophagus. 2014;27:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med. 1992;92:173-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 198] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 14. | Rajani R, Björnsson E, Bergquist A, Danielsson A, Gustavsson A, Grip O, Melin T, Sangfelt P, Wallerstedt S, Almer S. The epidemiology and clinical features of portal vein thrombosis: a multicentre study. Aliment Pharmacol Ther. 2010;32:1154-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Amitrano L, Guardascione MA. Management of portal vein thrombosis in cirrhotic patients. Mediterr J Hematol Infect Dis. 2009;1:e2009014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Noble SI, Shelley MD, Coles B, Williams SM, Wilcock A, Johnson MJ. Management of venous thromboembolism in patients with advanced cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:577-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484-3488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1246] [Cited by in F6Publishing: 1255] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 18. | Akl EA, Labedi N, Barba M, Terrenato I, Sperati F, Muti P, Schünemann H. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;7:CD006650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189-2204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 581] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 20. | Mandalà M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 Suppl 6:vi85-vi92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 21. | Debourdeau P, Farge D, Beckers M, Baglin C, Bauersachs RM, Brenner B, Brilhante D, Falanga A, Gerotzafias GT, Haim N. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Watson HG, Keeling DM, Laffan M, Tait RC, Makris M. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol. 2015;170:640-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Wickham N, Gallus AS, Walters BN, Wilson A. Prevention of venous thromboembolism in patients admitted to Australian hospitals: summary of National Health and Medical Research Council clinical practice guideline. Intern Med J. 2012;42:698-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Pollak AW, McBane RD. Succinct review of the new VTE prevention and management guidelines. Mayo Clin Proc. 2014;89:394-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Connolly GC, Francis CW. Cancer-associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | S3-Leitlinie Prophylaxe der Venösen Thromboembolie (VTE), Version vom 18. März 2009 mit Addendum vom 08. Mai 2010, ©AWMF Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. Autorisiert für elektronische Publikation: AWMF online Aktuelle PDF-Datei erzeugt: 14. 07 2011; AWMF-Register Nr. 003-001. [Cited in This Article: ] |
| 27. | Interdisziplinäre S2-Leitlinie- Diagnostik und Therapie der Venenthrombose und der Lungenembolie. European Journal of Vascular Medicine, Volume 39 S/78. August 2010, AWMF-Registernummer 065/002, VASA. Autorisiert für elektronische Publikation: AWMF online Aktuelle PDF-Datei erzeugt: 14. 07 2010; S/78 ©2010 by Verlag Hans Huber, Hogrefe AG, Bern Supplement 78, August 2010. [Cited in This Article: ] |
| 28. | Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, Moia M, Parazzini F, Rossi R, Sonaglia F. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 478] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 29. | Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92:1212-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Bergqvist D, Burmark US, Flordal PA, Frisell J, Hallböök T, Hedberg M, Horn A, Kelty E, Kvitting P, Lindhagen A. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 178] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 734] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 32. | Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;CD004318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Kakkar VV, Balibrea JL, Martínez-González J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | National Health and Medical Research Council. Clinical practice guideline for the prevention of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to Australian hospitals. Melbourne: National Health and Medical Research Council; 2009. ACT 2600. Available from: http://www.ag.gov.au/cca. [Cited in This Article: ] |
| 35. | Vedovati MC, Becattini C, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, Mariani E, Flamini O, Pucciarelli S, Donini A. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg. 2014;259:665-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Verso M, Agnelli G. New strategies of VTE prevention in cancer patients. Thromb Res. 2014;133 Suppl 2:S128-S132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S-e277S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1495] [Cited by in F6Publishing: 1350] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 38. | Søgaard KK, Horváth-Puhó E, Grønbaek H, Jepsen P, Vilstrup H, Sørensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 39. | Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, Riccardi L, Lancellotti S, Santoliquido A, Flore R. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 40. | Thomas RM, Ahmad SA. Management of acute post-operative portal venous thrombosis. J Gastrointest Surg. 2010;14:570-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Yoshiya S, Shirabe K, Nakagawara H, Soejima Y, Yoshizumi T, Ikegami T, Yamashita Y, Harimoto N, Nishie A, Yamanaka T. Portal vein thrombosis after hepatectomy. World J Surg. 2014;38:1491-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Yamashita Y, Bekki Y, Imai D, Ikegami T, Yoshizumi T, Ikeda T, Kawanaka H, Nishie A, Shirabe K, Maehara Y. Efficacy of postoperative anticoagulation therapy with enoxaparin for portal vein thrombosis after hepatic resection in patients with liver cancer. Thromb Res. 2014;134:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Nguyen H, Olsson CG, Turpie AG. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1159] [Cited by in F6Publishing: 1130] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 44. | Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 697] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 45. | Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 619] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 46. | Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 47. | Riess H, Haas S, Tebbe U, Gerlach HE, Abletshauser C, Sieder C, Rossol S, Pfeiffer B, Schellong SM. A randomized, double-blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non-surgical patients: CERTIFY Study. J Thromb Haemost. 2010;8:1209-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Haas S, Schellong SM, Tebbe U, Gerlach HE, Bauersachs R, Melzer N, Abletshauser C, Sieder C, Bramlage P, Riess H. Heparin based prophylaxis to prevent venous thromboembolic events and death in patients with cancer - a subgroup analysis of CERTIFY. BMC Cancer. 2011;11:316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Hull RD, Schellong SM, Tapson VF, Monreal M, Samama MM, Nicol P, Vicaut E, Turpie AG, Yusen RD. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 50. | Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, Weitz JI. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 51. | Cohen AT, Spiro TE, Spyropoulos AC. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:1945-1946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Carrier M, Khorana AA, Moretto P, Le Gal G, Karp R, Zwicker JI. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. 2014;127:82-86.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Cohen AT, Nandini B, Wills JO, Ota S. VTE prophylaxis for the medical patient: where do we stand? - a focus on cancer patients. Thromb Res. 2010;125 Suppl 2:S21-S29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Francis CW. Prevention of venous thromboembolism in hospitalized patients with cancer. J Clin Oncol. 2009;27:4874-4880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 56. | Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450-2457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 715] [Cited by in F6Publishing: 709] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 57. | Farge D, Durant C, Villiers S, Long A, Mahr A, Marty M, Debourdeau P. Lessons from French National Guidelines on the treatment of venous thrombosis and central venous catheter thrombosis in cancer patients. Thromb Res. 2010;125 Suppl 2:S108-S116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol. 2014;8:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 59. | Akl EA, Karmath G, Yosuico V, Kim SY, Barba M, Sperati F, Cook D, Schünemann HJ. Anticoagulation for thrombosis prophylaxis in cancer patients with central venous catheters. Cochrane Database Syst Rev. 2007;CD006468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Couban S, Goodyear M, Burnell M, Dolan S, Wasi P, Barnes D, Macleod D, Burton E, Andreou P, Anderson DR. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063-4069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 61. | Debourdeau P, Farge-Bancel D, Bosquet L, Kassab-Chahmi D, Cajfinger F, Desmurs-Clavel H, Desruennes E, Douard MC, Elias A, Elalamy I. [2008 Standards, Options: recommendations for venous thromboembolic events (VTE) treatment and central venous catheter thrombosis (CVCT) management in cancer patients]. Bull Cancer. 2008;95:750-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 62. | Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S-e226S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1087] [Cited by in F6Publishing: 1058] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 63. | Karthaus M, Kretzschmar A, Kröning H, Biakhov M, Irwin D, Marschner N, Slabber C, Fountzilas G, Garin A, Abecasis NG. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, Shepherd S, Stanley A, Sweeney A, Wilde J. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009;373:567-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 65. | Johnson MJ, Sproule MW, Paul J. The prevalence and associated variables of deep venous thrombosis in patients with advanced cancer. Clin Oncol (R Coll Radiol). 1999;11:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, Samosh M, Bramwell V, Pritchard KI, Stewart D. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 396] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, Melzer N, Kakkar AK. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 68. | Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 69. | Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 70. | Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, Sgouros J, Gardiner E, Wedgwood K, Ettelaie C. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 71. | Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, Müller L, Grunewald M, Stieler JM, Sinn M. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J Clin Oncol. 2015;33:2028-2034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 72. | Ben-Aharon I, Stemmer SM, Leibovici L, Shpilberg O, Sulkes A, Gafter-Gvili A. Low molecular weight heparin (LMWH) for primary thrombo-prophylaxis in patients with solid malignancies - systematic review and meta-analysis. Acta Oncol. 2014;53:1230-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1362] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 74. | Khorana AA, Otten HM, Zwicker JI, Connolly GC, Bancel DF, Pabinger I. Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1928-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 76. | Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7:291-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 77. | Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 574] [Cited by in F6Publishing: 546] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 78. | Suenaga M, Mizunuma N, Shinozaki E, Matsusaka S, Ozaka M, Ogura M, Chin K, Yamaguchi T. Anticoagulant therapy for venous thromboembolism detected by Doppler ultrasound in patients with metastatic colorectal cancer receiving bevacizumab. Onco Targets Ther. 2015;8:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 79. | Hingorani SR, Harris WP, Hendifar AE, Bullock AJ, Wu XW, Huang Y, Jiang P. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. J Clin Oncol. 2015;suppl 33:abstr 4006. [Cited in This Article: ] |
| 80. | Lee A, Kamphuisen W, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA. A randomized trial of longterm tinzaparin a low molecular weight heparin versus warfarin for treatment of acute venous thromboembolism in cancer patients - the CATCH study. Autorisiert für elektronische Publikation: AWMF online Aktuelle PDF-Datei erzeugt: 14. 07 2014; 2. [Cited in This Article: ] |
| 81. | Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1831] [Cited by in F6Publishing: 1689] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 82. | Noble S. The challenges of managing cancer related venous thromboembolism in the palliative care setting. Postgrad Med J. 2007;83:671-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Johnson MJ. Problems of anticoagulation within a palliative care setting: an audit of hospice patients taking warfarin. Palliat Med. 1997;11:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Lee AY. Thrombosis in cancer: an update on prevention, treatment, and survival benefits of anticoagulants. Hematology Am Soc Hematol Educ Program. 2010;2010:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, Beltrami CA, Bartoli E. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397-400. [PubMed] [Cited in This Article: ] |
| 86. | Rodriguez-Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;32:1465-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Senzolo M, M Sartori T, Rossetto V, Burra P, Cillo U, Boccagni P, Gasparini D, Miotto D, Simioni P, Tsochatzis E. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 88. | Aldawood A, Arabi Y, Aljumah A, Alsaadi A, Rishu A, Aldorzi H, Alqahtani S, Alsultan M, Felemban A. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Saccullo G, Marietta M, Carpenedo M, De stefano V, Falanga A, Federici A, Rodeghiero F, Tosetto A, Siragusa S. Platelet Cut-Off For Anticoagulant Therapy In Cancer Patients With Venous Thromboembolism and Thrombocytopenia: An Expert Opinion Based On RAND/UCLA Appropriateness Method (RAM). Blood. 2013;122:581. [Cited in This Article: ] |
| 90. | Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 91. | Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 963] [Cited by in F6Publishing: 877] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 92. | Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol. 2000;32:865-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 93. | Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 94. | Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S-e88S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1106] [Cited by in F6Publishing: 1016] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 95. | Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, Ramirez L, Julian J. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012;10:807-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 96. | Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1533] [Cited by in F6Publishing: 1519] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 97. | Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2218] [Cited by in F6Publishing: 2161] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 98. | Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1250] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 99. | Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1646] [Cited by in F6Publishing: 1608] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 100. | Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1838] [Cited by in F6Publishing: 1743] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 101. | Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZ. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 102. | Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134:774-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 103. | van der Hulle T, den Exter PL, Kooiman J, van der Hoeven JJ, Huisman MV, Klok FA. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J Thromb Haemost. 2014;12:1116-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 104. | Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147:475-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 105. | Larsen TB, Nielsen PB, Skjøth F, Rasmussen LH, Lip GY. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: a semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS One. 2014;9:e114445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatientchemotherapy. J Thromb Haemost. 2007;5:632-634. [PubMed] [Cited in This Article: ] |