Abstract
Catheter-based renal denervation (RDN) has been shown to reduce sympathetic nerve activity and blood pressure in patients with resistant hypertension1-3. Increased central sympathetic activity is a main contributor to the pathophysiology of several important chronic cardiovascular diseases, including diabetes and the metabolic syndrome. Indeed several recently published pilot studies and case reports suggest beneficial effects of RDN on glucose metabolism in patients with resistant hypertension. This review highlights the background of approaching the renal sympathetic nerves as a potential new therapeutic option to improve glycaemic control in patients with resistant hypertension.
Introduction
The benefit of renal denervation may not be restricted to blood pressure lowering alone since several cardiovascular diseases are characterised by excessive central sympathetic drive4. Indeed, chronic activation of the sympathetic nervous system has been associated with the components of the metabolic syndrome, such as hyperinsulinaemia, type 2 diabetes, and obesity5,6. The metabolic syndrome, which is extremely common worldwide, can be found in approximately 30% of patients with essential hypertension, identifying patients at high cardiovascular risk7,8. Over 50% of patients with essential hypertension are hyperinsulinaemic, regardless of whether they are untreated or in a stable programme of treatment9. Elevated fasting glucose levels, impaired glucose tolerance and diabetes have been associated with an increased risk of cardiovascular disease10-13, due to stimulation of inflammation, oxidative stress and thrombotic potential14, as well as inhibition of vascular smooth muscle cell apoptosis15. Insulin resistance is involved in the pathogenesis of type 2 diabetes mellitus with a progression from impaired fasting glycaemia to impaired glucose tolerance and finally to overt diabetes. The Spanish Ambulatory Pressure Monitoring registry16 included 70,000 patients and identified 8,300 (12%) to be resistant to drug treatment: of these, 35% were diagnosed as diabetics, representing the most common comorbidity. The present review highlights the background of sympathoinhibition and potential beneficial effects of renal denervation in patients with resistant hypertension.
Pathophysiology
Heightened central sympathetic activity is an accepted contributor to insulin resistance17, metabolic syndrome18, associated with central obesity, and risk of developing diabetes5. Data confirm a reciprocal relation wherein heightened central sympathetic activity contributes to insulin resistance, metabolic syndrome and risk of developing diagnosed diabetes, as well as the converse, where these clinical conditions themselves contribute to central sympathetic drive7. The combination of essential hypertension and diabetes mellitus type 2 has been associated with the greatest sympathetic hyperactivity as measured by resting muscle sympathetic nerve activity (MSNA) compared to each condition by itself (Figure 1)5.
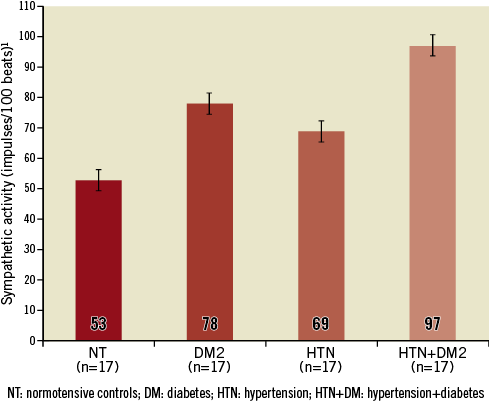
Figure 1. Sympathetic activity measured by MSNA in normotensive controls (NT), diabetes mellitus type 2 (DM2), hypertension (HTN) and metabolic syndrome (HTN+DM2). Modified from Huggett et al5.
The predominant mechanism linking sympathetic drive to insulin resistance is likely related to sympathetically mediated redistribution of blood flow from insulin-sensitive striated muscle, towards insulin-insensitive fat tissue7. As such, in the human forearm, increased noradrenaline release results in a substantial reduction in forearm blood flow19. It has been proposed that pressure-induced restriction of the microcirculation limits nutritional flow, and thereby impairs glucose uptake in the skeletal muscle20. There is clinical and experimental data indicating: i) that there is a direct relationship between sympathetic nerve activity to the skeletal muscle tissue and insulin resistance, and ii) that insulin resistance is inversely related to the number of open capillaries21. In turn, increased levels of insulin exhibit sympathoexcitatory effects22,23, contributing to activation of the sympathetic nervous system with its pathophysiological consequences. There is a bidirectional relationship between sympathetic overactivity inducing insulin resistance and hyperinsulinaemia producing sympathetic activation, thus initiating a vicious cycle (Figure 2).
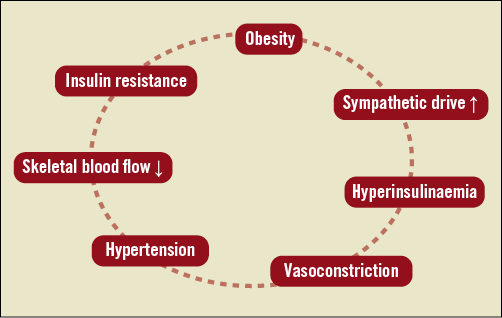
Figure 2. Vicious circle initiated by increased sympathetic activity.
The gold standard in diagnosing insulin resistance is the hyperinsulinaemic-euglycaemic clamp method, but this approach is not suitable for routine clinical practice. Thus, less invasive methods for evaluation, such as homeostasis model assessment (such as HOMA-IR = [Glucose x Insulin]/405), were developed. HOMA-IR is an established parameter for evaluation of insulin resistance24,25, correlating to the results of hyperinsulinaemic-euglycaemic clamp26. There is a direct relationship between the central sympathetic activity, measured by MSNA, and the HOMA-IR17.
In line with this, inhibition of the sympathetic nervous system by moxonidine has been shown to improve glucose metabolism by decreasing glucagon secretion and increasing skeletal blood flow with less glycogenolysis and gluconeogenesis27, which confirms the pathophysiological relation between the central nervous system and insulin resistance28. However, the use of centrally acting sympatholytics is limited by adverse effects, leading to high non-adherence rates29.
Effects of renal denervation on glucose metabolism
Since renal denervation has a favourable safety profile and results in a marked reduction of the sympathetic activity measured by MSNA30, the benefits of the procedure may not be restricted to treating resistant hypertension. A recently published pilot study investigated the effect of renal denervation on glucose metabolism and insulin resistance in patients with resistant hypertension31. Fifty patients with resistant hypertension were included in the study (37 underwent renal denervation, 13 served as controls), and 40% were diabetics. Besides significant blood pressure reduction observed in the treatment group (–32/–12 mmHg; p<0.001) after three months, fasting glucose (from 118±3.4 mg/dl to 108±3.8 mg/dl; p=0.039), insulin levels (from 20.8±3.0 μIU/ml to 9.3±2.5 μIU/ml; p=0.006), C-peptide levels (from 5.3±0.6 ng/ml to 3.0±0.9 ng/ml; p=0.002) and the HOMA-IR improved significantly from 6.0±0.9 to 2.4±0.8 (p=0.001) after three months (Figure 3).
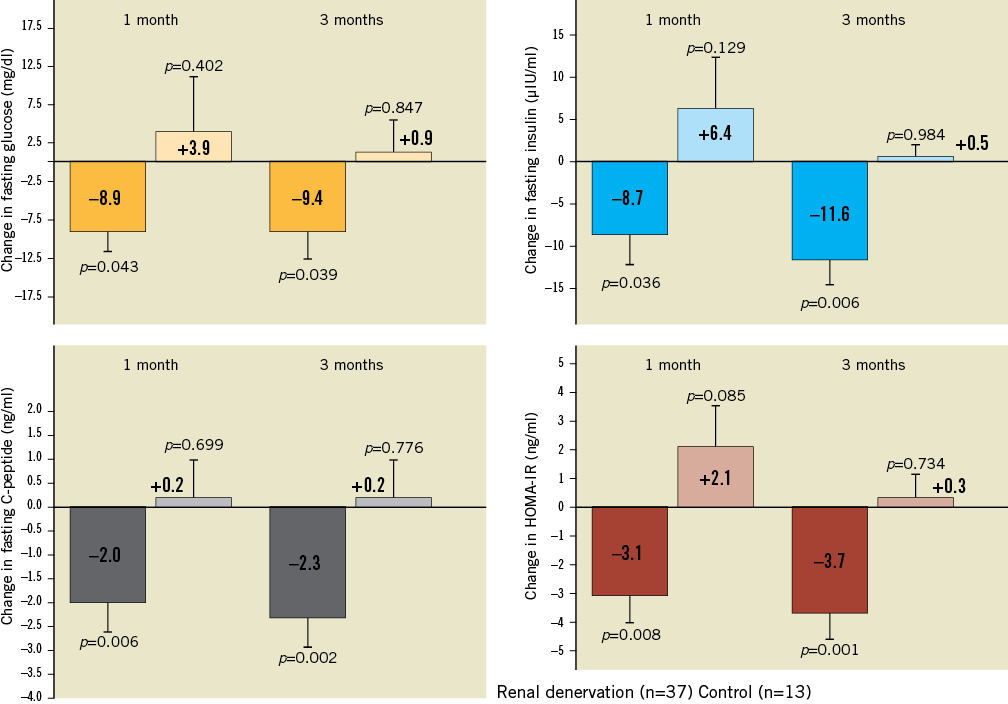
Figure 3. Change in fasting glucose, insulin levels, C-peptide levels, and the HOMA-IR in patients undergoing renal denervation and in the control group. Modified from Mahfoud et al38.
Additionally, mean two-hour glucose levels during an oral glucose tolerance test were reduced by 27 mg/dl (p=0.012, Figure 4) while there were no significant changes in blood pressure or any of the metabolic markers described above in the control group. Body mass index and antihypertensive background medication remained unchanged during the study period.
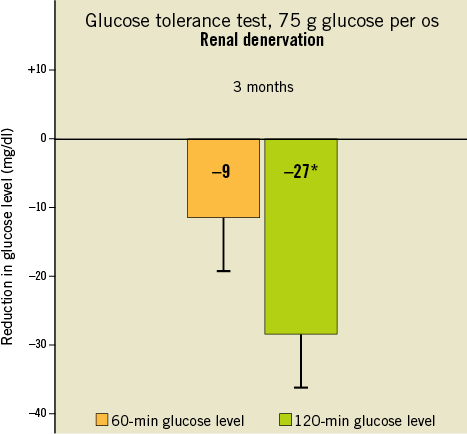
Figure 4. Change in 60-min and 120-min glucose level (n=37) after administration of glucose 3 months after renal denervation. Modified from Mahfoud et al38.
POLYCYSTIC OVARY SYNDROME
These findings are supported by investigations of patients with polycystic ovary syndrome (PCOS)32. PCOS is characterised by obesity, insulin resistance and BP elevation related to sympathetic nervous activation. Using the hyperinsulinaemic-euglycaemic clamp methodology it was demonstrated that insulin sensitivity improved by 17.5% in the absence of any weight change at three months following renal denervation. Besides the improvements in glucose metabolism, authors also report a reduction in sympathetic activity measured by MSNA, urinary albumin excretion and glomerular hyperfiltration, indicating beneficial effects of renal denervation on renal structure and function. These findings are of interest as renal denervation has been shown to prevent the development of structural renal changes due to early diabetic nephropathy in an animal model33. Functional and anatomic studies performed two weeks after the onset of streptozotocin-induced diabetes in denervated rats revealed attenuation of physiologic and anatomic findings of early diabetic nephropathy. In line with this, studies in humans demonstrated that sympathoinhibition with the centrally acting drug moxonidine reduced microalbuminuria in normotensive patients with type 1 diabetes, in the absence of any significant blood pressure changes34. This is supported by recently published data, investigating the effects of renal denervation on urinary albumin excretion in 100 patients with resistant hypertension and preserved renal function. The study demonstrated a reduced number of patients with micro- and macroalbuminuria after renal denervation and improvements in renal haemodynamics35.
OBSTRUCTIVE SLEEP APNOEA
Individuals with obstructive sleep apnoea syndrome (OSAS) are usually obese and have a high prevalence of the metabolic syndrome7. OSAS itself is characterised by an increased sympathetic activity36. A recently published pilot study investigated the effects of renal denervation on OSAS severity and glucose metabolism in patients with resistant hypertension37. This observational series has confirmed significant reduction in blood pressure (-34/-13 mmHg; p<0.01) and improvement in the OSAS severity in eight of 10 enrolled patients six months after renal denervation. Decreases were also observed in plasma glucose concentration two hours after glucose administration (median: 7.0 versus 6.4 mmol/L; p=0.05) and in haemoglobin A1C level (median: 6.1% versus 5.6%; p<0.05) at six months. A larger randomised controlled trial investigating the effect of renal denervation in patients with OSAS is currently ongoing (NCT01366625).
Outlook
Since renal denervation in patients with resistant hypertension has been shown to reduce sympathetic activity and blood pressure, and to improve glucose metabolism and glucose tolerance, it may be speculated that this treatment prolongs or even prevents the progression of type 2 diabetes and associated cardiovascular complications. The estimated change in cardiovascular risk associated with blood pressure reduction and improvement in diabetic state appears to be more than additive. Preclinical observations on renal protection against diabetic glomerular sclerosis coupled with the human data on renal denervation resulting in reduced blood pressure with improved insulin sensitivity and urinary albumin excretion justify additional studies in this area. Substantial research is needed i) to enhance the understanding of the potential retinal, renal and cardiovascular consequences of these findings, and ii) to document the durability of the results. The DREAMS (Denervation of the REnal Artery in Metabolic Syndrome, NCT 01465724) study has been conducted to investigate the effects of renal denervation in patients with metabolic syndrome.
Funding
F. Mahfoud, C. Ukena and M. Böhm are supported by the Ministry of Science and Economy of the Saarland. F. Mahfoud and M. Böhm are supported by the Deutsche Forschungsgemeinschaft (KFO 196). F. Mahfoud and S. Ewen are supported by Deutsche Hochdruckliga. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie.
Conflict of interest statement
F. Mahfoud, C. Ukena, B. Cremers and M. Böhm received speakers’ honoraria from Medtronic/Ardian, St. Jude Medical and/or Cordis. P. A. Sobotka was an employee of Medtronic/Ardian. S. Ewen and D. Linz have no conflicts of interest to declare.




