Abstract
Pulmonary arterial hypertension (PAH) is a severe, progressive and fatal disease. The creation of an interatrial right-to-left shunt in patients with PAH may enhance systemic ventricular output at the expense of desaturation. However, creating sustainable restricted interatrial communication is challenging. We describe the successful use of an atrial flow regulator, a novel implantable atrial communication device, in a 54-year-old female with severe irreversible PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a severe progressive disease leading to a gradual increase in right ventricular (RV) pressure, right heart failure (HF), and death1. Despite recent advances in treatment, many patients continue to deteriorate while on optimal medical therapy with decline in quality of life, a high rate of hospitalisations and increased medical costs. Thus, there is a need for innovative therapies and interventions to improve long-term outcomes.
Increasing systemic ventricular output by promoting right-to-left shunt via atrial fenestration may improve effective systemic oxygen transport and delivery despite arterial oxygen desaturation1. Percutaneous stent implantation and balloon dilatation of the interatrial septum (IAS) are well-established techniques to create or enlarge atrial communication in a variety of conditions in order to improve cardiac output2,3. However, complications experienced with these standard techniques include early spontaneous closure of the fenestration, excessive desaturation, stent occlusion or migration, and difficulties in adjusting shunt size to achieve the desired haemodynamic effects2. We describe the successful emergency use of an atrial flow regulator (AFR; Mia Medical, Istanbul, Turkey), a novel implantable device with central fenestration, in a 54-year-old female with severe PAH.
CASE REPORT
A 54-year-old female with progressive PAH was admitted for management of acute worsening chronic refractory right HF with progressive ascites and gross bilateral pitting oedema. Her history included insulin-dependent type 2 diabetes mellitus, dyslipidaemia and ongoing tobacco use. At age 43, she was diagnosed with severe pulmonary hypertension with moderately elevated pulmonary vascular resistance (PVR). Right atrial (RA) pressure was 21/18 (16) mmHg, pulmonary artery (PA) pressure was 70/32 (46) mmHg with systemic blood pressure of 98/52 (66) mmHg. Cardiac output was 6.5 L/min (Fick method) with PVR of 4.5 Wood units. She had an ostium secundum atrial septal defect (ASD) percutaneously occluded with an unfenestrated 22 mm Amplatzer™ septal occluder (ASO) (St. Jude Medical, St. Paul, MN, USA) with a left disc diameter of 36 mm at another institution. Prior to device implantation, a balloon test occlusion was performed for 20 minutes without any change in PA pressure or PVR.
A three-dimensional transoesophageal echocardiogram (3D TEE) showed severe right heart dilatation with moderate reduction in the RV systolic function. Prior to the procedure, she was on riociguat, high-dose furosemide and continuous ambulatory intravenous epoprostenol. In view of refractory ascites and poor RV function, she was hospitalised and placed on intravenous diuretic therapy and dopamine. Her exercise tolerance was limited by shortness of breath, New York Heart Association (NYHA) functional Class III. Her six-minute walk test distance was 363 metres two years prior to the intervention, and she was progressively becoming restricted in her mobility.
As a result of worsening PAH, a decision was made by a multidisciplinary team including a pulmonologist and lung transplant director, congenital heart specialists and an anaesthesiologist, to decompress and improve her right heart function with an atrial fenestration as the patient refused lung transplantation and smoking cessation. The AFR device was selected for use under the United States Food and Drug Administration’s (FDA) emergency use guidance. The patient was given detailed information on the risks and benefits of the procedure, including complications of general anaesthesia, TEE, cardiac catheterisation and the device itself. The potential risks associated with the AFR device during or after implantation include air embolus, allergic reaction to nickel, arrhythmia, bleeding, injury to vessels, device embolisation and migration, and thromboembolic events. She signed an emergency use informed consent form after all her questions were answered to her satisfaction prior to the procedure.
AFR DEVICE DETAILS
The AFR is a self-expandable double-disc wire mesh device constructed from 0.004–0.0075 inch nitinol (51% nickel, 49% titanium) tightly braided into two flat discs (Figure 1). A 1-2 mm connecting waist exists between the two discs, corresponding to the thickness of the atrial septum (Figure 2). The device is available in 6, 8 and 10 mm fenestration diameters with a total device diameter of 18, 24 and 30 mm requiring a delivery system of 10, 11 and 12 Fr, respectively (Figure 3).
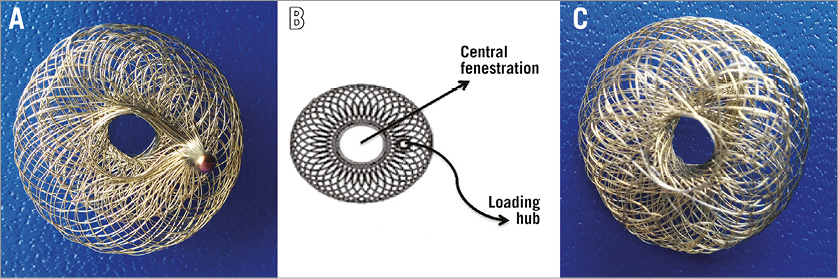
Figure 1. Atrial flow regulator. A) Right atrial disc with loading hub. B) Technical drawing of right atrial disc with loading hub. C) Left atrial disc.
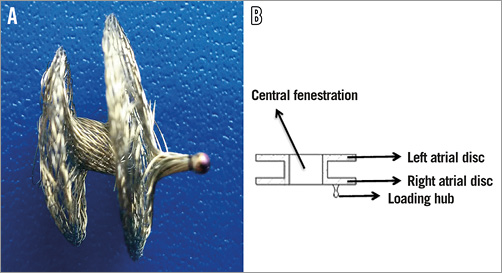
Figure 2. Atrial flow regulator. A) The 1-2 mm connecting waist shown between the two discs. B) Technical drawing depicting the connecting waist between the two discs.
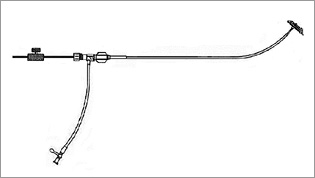
Figure 3. Technical drawing of delivery system.
PROCEDURE
Under general anaesthesia, 3D TEE showed the ASO device occupying the majority of the IAS, leaving approximately 9-12 mm near the inferior vena cava (IVC) rim for AFR implantation. A detailed right heart catheterisation was performed under aseptic precautions with stepwise oximetry and haemodynamics. The patient’s RA pressure was 19/14 (12) mmHg, PA pressure was 72/18 (34) with systemic blood pressure of 99/46 (62) mmHg. Oximetry in 30% oxygen included high superior vena cava (SVC) 74%, low SVC 70%, high RA 69.5%, low RA 69.5%, RV 68%, PA 67%, and aorta 90%. Selective angiograms were performed in the right and left pulmonary arteries to delineate the pulmonary vasculature and venous return. The levo phase of the left pulmonary angiogram at left anterior oblique (LAO) 30, cranial 30 defined the specific region below the ASO where transseptal puncture could be performed (Moving image 1A, Moving image 1B). Using 3D TEE guidance, a transseptal puncture was performed (Moving image 2). A 0.035 inch extra-stiff guidewire was introduced into the left upper pulmonary vein and a 12 Fr ASD device delivery system was advanced into place. The AFR was loaded on the proprietary delivery cable system and advanced through the sheath. The left atrial (LA) disc was deployed and the entire assembly withdrawn until it reached the IAS followed by deployment of the RA disc. After confirming secure deployment of the AFR device on 3D TEE (Figure 4), a hand injection into the sheath from the RA demonstrated adequate right-to-left shunting (Moving image 3). The delivery cable was then released from the device and withdrawn. As the discs of the AFR device were stretched between the inferior limbs of the previously placed oversized ASO, the central fenestration was smaller than its original size of 6 mm. Therefore, the fenestration of the AFR was serially dilated with 6 mm and then 8 mm non-compliant balloons at a maximum of 18 atmospheres to enlarge the central opening to its original size and ensure adequate right-to-left shunting (Moving image 4). The patient’s oxygen saturation measured prior to dilatation dropped from 95% to 89% immediately after the procedure.
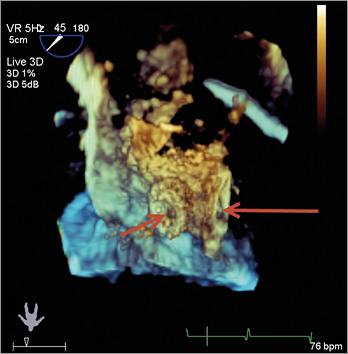
Figure 4. Post-deployment 3D TEE shows a well-seated AFR.
A post-deployment 3D TEE demonstrated a well-seated device with colour Doppler showing right-to-left shunting. There was phasic flow through the device without significant gradient, but noteworthy changes were observed with even minimal increase in tidal volume. The patient’s haemodynamics were stable throughout the procedure and there were no arrhythmias or other complications. She was extubated without any complications and discharged home the next day. As PAH warrants anticoagulation therapy, the patient’s warfarin regimen was continued after the deployment of the device. At her six-week follow-up visit, the patient reported that she had more energy with recorded resting saturation of 98%. The six-minute walk test distance was recorded at 319.74 metres. She remained without ascites and pedal oedema without any increase in her diuretic therapy.
Discussion
There is a known association between PAH and ASD in young adults, especially in females. This is independent of the degree of shunting and increased pulmonary blood flow from the ASD. Although it may be slow in its evolution, these patients can develop progressive PAH. It is essential to differentiate these patients from those who develop PAH related to large ASDs. The latter typically resolves after intervention and rarely progresses, especially with early intervention. Considering our patient’s clinical presentation and catheter findings at the time of ASD closure, she is likely to have had ASD-associated PAH. In addition, her defect was inappropriately closed with an unfenestrated device without comprehensive evaluation using pulmonary vasoreactivity testing. Patients developing PAH immediately or several months or years after shunt closure have a poorer prognosis when compared to congenital heart disease patients with PAH4,5. It is now well established that fenestrated ASD closure is preferable in the setting of ASD with moderate to severe PAH. The use of a fenestrated ASO is described in a 66-year-old female with secundum-type ASD and PAH. Twelve months after implantation, an exclusive left-to-right shunt was observed on the transthoracic echocardiogram6. As our patient received an atrial fenestration after previous ASD closure, it is important to evaluate whether a fenestrated closure device would be a better approach in patients with elevated PVR and bidirectional shunt to cease significant left-to-right shunting and at the same time allow possible overflow for right HF in the future6. However, spontaneous closure may occur in fenestrated devices2,3,6.
Contemporary medical management for patients with chronic PAH is confounded by the lack of long-term survival and has compelled the need for alternative and sustained ways for palliation. Alternative options for patients worsening despite receiving optimal medical therapy include balloon atrial septostomy and lung or heart-lung transplantation2. The trade-off to achieve a higher systemic ventricular output with marginal increase in cyanosis is desirable with an optimal saturation range of 87-90% at rest. However, creating a sustained IAS communication is a challenge due to sudden deterioration with severe desaturation and/or spontaneous closure after balloon atrial septostomy over a period of time. Thus, the problems related to maintaining an IAS opening compelled us to use a novel device that could maintain communication.
The most important advantages of the AFR device include easy deployment and reduced bulk of foreign material compared to current devices utilised for atrial fenestration. Moreover, the AFR has a central opening with no connecting area between the discs, allowing smooth internal endothelialisation of the fenestration to maintain its patency. There is no patch material sewn within the device. A stainless steel sleeve with a female thread is laser welded to the RA disc eccentrically at the periphery to allow unobstructed flow through the central fenestration. This microscrew hub is used to load the device onto the proprietary delivery cable.
FUTURE APPLICATIONS OF AFR DEVICE
Besides its use in PAH patients, the application of the AFR device may well be extended to other HF populations, especially those with severe restrictive cardiomyopathy or severe diastolic dysfunction of the LV, end-stage systolic dysfunction awaiting left ventricular assist device (LVAD), and in patients who remain symptomatic despite ambulatory inotropes with elevated LA pressures. Elevated LA pressure in chronic HF may permit left heart decompression via the atrial fenestration and recovery of LV function over time.
Conclusion
Palliative transcatheter AFR implantation to establish IAS communication is an alternative approach to increase systemic ventricular output and treat symptomatic patients with PAH. There was immediate haemodynamic and symptomatic improvement following the procedure in our patient. The application of this device may well be extended to patients with chronic refractory right and left-sided HF with elevated atrial pressures. However, further long-term multicentre clinical trials will be required to evaluate its efficacy and long-term outcomes. The use of AFR as a device-based approach to treat PAH provides a unique alternative to improve haemodynamics and symptomatic status.
| Impact on daily practice The AFR device is designed to decompress the RA or LA chambers to enhance cardiac output and improve symptomatology. It is likely to be useful in creating a fenestration in the Fontan circulation where a pop-off from the pulmonary circulation to the systemic circulation is desirable. The device may also be useful in diastolic HF of the RV or LV and provide access to the LA when repeated procedures are expected. |
Conflict of interest statement
J. Vettukattil holds the patent for the atrial flow regulator device. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1A. Levo phase of the left pulmonary angiogram at left anterior oblique 30, cranial 30.
Moving image 1B. Left pulmonary angiogram showing the left atrium and the potential location of interatrial septal wall below the Amplatzer ASO for transseptal puncture.
Moving image 2. Cineangiogram shows the site of successful transseptal puncture.
Moving image 3. RA angiogram showing right-to-left shunt through the AFR.
Moving image 4. Cineangiogram showing balloon 8×20 mm inflated through the AFR fenestration.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1a. Levo phase of the left pulmonary angiogram at left anterior oblique 30, cranial 30.
Moving image 1B. Left pulmonary angiogram showing the left atrium and the potential location of interatrial septal wall below the Amplatzer ASO for transseptal puncture.
Moving image 2. Cineangiogram shows the site of successful transseptal puncture.
Moving image 3. RA angiogram showing right-to-left shunt through the AFR.
Moving image 4. Cineangiogram showing balloon 8x20 mm inflated through the AFR fenestration.




