Is interventional cardiology largely sorted out? Can we further improve results of currently available percutaneous revascularisation techniques and devices? More poignantly, in this time of economic hardship, can we afford further developments?1,2
Science and technology are moving forward, and most likely for the better. Recent innovations in bioabsorbable endoprostheses will surely have a major impact on the management of patients with coronary artery disease, when their high price will have been decreased or will have been proved worthwhile.3 Moreover, ultra-deliverable stents have been developed4 with crossing profiles as small as 0.014”.5 Finally, the combination of antirestenotic and pro-healing effects will play an ever increasing role in achieving a safe and effective percutaneous coronary intervention (PCI).6
In the meanwhile, the key question for clinical practitioners and researchers is whether further improvements in coronary stents (i.e., metallic prostheses) are possible and clinically relevant. Indeed, recent results of randomised trials have clearly shown that first and second generation drug eluting stents (DES) can achieve very favourable clinical results, even in all comers,7-10 or when compared to the gold standard treatment for severe coronary artery disease (CAD), namely coronary artery bypass grafting (CABG).11
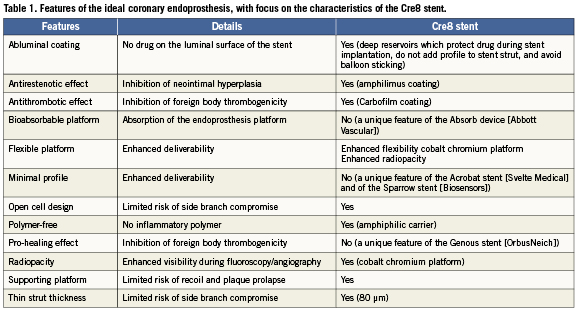
Despite such breakthroughs, restenosis and stent thrombosis may still occur after implantation of any DES,12 especially in challenging subsets such as left main disease13 or acute myocardial infarction.14 Thus, we welcome the recent preclinical study by Moretti et al which focuses on a novel DES, the Cre8 amphilimus-eluting stent (CID, Saluggia, Italy), published in this issue.15 Before focusing on the details of the Cre8 stent, it is however appropriate to reconsider what would be the ideal features of a coronary endoprostheses (Table1).
Dreaming the dream: the perfect stent
The ideal stent should most likely have a number of key features in order to become as safe and as effective as possible. First and foremost, it should be remarkably biocompatible and safe. Thus, abluminal coating, antithrombotic efficacy, absence of polymer coating, pro-healing action, open cell design and thin struts should be mandatory features. In addition, it should be easy to use and deliver. Thus, the device should be extremely flexible, with a minimal crossing profile and supporting platform, yet suitably radiopaque to be visualised under fluoroscopic imaging. Moreover, any coronary endoprosthesis should minimise neointimal hyperplasia, and thus exert a potent, yet controlled, antirestenotic effect. Finally, the ideal device would not be a stent at all (but should we still call it a stent?). Conversely, its platform and drug coating should hopefully dissolve with time without eliciting any inflammation.3,16
As “best is the enemy of the good” (le mieux est l’ennemi du bien–Voltaire), no currently available or imaginable device possesses simultaneously all the above features. Yet, several devices are available already –or will shortly be available– which can prove remarkably safe and effective, including several second generation DES, the Absorb prosthesis (Abbott Vascular, Abbott Park, IL, USA),3 the Genous stent (OrbusNeich, Ft. Lauderdale, FL, USA),6 the Acrobat stent (Svelte Medical, New Providence, NJ, USA),4 and the Sparrow stent (Biosensors, Singapore).5
Is the Cre8 stent a true creation?
The Cre8 stent is a mammalian target of rapamycin (m-TOR) inhibitor polymer-free eluting stent pre-mounted on a balloon catheter. The stent is a thin (80 µm) cobalt-chromium alloy L605 structure characterised by two main features: the presence of proprietary reservoirs on the stent’s outer surface, devoted to drug formulation containment, and a bio-inducer surface based on a permanent ultra-thin and high-density turbostratic carbon coating called i-Carbofilm, which has improved biocompatibility and is 0.03µm thick.17
The Cre8 uniqueness also consists in its formulation called amphilimus characterised by a non-polymeric amphiphilic carrier and an m-TOR inhibitor drug loaded at 0.9 µg/mm2. The m-TOR inhibitor family is widely applied in DES, while the carrier is a small molecule extensively used as an excipient in pharmaceutical field and widely present in the body. Thanks to its amphiphilic properties, it is able to modulate the drug elution from the abluminal reservoirs into the vessel wall. In fact in a preliminary pharmacokinetic study reported by the authors, the Cre8 stent presents a maximum drug blood release concentration in rabbit virtually equal to zero (Cmax=1.74±0.372 ng/mL). This was also confirmed by an extremely low drug systemic availability over 72 hours (AUC=35.8±19.4 ng×h/mL). The tissue drug concentration profile thus shows an initial peak followed by a sustained release for the subsequent 28 days. As further evidence of this device’s prolonged drug elution, quantification of the drug remaining on the stent revealed that only 50% of the drug total amount was released in about 18 days.
In the study by Moretti et al15, a total of 24 juvenile domestic pigs randomly received two stents (one stent in the left coronary artery and one in the right coronary artery) a total of 48 stents. The Cre8 stents were compared in vivo with the same stent platform loaded with just the amphiphilic carrier (R3 stent) and with sirolimus-eluting stents (SES, Cypher Select Plus, Cordis, Miami, FL, USA). Six pigs were sacrificed (12 stents) at seven days to conduct scanning electronic microscopy analysis to assess endothelisation extent, and in all groups the endothelial cells covered almost 100% of the strut surface. Only for the SES was the endothelial cell layer characterised by inhomogeneous cell growth.
Eighteen stents were explanted at 30 days and an additional 18 stents were explanted at 90 days follow-up for histological and histomorphometric examinations. Notably at 30 days, the measured neointimal thickness was lower for Cre8 (0.10±0.07mm) as compared to the other two groups (R3 is 0.19±0.15 mm and SES is 0.17±0.13 mm, p>0.05), as was the neointimal area of the Cre8 group in relation to the SES group (Cre8 0.93±0.43mm2; R3 1.49±0.67mm2; SES 1.81±0.94mm2; Cre8 vs. SES, p<0.05). At 90 days, SES showed a statistically higher neointimal growth than Cre8 stent (Cre8 0.15±0.07mm; R3 0.21±0.12mm; SES 0.31±0.15mm; Cre8 vs. SES p<0.05), with neointimal area 1.27±0.56mm2 for Cre8, 1.87±0.60mm2 for R3 and 2.79±1.14mm2 for SES, respectively (Cre8 and R3 vs. SES p<0.05). Notably, acute and chronic inflammatory profile was significantly less with Cre8 compared to the R3 and SES groups.
Accordingly, in support of the enhanced biocompatibility of the i-Carbofilm coating, antiplatelet treatment was limited to aspirin from two days before to the seventh day after the intervention, with no supplementary antiplatelet drugs. Despite this premature antiplatelet discontinuation and the ensuing risks18, neither thrombus, incomplete strut apposition or myocardial infarction were detected in any animal either by angiography or by histology.
Notwithstanding the crucial role of preclinical research in guiding clinical research and, possibly, also informing clinical practice by explaining pathophysiologic mechanisms, data on clinical safety and efficacy are eagerly awaited.19,20 Further insights will certainly be provided by the upcoming presentation at EuroPCR 2012 of the NEXT study, a randomised trial comparing the Cre8 stent with the Taxus Liberté stent in 300 patients with de novo coronary lesions. Preliminary data have already been reported by Didier Carrié at the 2011 TCT meeting.
Conclusions
Despite major achievements in the development of coronary stents, further improvements can still be envisioned and realistically pursued. The novel Cre8 amphilimus-eluting stent holds the promise of becoming a useful adjunct in the interventional cardiologist’s armamentarium. Perhaps another paper on a new stent is not as important as that stent’s introduction into clinical practice.
Conflicts of interest
G. Biondi-Zoccai has consulted for Abbott Vascular, CID, Cordis, Medtronic; lectured for Boston Scientific and Medtronic; and received a career grant from Medtronic. G. Sangiorgi has consulted for Biotronik, Eurocor and Medtronic and lectured for Abbott Vascular, Cordis and Medtronic. The other authors have no conflict of interest to declare.
References




