Abstract
Background: The long-term prognostic implications of fractional flow reserve (FFR)-negative lesions hosting vulnerable plaques remain unsettled.
Aims: The aim of this study was to evaluate the association of non-ischaemic lesions hosting optical coherence tomography (OCT)-detected thin-cap fibroatheromas (TCFA) with first and recurrent cardiovascular events during follow-up up to 5 years in a diabetes mellitus (DM) patient population.
Methods: COMBINE OCT-FFR is a prospective, international, double-blind, natural history study. Patients with DM and with ≥1 FFR-negative lesion were classified into 2 groups based on the presence or absence of ≥1 TCFA lesion. The primary endpoint (PE) is a composite of cardiac mortality, target vessel-related myocardial infarction (TV-MI), clinically driven target lesion revascularisation (TLR), or unstable angina (UA) requiring hospitalisation during follow-up up to 5 years.
Results: Among 390 DM patients (age 67.5±9 years; 37% female) with ≥1 FFR-negative lesion, 292 (74.9%) were TCFA-negative while 98 (25.1%) were TCFA-positive. The PE occurred more frequently in TCFA-positive than in TCFA-negative patients (21.4% vs 8.2%, hazard ratio [HR] 2.89, 95% confidence interval [CI]: 1.61-5.20; p<0.001; 6.42 vs 2.46 events per 100 patient-years, rate ratio [RR] 2.61, 95% CI: 1.38-4.90; p=0.002). Furthermore, when TV-MI, TLR, and UA were treated as recurrent components of the PE, TCFA-positive patients experienced a higher risk of recurrent events (HR 2.89, 95% CI; 1.74-4.80; p<0.001; 13.45 vs 2.87 events per 100 patient-years, RR 4.69, 95% CI: 2.86-7.83; p<0.001). A multivariable analysis identified the presence of TCFA as an independent predictor of the PE (HR 2.76, 95% CI: 1.53-4.97; p<0.001).
Conclusions: OCT-detected TCFA-positive lesions, although not ischaemia-generating, are associated with an increased risk of adverse events during long-term follow-up. ClinicalTrials.gov: NCT02989740
Introduction
In chronic coronary syndrome (CCS), current guidelines reserve percutaneous coronary intervention (PCI) for the treatment of ischaemia-generating coronary stenoses, identified by haemodynamic interrogation with fractional flow reserve (FFR) or other ischaemia detection methods, and highlight the safety of pharmacological treatment for non-flow-limiting lesions1. Yet, in certain patient subgroups, the prognosis of intermediate coronary stenoses may not only be linked to their ischaemia-generating potential.
In patients with diabetes mellitus (DM), the destabilisation of angiographically intermediate medically treated lesions occurs frequently234, and the high prevalence of vulnerable plaques may account for the observed increase in clinical events, despite the apparent absence of myocardial ischaemia56. As shown by histopathological studies7, thin-cap fibroatheroma (TCFA) is the main type of vulnerable plaque that is responsible for coronary events. In vivo identification of such vulnerable plaques is feasible with optical coherence tomography (OCT), a high-resolution intracoronary imaging tool89.
It remains largely unknown whether vulnerable plaque morphology, such as TCFA, increases the risk of future cardiovascular events irrespective of the presence of significant flow-limiting lesions. To investigate the differential impact of plaque vulnerability and myocardial ischaemia, the COMBINE OCT-FFR study10, a prospective double-blind international natural history study, focused on the impact of OCT-detected TCFA on clinical outcomes of DM patients with angiographically intermediate but otherwise non-ischaemic (i.e., FFR-negative) lesions.
The COMBINE OCT-FFR study showed that OCT-detected TCFA in patients with DM predicted future adverse event risk up to 18 months, despite the absence of myocardial ischaemia. The aim of the present analysis was to investigate the impact of OCT-detected TCFA on clinical outcomes up to 5 years of follow-up of the patients enrolled in the COMBINE OCT-FFR study.
Methods
The COMBINE OCT-FFR (ClinicalTrials.gov: NCT02989740) is a prospective, double-blind, international, natural history study that was conducted at 14 sites in 7 countries. The design of the COMBINE OCT-FFR study11 and the main results have been published previously10. In brief, the study combined haemodynamic (by FFR) and morphologic (by OCT) assessments of intermediate lesions in patients with DM who had undergone angiography for any clinical indication and had at least 1 de novo native coronary lesion with a diameter stenosis of 40-80% by visual assessment (other than the culprit lesion, in patients presenting with myocardial infarction).
The full inclusion and exclusion criteria are reported in the Supplementary Appendix 1. In patients who presented with acute coronary syndrome (ACS), the culprit lesion was revascularised first. Lesions that were deemed by the operator to be clearly severe (>80% diameter stenosis) and/or had a Thrombolysis in Myocardial Infarction (TIMI) flow <3 were also eligible for revascularisation without the need for a physiological assessment.
Then, all the remaining intermediate lesions were assessed using FFR. Patients with exclusively FFR-positive lesions (i.e., FFR ≤0.80) underwent revascularisation of these lesions. Patients with ≥1 FFR-negative lesion (i.e., FFR>0.80) underwent OCT assessment and represent the study population of this analysis. Lesions that were assessed using combined FFR and OCT were defined as target lesions. The OCT core lab findings were blinded to patients, operators and the team that performed the clinical follow-up.
The study was approved by the national regulatory agencies and the institutional review boards of all the participating centres. All patients gave informed consent prior to enrolment.
Quantitative and qualitative OCT analysis
A summary of OCT definitions and analysis methodology has been reported previously1011. The OCT analysis was based on a consensus document by Tearney et al regarding the acquisition, measurement and reporting of OCT studies12. OCT image analysis scrutinised serial cross-sectional images of the vessel in every frame of OCT pullback, starting at 5 mm distal to and ending 5 mm proximal to the OCT-defined lesion border. Signal-rich homogeneous plaques were classified as fibrous, signal-poor regions with diffuse borders were classified as lipid-rich plaques (LRP), and signal-poor regions with well-defined borders as calcified plaques. TCFA was defined as any lesion with predominantly LRP, with the thinnest part of the atheroma cap ≤65 μm, and a lipid arc of more than 90°. Healed plaques were detected using the landmark of multiple heterogeneous signal-rich layers of different optical signal density located close to the luminal surface with clear demarcation from the underlying tissue13.
The inter-rater agreement analysis for OCT-defined TCFA identification was kappa=0.81 (95% confidence interval [CI]: 0.70-0.97), and the intra-rater agreement was kappa=0.78 (95% CI: 0.61-0.92). The analysis was performed using the CAAS IntraVascular 2.0 software (Pie Medical Imaging).
Endpoints
The primary endpoint was a composite of cardiac death (CD), target vessel-related myocardial infarction (TV-MI), target lesion revascularisation (TLR), or hospitalisation due to unstable angina (UA), assessed in the FFR-negative and TCFA-positive patients, as compared to the FFR-negative and TCFA-negative patients, during 5 years of follow-up. As a secondary analysis, TV-MI, TLR, and UA were treated as recurrent components of the endpoint, while we treated CD as a terminal event.
All adverse events were adjudicated by an independent clinical event committee with members who were blinded to the results of the OCT analysis.
Statistical analysis
At least 334 patients with ≥1 lesion with an FFR>0.80 needed to be enrolled in the study to ensure 80% power for the difference in the primary outcome at 18 months of follow-up. Details of the sample size calculation and study design justification have been previously published1011.
Categorical variables were expressed as absolute frequencies and percentages, while continuous variables were presented as mean±standard deviation or median (interquartile range [IQR]), as appropriate. The categorical data were compared using Fisher’s exact test or the chi-square test. Normally distributed data were compared using the Student’s t-test, and non-normally distributed data were compared using the Mann-Whitney U test.
The cumulative incidence of the primary and secondary endpoints, in relation to OCT-detected plaque morphology on a patient level, was estimated using the Kaplan–Meier method with an “at risk” table and log-rank test. For the primary endpoint analysis, Cox proportional hazards models were used to calculate the hazard ratios (HR) and respective 95% confidence intervals (CI). All variables of clinical importance were also used as potential covariates in multiple Cox regression models (TCFA, healed plaque, plaque rupture, macrophage infiltration, stenosis at the minimal lumen area [MLA] site, MLA, stenosis length, FFR, admission for ACS, insulin treatment, statin at discharge). The final model was obtained using a stepwise approach with minimisation of the Bayesian information criterion as the target. A model validation was performed using bootstrap resampling; proportional hazard assumption was tested by the examination of Schoenfeld residuals. The model’s goodness-of-fit was assessed using the C-statistic.
An additional analysis was performed using the Prentice-Williams-Peterson model1415, where TV-MI, TLR, and hospitalisation due to UA were reported as recurrent events and CD as a terminal event. Results were presented as HR with 95% CI and also presented as numbers per 100 patient-years with 95% CI; results were visualised using mean cumulative function estimates and plots16. Raw data for each patient’s events with their survival time were also shown separately for both TCFA-negative and TCFA-positive groups.
No missing data imputation was performed for the primary endpoint analysis. The time to last observation was used in Cox regression models. A 2-sided level of significance for the primary endpoint was set to 0.05. All statistical analyses were performed in R 4.1.1 (R Foundation for Statistical Computing) with the rms (version 6.2-0) and survminer (version 0.4.9) packages.
Results
Study population
A total of 390 patients with ≥1 FFR-negative lesion underwent OCT evaluation. The mean age of the patients was 67.5±9 years, 63% were male, and the mean FFR value at baseline was 0.88±0.05. Among all FFR-negative patients, 292 were TCFA-negative and 98 TCFA-positive, based on evaluation with OCT. The baseline characteristics of TCFA-positive and TCFA-negative patients were well balanced and are shown in Table 1. The majority of patients (75%) presented with CCS. The median follow-up was 1,309 days (IQR 995-1,555).
Interestingly, statin treatment at discharge was less frequent in patients with versus without TCFA lesions, while P2Y12 inhibitors at discharge were more frequent in patients with versus without TCFA lesions. However, both differences did not persist at the extended follow-up timepoint (Supplementary Table 1).
Table 1. Demographics and patient characteristics of patients with and without TCFA.
| Variables | FFR(−)/TCFA(+)n=98 | FFR(−)/TCFA(−)n=292 | p-value | |
|---|---|---|---|---|
| Median age (IQR), yrs | 70 (59-76) | 68 (62-74) | 0.87 | |
| Median BMI (IQR)a | 29 (27-33) | 29 (26-32) | 0.99 | |
| Male sex, n (%) | 65 (66.3) | 180 (61.6) | 0.41 | |
| Insulin dependent DM, n (%) | 35 (35.7) | 100 (34.2) | 0.79 | |
| Oral antidiabetics, n (%) | 82 (83.7) | 240 (82.2) | 0.74 | |
| Smoking status | Current smoking, n (%) |
22 (22.4) | 53 (18.7) | 0.42 |
| Previous smoking, n (%) |
23 (34.8) | 64 (31.1) | 0.57 | |
| Hypercholesterolaemia, n (%) | 61 (62.2) | 171 (58.8) | 0.54 | |
| Hypertension, n (%) | 75 (76.5) | 214 (73.8) | 0.59 | |
| Previous ACS, n (%) | 42 (42.9) | 97 (33.2) | 0.08 | |
| Previous PCI, n (%) | 41 (41.8) | 103 (35.3) | 0.24 | |
| Previous CABG, n (%) | 4 (4.1) | 8 (2.7) | 0.51 | |
| Previous CVA, n (%) | 12 (12.2) | 20 (6.8) | 0.09 | |
| CCS at presentation, n (%) | 77 (78.6) | 215 (73.6) | 0.78 | |
| ACS at presentation, n (%) | 21 (21.4) | 77 (26.4) | 0.78 | |
| MI at presentation, n (%) | 12 (12.2) | 50 (17.1) | 0.25 | |
| Total no. of lesions, n (per patient)b | 204 (2.08) | 493 (1.69) | 0.02 | |
| 1-vessel disease | 38 (38.8%) | 157 (53.8%) | 0.01 | |
| 2-vessel disease | 49 (50.0%) | 114 (39.0%) | 0.07 | |
| 3-vessel disease | 11 (11.2%) | 21 (7.2%) | 0.29 | |
| Lesions revascularised, n (per patient) |
81 (0.83) | 152 (0.52) | 0.003 | |
| FFR-negative target lesions, n (per patient) |
123 (1.26) | 341 (1.17) | 0.50 | |
| Distribution FFR-negative lesions | Left main | 1 (0.8%) | 5 (1.5%) | 0.14 |
| LAD | 45 (36.6%) | 156 (45.7%) | ||
| Cx | 33 (26.8%) | 93 (27.3%) | ||
| RCA | 44 (35.8%) | 87 (25.5%) | ||
| Median total cholesterol (IQR), mg/ml | 161 (142-189) | 154 (135-193) | 0.18 | |
| Median LDL (IQR), mg/dl | 88 (82-93) | 91 (81-99) | 0.52 | |
| Median triglycerides (IQR), mg/ml | 168 (120-242) | 150 (106-231) | 0.25 | |
| Median haemoglobin A1c (IQR), % | 7.3 (6.7-7.9) | 7.3 (6.6-8.1) | 0.78 | |
| aThe body mass index (BMI) is the weight in kilograms divided by the square of the height in metres. bTotal number of lesions refers to both angiographically moderate lesions that underwent FFR-OCT and lesions that were treated as culprit lesions in ACS patients. ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome; CVA: cerebrovascular accident; Cx: circumflex artery; DM: diabetes mellitus; FFR: fractional flow reserve; IQR: interquartile range; LAD: left anterior descending artery; LDL: low-density lipoprotein; MI: myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery; TCFA: thin-cap fibroatheroma; (-): negative; (+): positive | ||||
OCT findings
Lesion level quantitative and qualitative OCT data for TCFA-positive as compared to TCFA-negative lesions are presented in Table 2. TCFA-positive lesions were longer and tended to have a smaller MLA and higher % area stenosis as compared to TCFA-negative lesions. The calcium arc was larger and the presence of protruding calcification more frequent in TCFA-negative lesions, while TCFA-positive lesions had a wider lipid arc and a higher prevalence of cholesterol clefts, neovascularisation, and macrophage infiltration.
Table 2. Lesion level quantitative and qualitative OCT analysis results of patients with and without TCFA.
| Variables | FFR(–)/TCFA(+)n=104a | FFR(–)/TCFA(–)n=341 | p-value |
|---|---|---|---|
| Quantitative OCT analysis | |||
| MLA (IQR), mm2 | 2.35 (1.70-3.18) | 2.60 (1.90-3.50) | 0.09 |
| % area stenosis (IQR), % | 65 (57-73) | 62 (53-70) | 0.07 |
| Lesion length (IQR), mm | 27.65 (18.10-36.10) | 20.10 (14.10-29.60) | <0.001 |
| Proximal RLD (IQR), mm | 3.10 (2.70-3.50) | 3.00 (2.60-3.50) | 0.63 |
| Distal RLD (IQR), mm | 2.50 (2.30-3.00) | 2.60 (2.20-3.00) | 0.68 |
| Qualitative OCT analysis | |||
| Fibrous cap thickness (IQR), µm | 60 (56-63) | 151 (109-218) | - |
| Calcification present, n (%) | 91 (87.5) | 292 (85.6) | 0.99 |
| Calcium arc (IQR), o | 112 (80-192) | 159 (88-244) | 0.02 |
| Calcified noduli, n (%) | 36 (34.6) | 157 (46.0) | 0.04 |
| Cholesterol clefts, n (%) | 75 (72.8) | 149 (44.1) | <0.001 |
| Lipidic plaque, n (%) | 104 (100) | 201 (58.9) | <0.001 |
| Lipidic arc (IQR), o | 241 (193-287) | 169 (126-214) | <0.001 |
| Healed plaque, n (%) | 25 (24.0) | 64 (18.9) | 0.26 |
| Neovascularisation, n (%) | 88 (84.6) | 232 (68.0) | 0.002 |
| Macrophage infiltration, n (%) |
72 (69.9) | 157 (46.0) | <0.001 |
| a Number represents only TCFA hosting lesions. FFR: fractional flow reserve; IQR: interquartile range; MLA: minimal lumen area; OCT: optical coherence tomography; RLD: reference lumen diameter; TCFA: thin-cap fibroatheroma | |||
Clinical outcomes
The composite primary endpoint occurred more frequently in TCFA-positive than in TCFA-negative patients (21.4% vs 8.2%, HR 2.89, 95% CI: 1.61-5.20; p<0.001; 6.42 vs 2.46 events per 100 patient-years, risk ratio [RR] 2.61, 95% CI: 1.38-4.90; p=0.002) (Table 3). The increased event risk in TCFA-positive patients was confirmed at the mean follow-up of 3.5 years (20.4 vs 7.2%, HR 3.09, 95% CI: 1.68-5.71; p<0.001) and in patients with at least 3 years of follow-up (235 patients, 66%; 22.6% vs 7.5%, HR 3.48, 95% CI:1.63-7.40; p=0.001). Kaplan-Meier curves for the primary endpoint are presented in Figure 1.
Furthermore, the composite endpoint of CD, TV-MI, or TLR occurred more often in TCFA-positive than in TCFA-negative patients (20.4% vs 6.51%, HR 3.43, 95% CI: 1.83-6.43; p<0.001) (Table 3). Similarly, the incidence of TV-MI, TLR, or unstable angina pectoris was significantly higher in TCFA-positive patients. There was no difference in CD between TCFA-positive and TCFA-negative patients.
Multivariable analysis identified TCFA-positive patients (HR 2.76, 95% CI: 1.53-4.97; p<0.001) to be independent predictors of the primary clinical endpoint, while the presence of healed plaque (HR 1.71, 95% CI: 0.91-3.21; p=0.09), and stenosis at the MLA site (HR 1.32, 95% CI: 0.995-1.74; p=0.054) showed a modest effect (overall model’s C-statistic: 0.7, 95% CI: 0.63-0.77).
Notably, when TV-MI, TLR, and UA were treated as recurrent components of major cardiovascular events (MACE) and CD as a terminal event, TCFA-positive patients experienced a higher risk of recurrent MACE (HR 2.89, 95% CI: 1.74-4.80; p<0.001; 13.45 vs 2.87 events per 100 patient-years, RR 4.69, 95% CI: 2.86-7.83; p<0.001). The mean cumulative function estimator for recurrent primary endpoint events is presented in Figure 2. Event curves started to diverge early and separated still further until the end of follow-up. After 5 years, TCFA-positive patients had an estimated 1.17 more events than TCFA-negative patients. The event plot for recurrent MACE components is presented in Figure 3.
Table 3. Clinical outcomes up to 5-year follow-up of patients with and without TCFA.
| Variables | Total | FFR(−)/TCFA(+)98 | FFR(−)/TCFA(−)292 | Hazard ratio95% confidence interval | p-value |
|---|---|---|---|---|---|
| Primary endpoint, n (%) | 45 (11.54) | 21 (21.43) | 24 (8.22) | 2.891 (1.609;5.195) | <0.001 |
| Cardiac death or TVR-MI or TLR | 39 (10.00) | 20 (20.41) | 19 (6.51) | 3.432 (1.831;6.433) | <0.001 |
| Cardiac death or TVR-MI | 19 (4.87) | 8 (8.16) | 11 (3.77) | 2.398 (0.964;5.962) | 0.06 |
| Cardiac death, n (%) | 12 (3.08) | 2 (2.04) | 10 (3.42) | 0.580 (0.127;2.648) | 0.48 |
| TVR-MI, n (%) | 7 (1.80) | 6 (6.12) | 1 (0.34) | 18.233 (2.195;151.458) | 0.007 |
| TLR | 26 (6.67) | 17 (17.35) | 9 (3.08) | 6.086 (2.712;13.656) | <0.001 |
| Unstable anginaa | 17 (4.36) | 10 (10.20) | 7 (2.40) | 4.504 (1.713;11.846) | 0.002 |
| MI spontaneous, n (%) | 20 (5.14) | 10 (10.20) | 10 (3.44) | 3.107 (1.293;7.466) | 0.011 |
| All revascularisation | 55 (14.10) | 27 (27.55) | 28 (9.59) | 3.199 (1.885;5.429) | <0.001 |
| Death or MI or revascularisation | 75 (19.23) | 31 (31.63) | 44 (15.07) | 2.390 (1.509;3.786) | <0.001 |
| Death or MI | 42 (10.77) | 16 (16.33) | 26 (8.90) | 1.968 (1.056;3.669) | 0.033 |
| Death, n (%) | 21 (5.38) | 5 (5.10) | 16 (5.48) | 0.931 (0.341;2.541) | 0.89 |
| aHospitalisation due to unstable angina. FFR: fractional flow reserve; MI: myocardial infarction; TCFA: thin-cap fibroatheroma; TLR target lesion revascularisation; TVR-MI: target vessel-related myocardial infarction | |||||
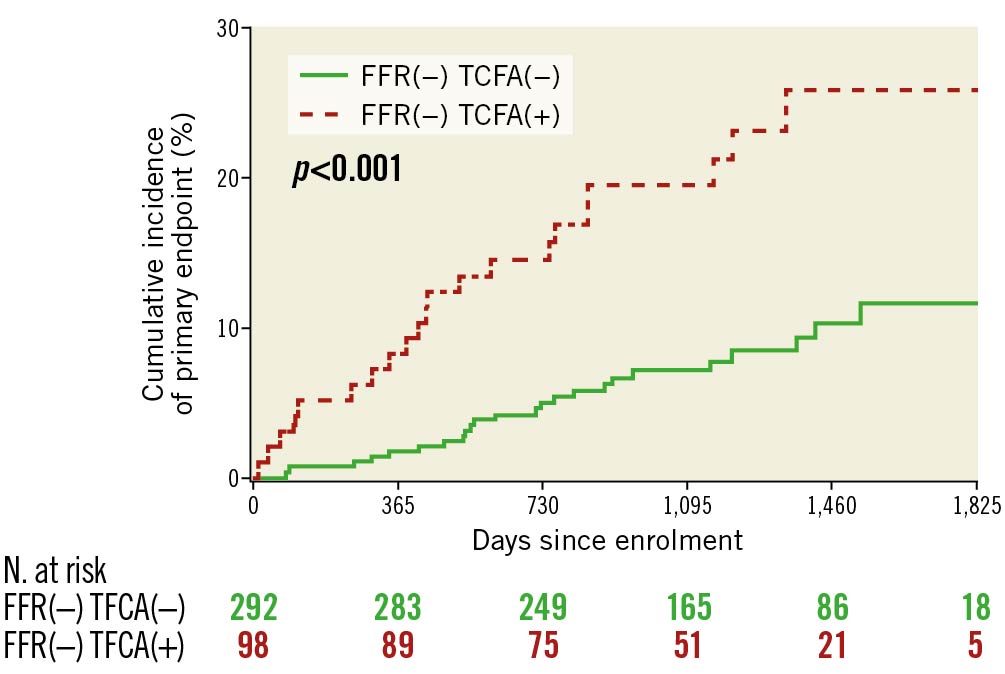
Figure 1. Kaplan-Meier curves for primary endpoint of FFR(−) TCFA(−) vs FFR(−) TCFA(+) groups. FFR: fractional flow reserve; TCFA: thin-cap fibroatheroma
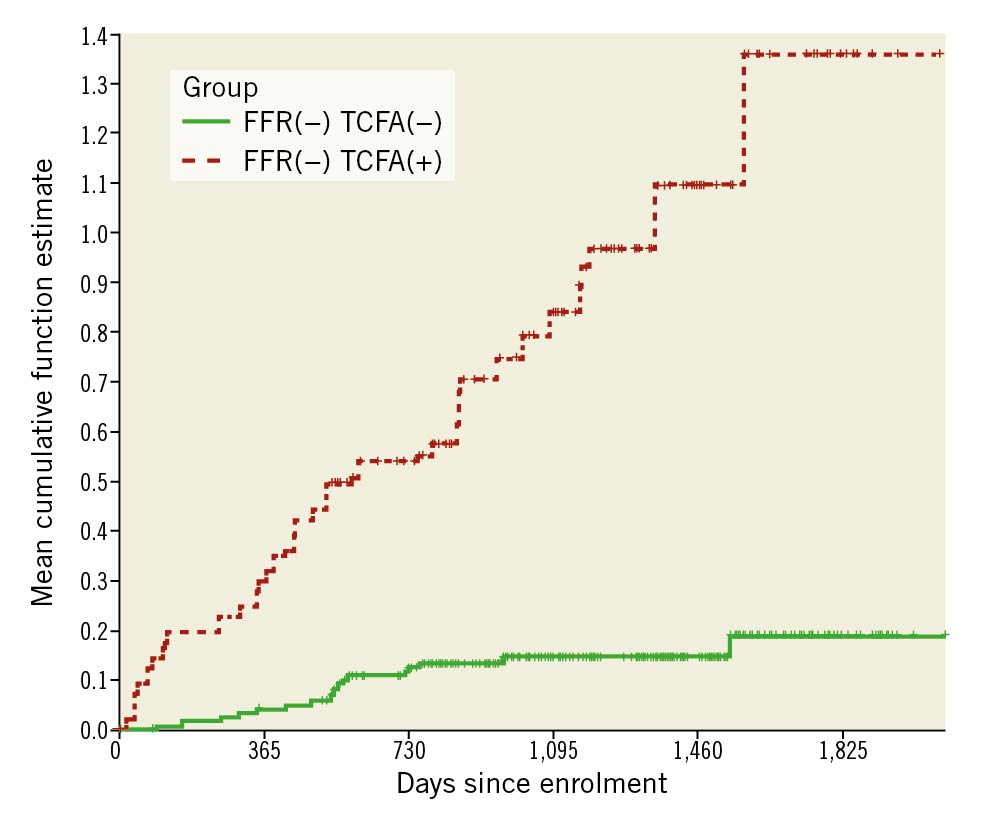
Figure 2. Mean cumulative function estimator for recurrent components of the primary endpoint in FFR(−) TCFA(−) vs FFR(−) TCFA(+) groups. On the vertical axis is the estimated number of events a patient can experience up to a specific timepoint on the horizontal axis. Over 3 years, TCFA-positive patients experienced 0.85 estimated events compared to 0.15 estimated events in TCFA-negative patients. Therefore, at 3 years, TCFA-positive patients had an estimated 0.7 more events than
TCFA-negative patients. At 5 years, TCFA-positive patients had an estimated 1.17 more events than TCFA-negative patients.
FFR: fractional flow reserve; TCFA: thin-cap fibroatheroma
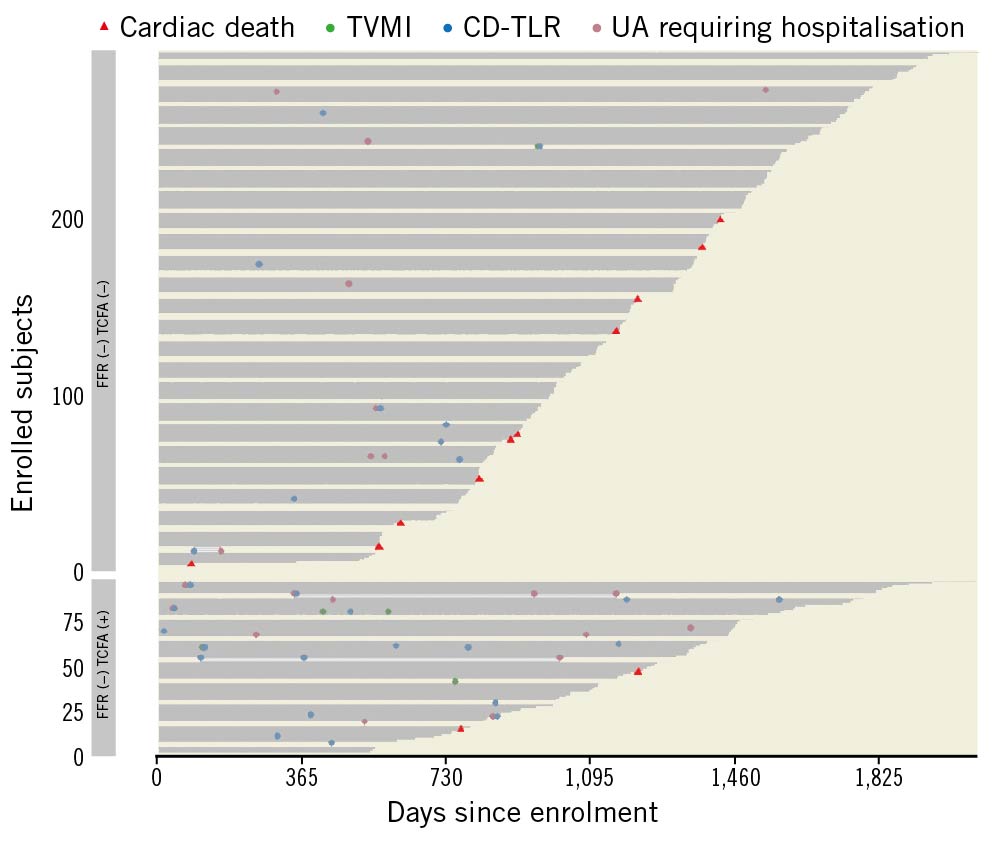
Figure 3. Event plot for recurrent MACE components and cardiac death in FFR(−) TCFA(−) vs FFR(−) TCFA(+) groups. Event plot presents events of all patients, sorted by the length of observation in each of study groups and each event marked separately. All observations start at time of enrolment and end either with censoring or death. CD-TLR: clinically driven target lesion revascularisation; FFR: fractional flow reserve; TCFA: thin-cap fibroatheroma; TVMI: target vessel myocardial infarction; UA: unstable angina
Discussion
The COMBINE OCT-FFR study is the largest prospective study with OCT assessment of plaque morphology to investigate the impact of OCT-detected TCFA in patients with DM and with angiographically intermediate but otherwise non-ischaemic lesions. The COMBINE OCT-FFR study already showed that, at 18 months, OCT-detected TCFA was associated with a significantly higher rate of future adverse events10. The present analysis addressed the question of whether these non-ischaemic TCFA-carrying lesions at baseline could also predict MACE at an extended (up to 5 years) follow-up.
The main findings of the present analysis are as follows: 1) the presence of TCFA in FFR-negative lesions was associated with a higher risk of long-term MACE; 2) recurrence of target lesion-related MACE was higher in TCFA-positive than in TCFA-negative patients; 3) the TCFA plaque morphology is an independent predictor of MACE in the long term. The novelty of the current analysis is based on the fact that OCT assessment succeeded in identifying patients that still remain at a higher risk of adverse events at a long-term follow-up, despite optimal medical treatment and the absence of myocardial ischaemia at baseline.
The TV-MI rate was higher in TCFA-positive than in TCFA-negative patients. This finding suggests that TCFA patients may be at higher risk for future MI related to the presence of a vulnerable plaque. A novel aspect of our observations is that the TLR rate at long-term follow-up was higher in TCFA-positive patients. This may indicate that TCFA could be a marker of fast plaque progression, a process that might result from “subclinical” episodes of silent plaque disruption and subsequent healing17. This stepwise, progressive scenario may be disrupted by the occurrence of acute ischaemic complications. Endothelial disruption is a potent stimulus for platelet activation and thrombus formation18, which may cause (sub)total lumen occlusion and clinical presentation as an acute MI. Conversely, an asymptomatic rupture that heals can lead to lumen diameter reduction and clinical presentation with progressive (unstable) angina pectoris19. Interestingly, TCFA-positive patients also had a higher rate of hospitalisation due to unstable angina as compared to TCFA-negative patients.
Our findings regarding the impact of TCFA on clinical outcomes are in line with the recently reported CLIMA (Relationship Between OCT Coronary Plaque Morphology and Clinical Outcome) study20, which identified a fibrous cap thickness <75 mm as the strongest predictor of events. Similarly, another study by Kubo et al21 identified patients with TCFA as those with the highest risk for future ACS. Recently, also Fang et al22, in a 3-vessel OCT study, showed that TCFA had a high propensity to cause coronary events, and non-culprit TCFA were independent predictors of 2-year MACE.
These findings are clinically relevant as they show that the evaluation with OCT may also add useful information for risk stratification in patients without myocardial ischaemia. As shown by the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial23, an ischaemia-guided revascularisation reduces angina frequency and improves quality of life. Yet, this approach was not sufficient to reduce hard ischaemic endpoints (e.g., cardiovascular mortality and myocardial infarction), as compared to optimal medical treatment.
Considering the high risk that TCFA-positive patients bear, the use of newer treatment strategies should be considered. Recent studies have shown that the addition of the proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9-i) to high-intensity statin therapy may favourably affect coronary atherosclerosis, inducing greater coronary plaque regression compared to placebo2425. In the HUYGENS (Imaging of Coronary Plaques in Participants Treated With Evolocumab) trial24, the addition of the PCSK9-i evolocumab to intensive statin therapy produced incremental benefits in OCT measures of plaque composition - a greater increase in minimum fibrous cap thickness (FCT) and a decrease in maximum lipid arc. In the PACMAN-AMI (Vascular Effects of Alirocumab in Acute MI-Patients) trial25, the PCSK9-i alirocumab, compared with placebo, resulted in a significantly greater reduction in the mean change in percent atheroma volume, as assessed by intravascular ultrasound (IVUS); a greater reduction in lipid burden, as assessed by near-infrared spectroscopy (NIRS); and a greater increase in minimal FCT, as assessed by OCT. However, further research is needed to evaluate whether these therapy-induced changes in plaque morphology would translate into a reduction of future adverse events.
Yet, inflammation is considered a relevant pathway in the pathogenesis of atherosclerosis. In the CANTOS (Cardiovascular Risk Reduction Study [Reduction in Recurrent Major CV Disease Events]) trial26, canakinumab, a monoclonal antibody targeting interleukin-1β, showed the clinical benefit of targeting inflammation compared to placebo in patients with previous MI and residual inflammatory risk. This trial validated the inflammation as a viable target for preventing cardiovascular events; however, what the impact is of canakinumab on plaque stability remains unknown.
Future clinical trials might want to assess the usefulness of plaque sealing by focal percutaneous coronary treatment, considering the very low event rates of contemporary drug-eluting stents. Some feasibility aspects of this strategy have been already tested in the PROSPECT-ABSORB (Providing Regional Observations to Study Predictors of Events in the Coronary Tree II Combined with a Randomized, Controlled, Intervention) Trial27. The study revealed that the use of bioresorbable scaffolds to seal mild stenoses with high lipidic content, identified with NIRS-IVUS in non-culprit vessels of patients with acute coronary syndromes, was not associated with an increased risk of periprocedural myocardial infarction and permitted compensatory vessel remodelling at follow-up.
In summary, we have reported the long-term follow-up of the COMBINE OCT-FFR trial in order to better understand the natural history of non-ischaemic atherosclerotic lesions and to assess the impact of plaques with high-risk features, identified by OCT, on future cardiovascular events in the long term. Among patients with DM, OCT enables the identification of patients at an increased long-term risk of adverse events, despite the absence of an ischaemic lesion at baseline (Central illustration). A combined haemodynamic and morphologic evaluation to guide and optimise the treatment of these high-risk patients should be tested by future studies in order to establish novel and more tailored treatment strategies.
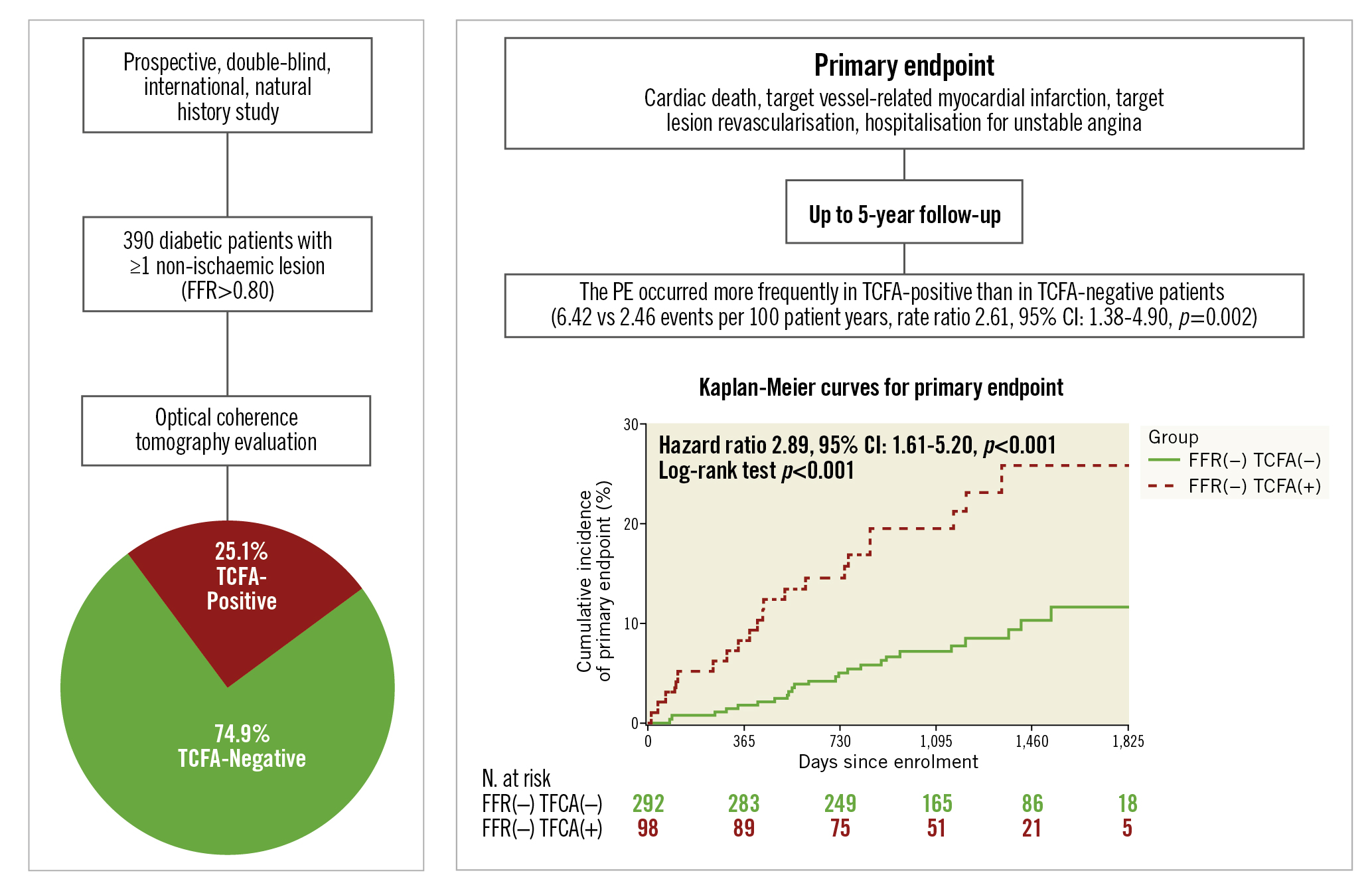
Central illustration. COMBINE OCT-FFR study extended follow-up. Among patients with diabetes mellitus and fractional flow reserve (FFR)-negative non-culprit lesions enrolled in the COMBINE OCT-FFR study, patients with thin-cap fibroatheroma (TCFA) had a higher rate of the composite primary endpoint (cardiac death, target vessel-related myocardial infarction, target lesion revascularisation, hospitalisation for unstable angina) than those who were TCFA-negative, up to 5 years of follow-up. CI: confidence interval; FFR: fractional flow reserve; OCT: optical coherence tomography; PE: primary endpoint; TCFA: thin-cap fibroatheroma
Limitations
The limitations of the COMBINE OCT-FFR study have been reported previously10. The study reflects the “real-world” treatment scenario, and the identification of intermediate angiographic lesions was based on visual assessment; nevertheless, OCT analysis confirmed that the area stenosis was intermediate in both groups. The study did not comprise routine angiographic follow-up but, instead, evaluated the impact of baseline FFR and OCT findings on the long-term outcome; this approach is much more similar to routine clinical practice than a study design with routine angiographic follow-up. The absence of strict criteria for the definition of “healed plaque” may have influenced the reproducibility of its identification. However, the presence of “healed plaque” in our study showed a borderline association to the primary clinical endpoint, suggesting that further research is needed to better understand the potential role of “healed plaque” and, also, to improve its definition with more stringent criteria. Although the present analysis is the largest to date in a cohort of patients with DM, absolute numbers remain limited. Finally, the impact of a stricter glycaemic or lipidic control on long-term adverse events cannot be deduced from this study.
Conclusions
Among patients with DM and FFR-negative non-culprit lesions, patients with TCFA had a higher MACE risk than TCFA-negative patients beyond 18-month follow-up and up to 5 years. The assessment with OCT allowed for the identification of high-risk patients who might benefit from more aggressive treatment.
Impact on daily practice
The extended follow-up of the COMBINE OCT-FFR study showed that among patients with diabetes mellitus and FFR-negative non-culprit lesions, OCT-detected TCFA-positive lesions, although not ischaemia-generating, are associated with a higher risk of adverse events during long-term follow-up. The recurrence of target lesion-related MACE are higher in TCFA-positive than in TCFA-negative patients. OCT may be able to identify patients hosting lesions at a higher risk for future adverse clinical events; future studies should assess the value of a tailored novel and more aggressive treatment strategy in these higher-risk patients.
Funding
This investigator-initiated study was sponsored by Isala Hartcentrum, Zwolle, the Netherlands, and supported by an unrestricted institutional grant from St. Jude Medical/Abbott Vascular. ClinicalTrials.gov: NCT02989740.
Conflict of interest statement
C. von Birgelen reports institutional research grants (to the research department of Thoraxcentrum Twente) from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic, outside the submitted work. H. Garcia-Garcia reports other from Medtronic, Boston Scientific, Abbott Vascular, Biotronik, Neovasc, CorFlow, Shockwave, Chiesi, outside the submitted work. W. Wojakowski reports personal fees from Abbott Vascular, outside the submitted work. E. Kedhi reports personal lecture and advisory fees from Abbott and Medtronic, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.




