
Abstract
Aims: We sought to evaluate procedural complications and one-year clinical outcomes for patients who underwent percutaneous coronary intervention (PCI) with orbital (OA) and rotational atherectomy (RA).
Methods and results: From a total of 13,467 patients who underwent PCI in our hospital between January 2013 and June 2016, 1,149 consecutive patients were treated with atherectomy for moderately-severely calcified lesions (184 with OA, 965 with RA). Procedural complications were similarly observed in the two groups except for higher dissection and perforation rates with OA. Major adverse cardiovascular events (MACE) were defined as the composite of death, myocardial infarction or target lesion revascularisation. Multivariable adjusted analysis showed that OA use was associated with comparable adjusted one-year MACE compared to RA use (hazard ratio 0.79 [95% confidence interval 0.54-1.17], p=0.25). There were no significant differences in individual MACE endpoints. Furthermore, we studied 67 patients with OCT images. OCT analysis showed comparable tissue modification with a trend towards higher stent expansion with OA vs. RA.
Conclusions: OA use was associated with lower unadjusted but similar adjusted one-year MACE outcomes compared to RA with higher rates of dissection and device-induced perforation.
Abbreviations
BMI: body mass index
CAC: coronary artery calcification
CSA: cross-section area
DM: diabetes mellitus
LAD: left anterior descending
MACE: major adverse cardiovascular events
MI: myocardial infarction
OA: orbital atherectomy
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
RA: rotational atherectomy
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Coronary artery calcification (CAC) is frequently detected at the time of angiography in approximately one third of target lesions1,2. CAC complicates device delivery and stent expansion during percutaneous coronary intervention (PCI)3,4. It is also reported to relate to adverse clinical outcomes after PCI including stent restenosis and target lesion revascularisation (TLR)5,6. PCI for severely calcified lesions still remains challenging despite the development of novel ablative devices. Coronary rotational atherectomy (RA) is considered to be reasonable for heavily calcified lesions that might not be crossed by a balloon catheter or adequately dilated before stent implantation7. Orbital atherectomy (OA) is a recently developed coronary atherectomy device that was approved by the Food and Drug Administration. The ORBIT II (Evaluate the Safety and Efficacy of OA system in Treating Severely Calcified Coronary Lesions) trial demonstrated favourable acute, 30-day, 1-year, 2-year, and 3-year clinical outcomes of OA followed by stenting for severely calcified coronary lesions8-11. OA also demonstrated similar 30-day clinical outcomes compared to RA in a small retrospective study12. Previously, we reported post-procedural complications, midterm (six months) clinical outcomes and a small OCT analysis of OA vs. RA in separate studies13,14. In this study, we evaluated procedural safety data, one-year clinical outcomes and additionally analysed OCT images of the patients who underwent OA vs. RA in a larger population.
Methods
STUDY POPULATION AND DESIGN
Among a total of 13,476 patients who underwent PCI at our institution from January 2013 to June 2016, 1,149 (8.52%) consecutive patients who were treated with coronary atherectomy followed by stent implantation for moderately-severely calcified lesions by angiography were studied. Lesion calcification was assessed by angiography. Moderate calcification was defined as radiopaque densities seen only during the cardiac cycle prior to contrast injection, generally affecting one side of the arterial wall. Severe calcification was defined as radiopacities noted without cardiac motion before contrast injection, usually involving both sides of the arterial wall. Calcification severity was judged by two independent observers. The atherectomy device was selected based on operator preference. We compared procedural safety data including device-induced perforation, periprocedural myocardial infarction (MI) and one-year clinical outcomes including death, target vessel revascularisation (TVR), TLR and major adverse cardiovascular events (MACE) between patients who underwent OA and RA. Asymptomatic presentation was defined as a condition without any ischaemic symptom. PCI for asymptomatic patients was generally performed based on a positive stress test; staged PCI was also classified as asymptomatic presentation, if the symptom disappeared after previous PCI. In our hospital, blood samples are routinely collected four to six hours after PCI. If troponin-I or CK-MB levels are elevated, follow-up blood tests are added. MI was defined based on the third universal definition of MI15. Dissection was defined as flow-limiting dissection consistent with the NHLBI category for type C or greater. Any coronary perforation within the ablated segment after atherectomy during the procedure was classified as device-induced perforation. Death was all-cause death and stent thrombosis was definite/probable stent thrombosis based on the Academic Research Consortium criteria16. The primary one-year clinical outcome was MACE, defined as a composite of death, MI or TLR. Patients with cardiac shock and missing data for angiographic calcification were excluded. All the data were retrospectively acquired from the Mount Sinai institutional PCI registry14. Demographic, procedural, laboratory, and medical history data at baseline were collected through a review of medical records. Patients with missing data were excluded from statistical analysis. Clinical follow-up included a telephone follow-up at 30 days and one year after PCI and a review of medical records and clinical visits by trained research coordinators. Our PCI registry has institutional review board approval to contact patients and to collect data on all patients who have undergone PCI. In addition, we extracted a subset of patients who underwent coronary atherectomy with OA or RA in whom OCT examination was performed from the institutional imaging database.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
The target lesion was evaluated by QCA before any procedure, after atherectomy, and after stenting using QAngio XA 7.3 (Medis, Leiden, the Netherlands) to estimate minimum lumen diameter, reference lumen diameter, and % diameter stenosis according to the standard analytic protocols by experienced analysts (N. Okamoto and Y. Vengrenyuk).
OPTICAL COHERENCE TOMOGRAPHY (OCT) IMAGE ACQUISITION AND ANALYSIS
OCT evaluation was performed after atherectomy and after stenting with the commercially available C7-XR™ OCT Intravascular Imaging System and Dragonfly™ imaging catheter (Abbott Vascular, Santa Clara, CA, USA). OCT images were acquired after crossing the OCT catheter distally to the target lesion with continuous contrast injection with a 20 mm/s pullback and analysed offline at 1 mm intervals according to current guidelines, as previously described13,17 (Supplementary Appendix 1). Post-atherectomy tissue modifications were morphologically classified as (a) nodules (Type I), superficial intimal flaps with smooth luminal border, (b) deep intimal cuts (Type II), cuts with less than 0.5 mm depth for RA and with less than 1.0 mm depth for OA, and (c) deeper intimal-medial dissections (Type III), defined as dissections with more than 0.5 mm depth for RA and with more than 1.0 mm depth for OA (Figure 1).
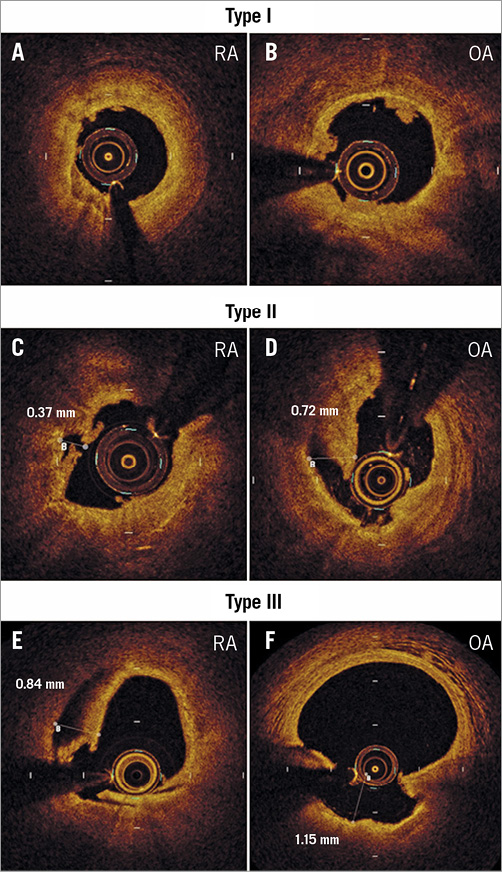
Figure 1. OCT images of tissue modifications after atherectomy. Representative OCT images and measurements of tissue modification after atherectomy. Type I tissue modifications are defined as superficial intimal flaps with smooth luminal border (A, B). Type II tissue modifications are deep intimal cuts with less than 0.5 mm depth for RA (C) and with less than 1.0 mm depth for OA (D). Type III tissue modifications are deeper intimal-medial dissections with more than 0.5 mm depth for RA (E) and with more than 1.0 mm depth for OA (F). OA: orbital atherectomy; OCT: optical coherence tomography; RA: rotational atherectomy
STATISTICAL ANALYSIS
Continuous data were expressed as mean±standard deviation and compared across the two groups with a t-test for normally distributed variables and with a Mann-Whitney U test for non-normally distributed variables. The chi-square test or Fisher’s exact test was used for categorical data and presented as numbers and percentages. Event rates at one year were generated by the Kaplan-Meier method and compared using the log-rank test. Hazard ratios were calculated using multivariable Cox proportional hazards regression. Multivariable models were generated using backward elimination with an exit criterion of 0.1. The following candidate covariates were considered according to baseline imbalance between groups or as established correlates of risk after PCI: age, gender, body mass index (BMI), diabetes mellitus (DM), chronic kidney disease, presentation severity (stable angina versus acute coronary syndrome), presence of lesion in left anterior descending (LAD) artery, and coronary calcification were selected by backward elimination. A propensity model was generated using logistic regression with OA (versus RA) serving as the dependent outcome. Covariates included all variables demonstrating baseline imbalance between groups. We then performed 1:1 nearest neighbour matching to generate a matched cohort. Statistical analysis was performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A two-tailed probability value <0.05 was considered statistically significant.
Results
STUDY POPULATION FOR CLINICAL OUTCOMES
Of the 1,149 patients with moderately to severely calcified lesions treated with atherectomy, 184 (16%) patients were treated with OA, and 965 (84%) patients were treated with RA prior to stenting. Baseline characteristics, medications and procedural data are shown in Table 1 and Table 2. The data were available for 100% of patients except for BMI (99.7%), serum creatinine (93.5%), LDL cholesterol (96.8%) and left ventricular ejection fraction (95.0%). Age, gender and BMI were similar in the two groups. DM was more frequently observed in the RA group than in the OA group. The patients in the RA group had a lower prevalence of asymptomatic presentation and a higher degree of angiographic severe lesion calcification than those in the OA group. RA was less frequently used in the LAD but more frequently in the left main artery than OA. Twenty-five percent of OA cases were performed at high speed (120,000 rpm), especially in the earlier time period (Figure 2).
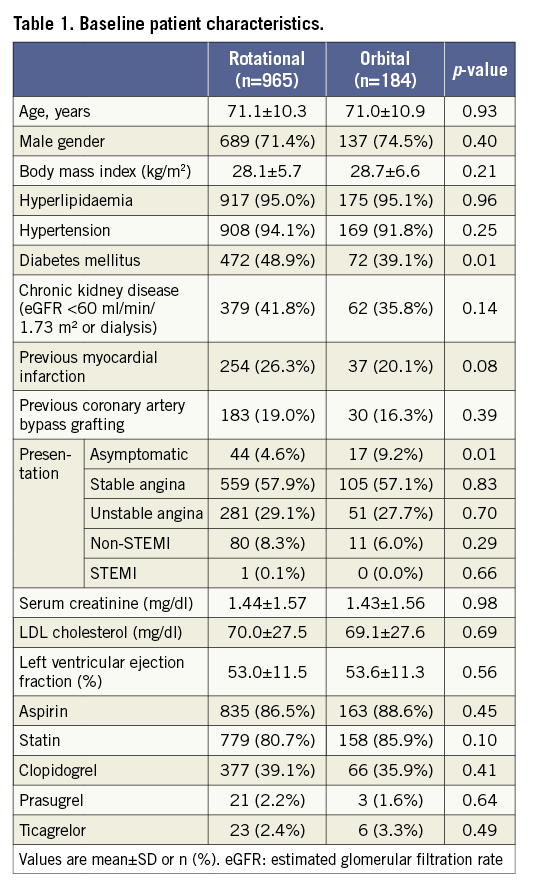
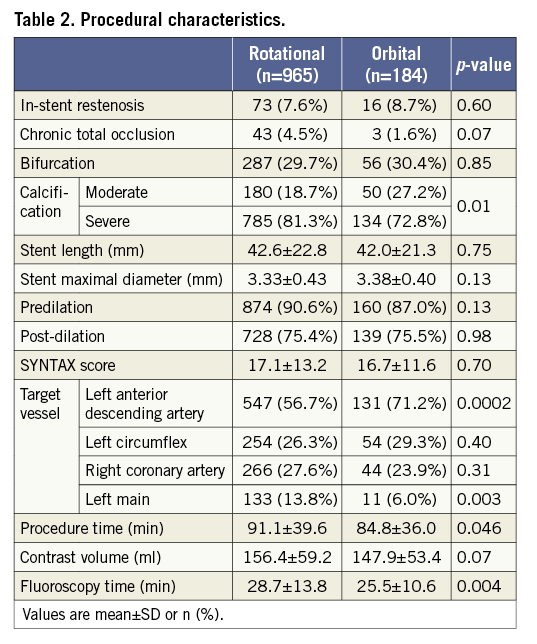
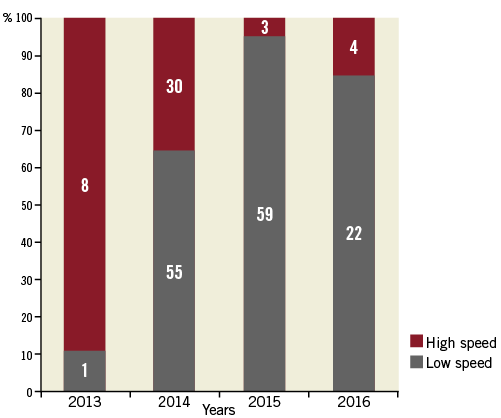
Figure 2. Transition of OA speeds at the institution. The bar graph indicates the percentage of OA speeds by year. OA: orbital atherectomy
CLINICAL OUTCOMES
Clinical safety outcomes are summarised in Table 3. Dissection and device-induced perforation were more frequently observed in the OA group compared to the RA group (3 [1.6%] vs. 3 [0.3%], p=0.02; 3 [1.6%] vs. 2 [0.2%], p=0.03). An additional stent was implanted in four lesions with main vessel dissection, OA (n=2) and RA (n=2), which resulted in TIMI 3 flow in all cases. Side branch dissections were observed in two patients, OA (n=1) and RA (n=1), and treated with balloon angioplasty. TIMI 3 flow was achieved in the OA treated lesion, while a final TIMI 1 flow was obtained for the RA patient. With regard to device perforation, one perforation occurred right after OA use and was treated with pericardiocentesis and covered stent implantation. The remaining two OA perforations were observed after stenting or post-OA balloon angioplasty and were treated by prolonged balloon dilation without pericardiocentesis. In the case of RA, both device perforations happened right after the atherectomy procedure and were treated with covered stent deployment without pericardiocentesis. Side branch closure, tamponade, slow/no flow and vessel closure were not different between the two groups. Periprocedural MI and CK-MB release were also comparable in the two groups (cardiac enzyme data were available for all patients).
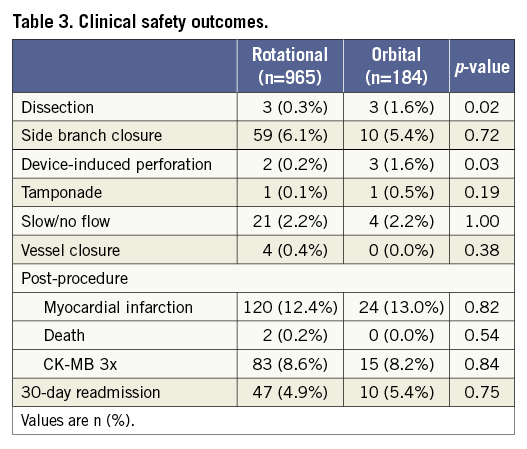
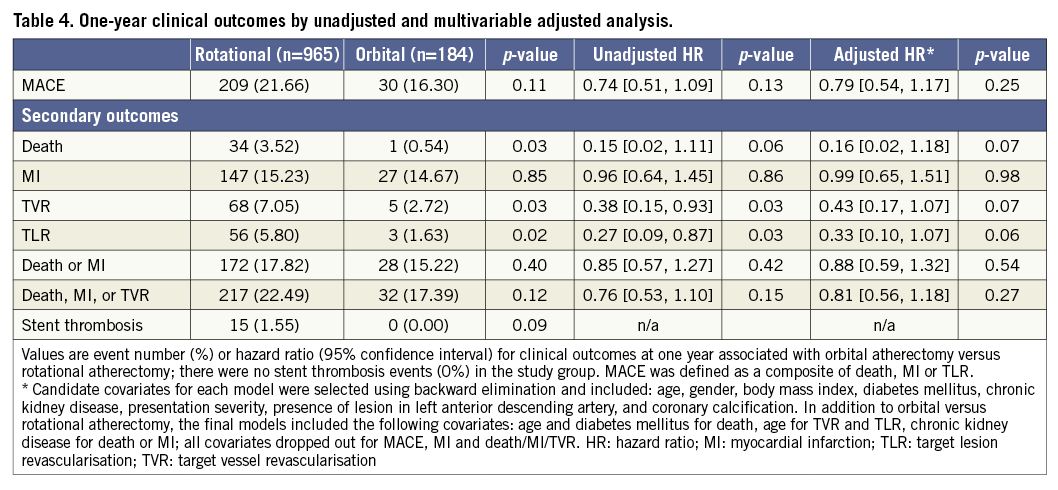
Follow-up data were available for 1,058 (92.1%) patients at one year with a median follow-up duration of 365 days. Unadjusted rates of death, TVR and TLR at one year in the OA group were significantly lower compared to the RA group; however, the rate of MACE was comparable between the groups (16.30% vs. 21.66%, p=0.11) (Table 4). After multivariable adjustment, OA use was associated with similar one-year MACE compared to RA use (adjusted hazard ratio 0.79 [95% confidence interval 0.54 to 1.17], p=0.25) and statistical significance in death, TVR and TLR was attenuated. In the propensity model, baseline and lesion characteristics were well balanced between the groups (Supplementary Table 1), and one-year MACE after OA was comparable to that of RA (Supplementary Table 2).
STUDY POPULATION FOR IMAGING ANALYSIS
OCT analysis included 67 consecutive patients with de novo lesions who underwent coronary atherectomy and OCT examination after drug-eluting stent implantation. OA was performed in 36 patients and RA in 32 patients. In addition to post-stent OCT, 42 patients had OCT pullbacks performed after atherectomy before stenting (OA: n=26, RA: n=17). Staged PCI using OA and RA for multivessel disease was performed in one patient. Baseline clinical and procedural characteristics for the imaging subgroup are presented in Supplementary Table 3. Clinical patient characteristics were not significantly different between the two groups. The rotation speed of RA was 150,000 rpm in all patients and that of OA was 80,000 rpm in 29 patients and 120,000 rpm in seven patients. The number, length and diameter of implanted stents were similar between the groups.
QCA AND OCT ANALYSIS
Reference diameter, minimum lumen diameter and % diameter stenosis before atherectomy, after atherectomy and after stenting by QCA were similar between the OA and RA groups (Supplementary Table 4).
The results of OCT analyses are summarised in Supplementary Table 5. Both groups had a similar minimal lesion lumen cross-section area (CSA) and percent lumen area stenosis after atherectomy. Calcification was detected in all lesions by OCT and the maximal total calcium arc was comparable in the OA vs. the RA group (223.6±105.1° vs. 279.7±82.3°, p=0.06). Similarly, average arc and maximal length of calcification were not different between the two groups. While the frequency of lipid-rich plaques was similar, there was a greater maximal lipid arc in the RA group. Tissue modification depth and length were similar after OA and RA. The prevalence of Type I and Type III was not different between the groups; however, Type II deep intimal cuts were more frequently observed after OA compared to RA (13 [50%] vs. 3 [18%], p=0.03). OCT stent analysis showed a similar reference area and in-stent lumen area in the two groups (Supplementary Table 5). Minimal and mean stent area were also comparable; however, there was a trend towards a higher stent expansion after OA compared to RA (102.5±23.7 vs. 93.3±16.1, p=0.08). Furthermore, the OA and RA groups had similar characteristics of stent apposition including percentage of malapposed struts, malapposition distance, length, area and volume.
Discussion
To the best of our knowledge, the present report is the first attempt to compare procedural safety, one-year clinical outcomes and OCT imaging data in a large population of patients who underwent PCI with OA vs. RA. The main findings of the study include the following. (1) The risks of procedural complications including periprocedural MI, CK-MB release, and slow/no flow were similar between the groups; (2) higher rates of dissection and device perforation were observed in the OA group; (3) risk-adjusted one-year outcomes including MACE were not significantly different between patients treated with OA vs. RA; (4) OA and RA resulted in comparable depth and length of tissue modifications assessed by OCT; (5) stent expansion and apposition by OCT were similar in the calcified lesions modified with OA and RA with a trend towards higher stent expansion with OA.
In the present study, OA was associated with significantly higher rates of dissection and device-induced perforation than RA. As the number of such events was small, however, a cautious interpretation regarding these results is warranted. With respect to longitudinal outcomes, unadjusted rates of death, TVR and TLR at one year were significantly lower in the OA group. Differences were attenuated after multivariable adjustment, suggesting that unadjusted reductions in adverse events with OA use reflect confounding rather than a true safety advantage compared with RA.
The OA system has an eccentric diamond-coated crown that orbits and sands calcified plaque and the size of ablation depends on the rotational speed18. OA has been shown to ablate calcified tissue effectively in a small single-arm OCT study17. Previously, we demonstrated deeper tissue modifications and fewer malapposed struts after OA compared to RA using OCT13. At the time, 60% of OA cases were performed at high speed, while only about 20% were treated at high speed in the present study. Lower frequency of high speed OA in the present report may be responsible for comparable tissue modification with only Type II more frequently observed after OA.
Previous OCT studies have shown that small in-stent minimal lumen area and small minimal stent area are predictors of adverse events after stent implantation19,20. Several studies have suggested that malapposed struts might be a possible mechanism of stent thrombosis21,22. Our comparable post-stent OCT findings might provide an underlying mechanism for similar one-year clinical outcomes in OA and RA groups.
Limitations
This study is a retrospective, single-centre study. The baseline differences in the complexity of the cases, with OA performed less frequently in diabetic patients, LM and severely calcified lesions, might have had an effect on study outcomes. Pre-atherectomy OCT images were not available and post-atherectomy OCT was not performed for all patients. In addition, we could not compare the clinical outcomes and OCT findings between high- and low-speed OA due to the small number of patients.
Conclusions
The use of OA achieved comparable one-year clinical outcomes including MACE, with higher rates of dissection and device-induced perforation.
| Impact on daily practice OA has procedural complications comparable to RA except for higher dissections and perforations. After OA, risk-adjusted one-year MACE is similar to RA. In line with the clinical findings, OCT demonstrated comparable tissue modification, stent expansion and apposition between RA and OA with a trend towards higher stent expansion after OA. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. OCT image analysis.
Supplementary Table 1. Baseline clinical and procedural characteristics in the propensity-matched groups.
Supplementary Table 2. One-year clinical outcomes by propensity matching analysis.
Supplementary Table 3. Baseline clinical and procedural characteristics of the patient subgroup for QCA/OCT analysis.
Supplementary Table 4. Quantitative coronary angiography analysis.
Supplementary Table 5. OCT lesion and stent analysis after RA and OA.
To read the full content of this article, please download the PDF.




