
Abstract
Aims: This study aimed to assess the safety and efficacy at midterm follow-up of left atrial appendage occlusion (LAAO) using different devices, in real life in Belgium.
Methods and results: Between June 2009 and November 2016, 457 consecutive patients (63% male, 75±12 yrs, CHA2DS2-VASc 4±0.6, HAS-BLED 3.5±0.7) undergoing LAAO were included. Technical success was 97.1%. There were 19 periprocedural major adverse events (4.1%) including three deaths (0.6%), nine tamponades (1.9%), four major bleedings (0.8%) and two device embolisations (0.4%). Among patients successfully implanted having a complete follow-up (672 patient-years, median follow-up 370 days), the actual annual stroke rate was 1.2%, lower than the expected stroke risk of 4% (70% reduction). The observed bleeding rate was 2%, while the calculated risk was 3.7% (46% reduction). Kaplan-Meier analysis showed a similar overall survival (93±2% and 87±3% versus 91±3% and 87±4%; p=0.35) and event-free survival (92±2% and 84±3% versus 88±3% and 80±5%; p=0.17) at one and two years, for the ACP/Amulet versus the WATCHMAN groups of patients, respectively.
Conclusions: The data from the Belgian left atrial appendage occlusion registry suggest that the procedure is effective and relatively safe in a real-world setting, using either the WATCHMAN or the ACP/Amulet device.
Abbreviations
ACP: AMPLATZER Cardiac Plug
AF: atrial fibrillation
DAPT: dual antiplatelet therapy
LAAO: left atrial appendage occlusion
MAE: major adverse events
NOAC: new oral anticoagulant
NS: not significant
OAC: oral anticoagulant
TEE: transoesophageal echocardiography
TIA: transient ischaemic attack
Introduction
Transcatheter percutaneous left atrial appendage occlusion (LAAO) is an alternative therapeutic option for stroke prevention in patients with atrial fibrillation (AF)1-4. LAAO has been demonstrated to be non-inferior to warfarin in two randomised controlled trials that included only patients without contraindications to oral anticoagulants (OACs)5,6. Large observational studies described the safety and short-term outcome after using only one device7-14. Until now, there have been no randomised trials comparing the results of the currently available prostheses. Real-world data on the long-term safety and efficacy of LAAO using different device technologies are limited15,16. The aim of our study was to collect the baseline, procedural and follow-up characteristics of patients undergoing LAAO in Belgium, and to assess the safety and efficacy of the procedure in the real world of patients not candidates for long-term OAC, allowing a comparison of the different types of device used.
Methods
Between June 2009 and November 2016, 457 consecutive patients undergoing LAAO in 21 centres in Belgium were prospectively included in the registry. Demographics, baseline characteristics, indications for LAAO, CHA2DS2-VASc and HAS-BLED scores, antithrombotic medication, procedural details, periprocedural adverse events and clinical follow-up were prospectively collected in a dedicated database. The protocol was approved by the Ethics Committee of the Université Catholique de Louvain. Three different devices available in Belgium were implanted according to operator preference –the AMPLATZER™ Cardiac Plug (ACP), the AMPLATZER™ Amulet™ (both St. Jude Medical, St. Paul, MN, USA) and the WATCHMAN® (Boston Scientific, Marlborough, MA, USA). The results of the first 90 patients implanted with the ACP device have been reported previously10.
DEFINITIONS OF SUCCESS
According to the Munich consensus document17, device success was defined as successful implantation of the device in the correct position. Technical success is a device success with no large leak and no device-related complications. Device-related complications are embolisation, erosion, interference with surrounding structures, thrombus, fracture, infection, perforation or allergy. Procedural success is a technical success without any major periprocedural complications.
PERIPROCEDURAL COMPLICATIONS
Periprocedural complications (occurring during zero to seven days after the procedure or before hospital discharge, whichever was last) included death, myocardial infarction, stroke, transient ischaemic attack (TIA) according to VARC criteria18, systemic embolism, air embolism, device embolisation, major bleeding according to the BARC 3 and 5 criteria19, and cardiac tamponade.
Periprocedural major adverse events (MAE) included death, stroke, systemic embolism and complication requiring major surgical or endovascular intervention (major bleeding, tamponade, device migration treated by snare or surgery) occurring between zero and seven days post procedure or before hospital discharge, whichever was last.
CLINICAL FOLLOW-UP
Patient survival and clinical events during the follow-up were determined by review of medical records or phone contact of patients implanted successfully. Adverse events during follow-up included death (cardiovascular or non-cardiovascular), stroke, TIA, systemic embolism, major bleeding, tamponade, myocardial infarction, and device-related complications. Antithrombotic medication was recorded at discharge and at last follow-up visit.
EFFICACY ON STROKE, TIA, SYSTEMIC EMBOLISM AND BLEEDING PREVENTION
LAAO efficacy on thromboembolism and bleeding prevention was tested by comparing the actual event rate with the predicted event rate by the CHA2DS2-VASc or HAS-BLED score, respectively20,21. Event reduction was calculated as follows – (estimated % – actual % event rate)/estimated % event rate.
ECHOCARDIOGRAPHY
Patients underwent a transoesophageal echocardiography (TEE) during the procedure allowing the grading of the potential residual leak after implantation. Despite the fact that several definitions have been proposed over time3,8-10, in the current study, according to the Munich consensus document, residual leak was graded using the width of the Doppler colour jet as none (absence of colour jet), mild (1-5 mm) or large (>5 mm) for all devices.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±1 standard deviation. Categorical variables are presented as counts and percentages. Continuous variables were tested using the independent samples t-test and categorical variables using Fisher’s exact test.
Univariate and multivariate analysis was carried out using the Cox proportional hazards method. Variables with a p<0.10 at univariate analysis were included in the backward stepwise multivariate analysis. Estimates for freedom from the composite of death and MAE were obtained by the Kaplan-Meier estimation method. A p-value <0.05 was considered statistically significant. Analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENTS
A total of 457 patients were included in the database and constituted the total cohort of the study. Baseline characteristics are listed in Table 1. The distribution of the CHA2DS2-VASc and HAS-BLED scores is detailed in Figure 1. The mean CHA2DS2-VASc (4.6±1.6) and HAS-BLED (3.2±1) scores were not significantly different between patients treated by ACP/Amulet (Group 1) and those undergoing a WATCHMAN implantation (Group 2; 4.5±1.7 and 3.1±1, respectively; p=NS). Patients in Group 1 more frequently experienced a previous major bleeding but had less diabetes mellitus than those in Group 2.
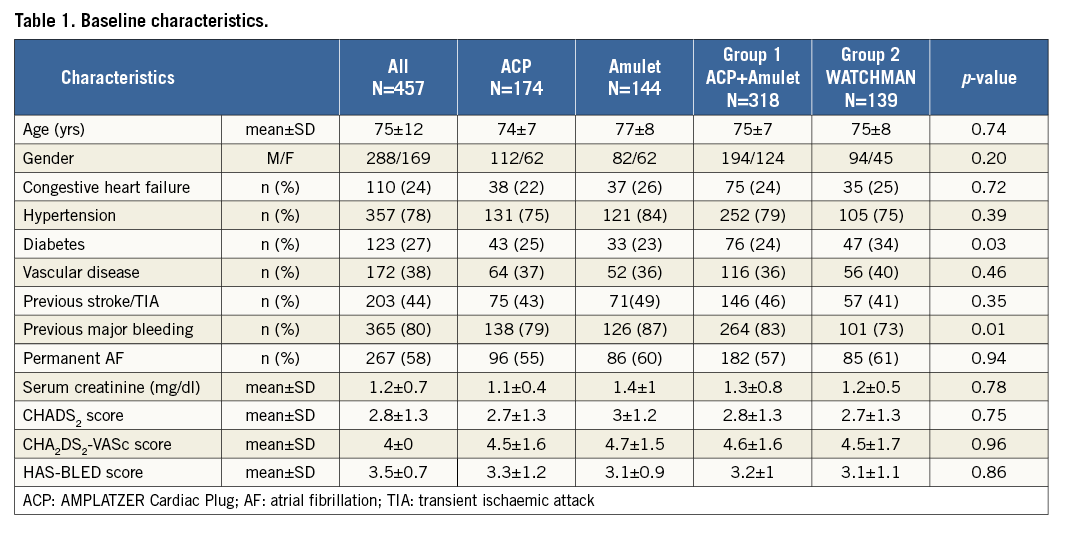
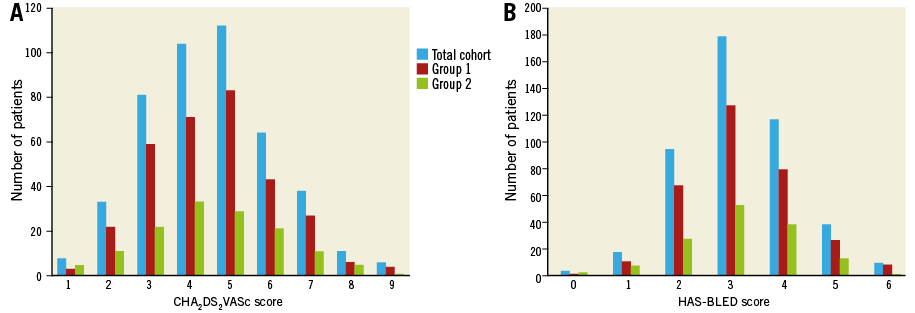
Figure 1. Distribution of the CHA2DS2-VASc (A) and the HAS-BLED (B) scores observed in the total cohort (in blue), Group 1 (in red), and Group 2 (in green).
Indications for the procedure in Belgium were mainly previous major bleeding (gastrointestinal and cerebral in 136 and 134 patients, respectively). The other reasons for LAAO were recurrent minor bleeding in 27%, recurrent stroke under anticoagulation in 11% and other cause in 18% of cases.
PROCEDURE
Procedures were performed in 21 centres reporting by themselves their complete data from the beginning (first implants were not excluded from the study). Only three centres performed more than 50 procedures, while six others started the LAAO programme in 2016. The device implanted was ACP in 174 patients, Amulet in 144 and WATCHMAN in 139 cases. One hundred and sixty-eight patients (37%) were in sinus rhythm at the time of implantation. An additional procedure was combined in 22% of cases - 93 coronary angiography, 11 atrial fibrillation ablation and one interatrial septal defect closure. The outcome of the patients was similar between patients with and those without a combined intervention. Device success was achieved in 97.5%; 11 device failures were reported due to inappropriate anatomy, four with the ACP device, zero with the Amulet and seven with the WATCHMAN. Large residual leak was observed after two WATCHMAN implantations (0.4% of the total cohort); technical success was achieved in 97.1%.
PERIPROCEDURAL COMPLICATIONS
The rate of periprocedural MAE was 4.1% (Table 2). Three procedural deaths occurred (0.6%), all related to tamponades treated by surgery but ultimately resulting in death. Additional tamponades were observed in nine cases: four of them were successfully treated by a percutaneous pericardiocentesis and five required surgery. There were significantly more MAE with the ACP device than after Amulet implantation (4.5% versus 0.7%, p=0.04), explained by the learning curve (centres started with the ACP and moved to the Amulet when it became available). Two device embolisations were reported: one was totally asymptomatic, discovered at day 1 and successfully retrieved by snare. The other one required a surgical removal of the implant with a good outcome. Major bleedings occurred only in Group 2 (four versus zero, p=0.008) – two groin haematoma and two recurrent bleedings (one from the lung, the other one from gastrointestinal angiodysplasia) still recurrent despite stopping anticoagulants after LAAO. Dual antiplatelet therapy (DAPT) was used in three of them, while the last one was under aspirin alone. This is the only statistically significant difference between Groups 1 and 2, but it is numerically not clinically relevant. There was no incidence of periprocedural stroke, TIA or myocardial infarction in our cohort. Procedural success was achieved in 96.6% of cases.
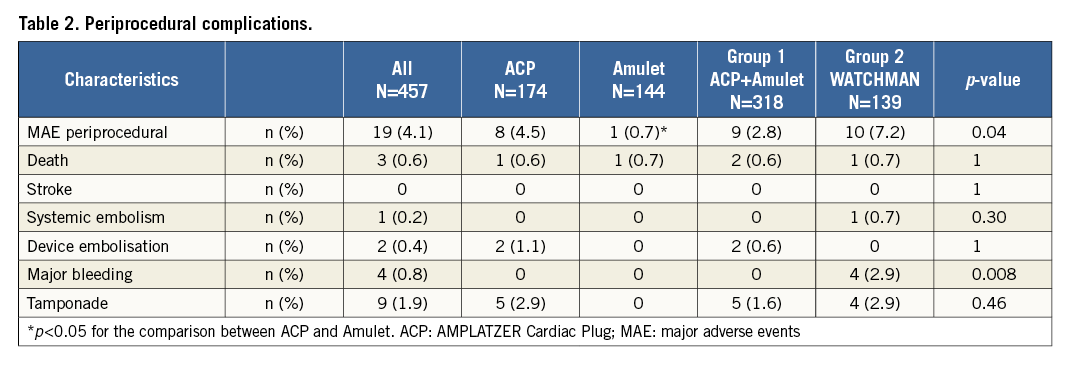
Medications at discharge were mainly DAPT, prescribed in 72% of cases (Figure 2A). Any form of anticoagulant therapy was more frequently used after WATCHMAN (24%) than after ACP/Amulet implantation (3%). Eighty-eight percent of patients were left untreated by any anticoagulant at discharge after LAAO.
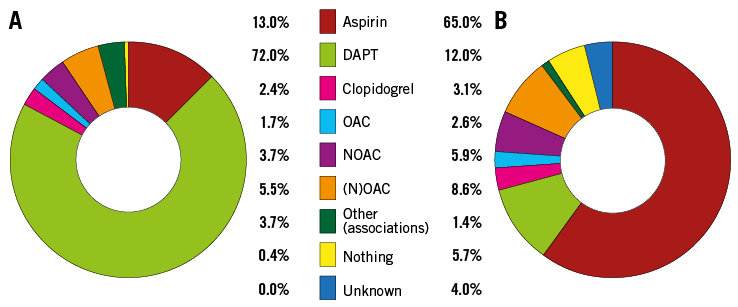
Figure 2. Medications given. A) At discharge. B) At last follow-up. DAPT: dual antiplatelet therapy; NOAC: new oral anticoagulant; (N)OAC: any form of anticoagulant; OAC: oral anticoagulant
FOLLOW-UP
The follow-up was complete in 417 of 444 patients with successful LAAO (94%). The mean duration of follow-up was 589 days, and the median value was 12.3 months (interquartile range 136-841 days), resulting in a total of 672 patient-years. There were 10 strokes (eight ischaemic, two haemorrhagic), seven TIAs, three fatal bleedings, nine major bleedings reported during the follow-up period, with no difference between groups (Table 3). Among the 49 deaths observed during this period, 14 were from an identified cardiovascular disease (Table 4). Only one death was procedure-related due to a delayed device embolisation at one month after an uneventful ACP implantation, treated by emergent surgery but resulting in death. The vast majority of deaths were related to the comorbidities of patients and occurred at a mean time of 594 (median 406) days after the procedure.
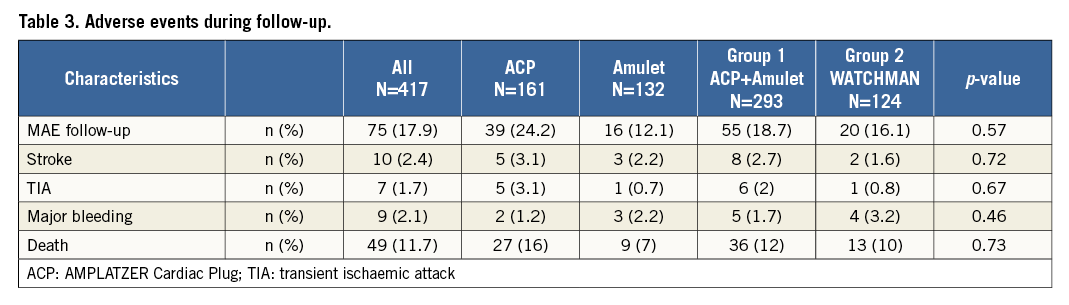
Overall survival of the total cohort was 89±2% (Figure 3). Kaplan-Meier analysis showed a similar overall and event-free survival, for the WATCHMAN versus the ACP/Amulet groups of patients, respectively (Figure 4).
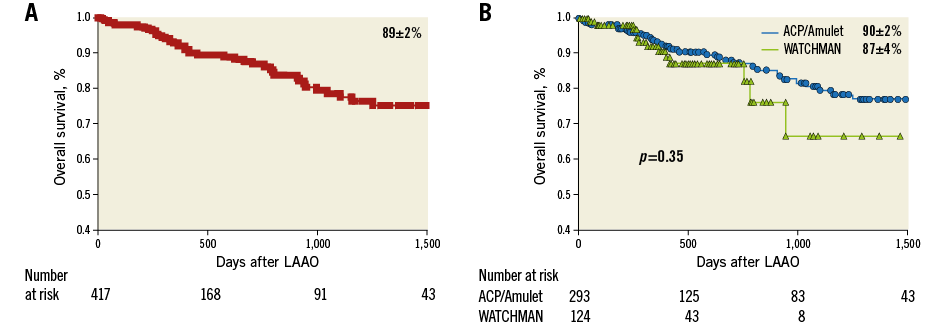
Figure 3. Overall survival after LAAO. A) Kaplan-Meier analysis showing the overall survival of the total cohort. B) Comparison of the overall survival between Group 1 and Group 2.
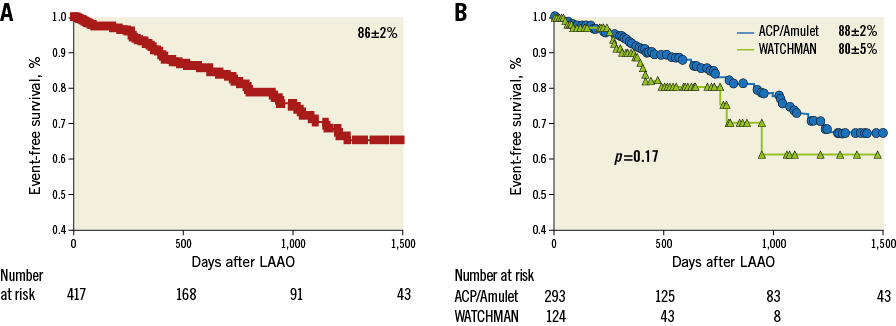
Figure 4. Event-free survival after LAAO. A) Kaplan-Meier analysis showing the event-free survival of the total cohort. B) Comparison of the event-free survival between Group 1 and Group 2.
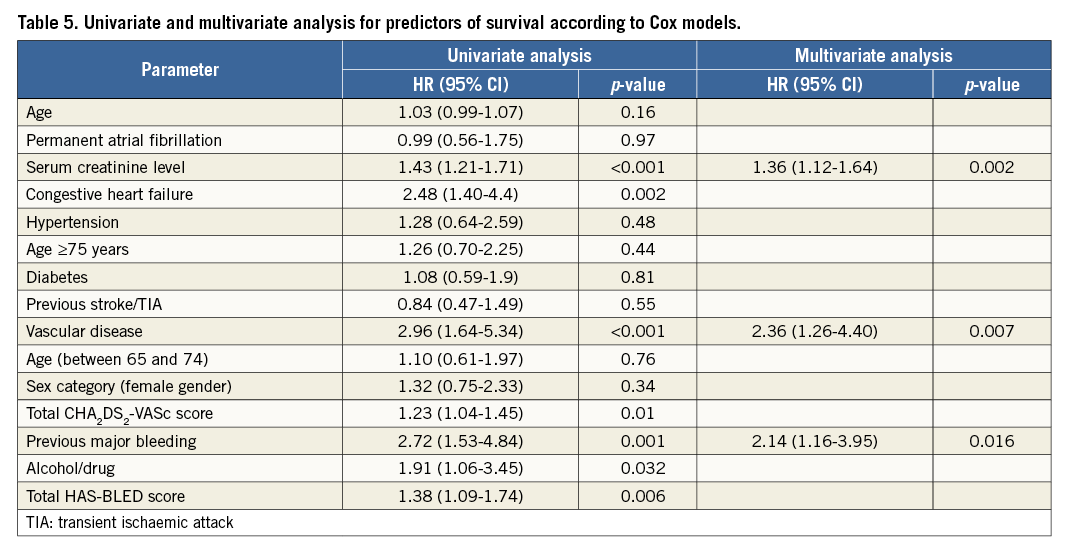
In univariate analysis, CHA2DS2VASc score, HAS-BLED score, congestive heart failure and alcohol abuse/use of drugs were associated with death at follow-up, while in multivariate analysis serum creatinine level, vascular disease and previous major bleeding were independent predictors of mortality at follow-up (Table 5).
The actual annual rate (periprocedural and follow-up period) of stroke was 1.2%, and of thromboembolism 2.2%, while the expected annual thromboembolism risk was calculated by the CHA2DS2-VASc score at 4%, which translates into a 45% risk reduction (Figure 5).
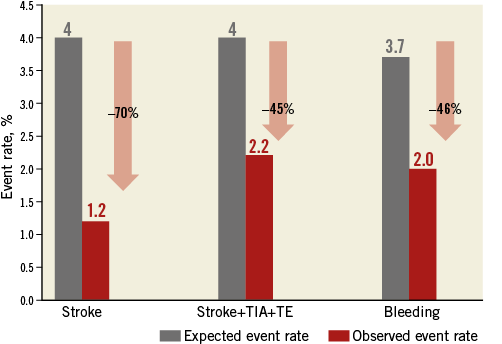
Figure 5. Effectiveness of LAAO in reduction of thromboembolism and bleeding based on annual rate predicted by CHA2DS2-VASc and the HAS-BLED score. Observed event rate in red; expected event rate in grey. TE: thromboembolism
The observed annual major bleeding rate (periprocedural and follow-up period) was 2% and the annual risk of bleeding estimated by the HAS-BLED score was 3.7% (46% reduction).
Medication at last follow-up was limited to acetylsalicylic acid in 65% of patients, while only 10% of them were under anticoagulant therapy (Figure 2B). In detail, medication used in patients of Group 1 vs. Group 2 was OAC (5 vs. 6), NOAC (10 vs. 15), aspirin (207 vs. 63), DAPT (38 vs. 13), clopidogrel (9 vs. 4), combination (1 vs. 5), and nothing (14 vs. 10 patients), respectively.
Discussion
The main findings of this paper are:
1. The efficacy of LAAO in stroke prevention is very high in real-life settings.
2. The bleeding rate after LAAO, especially the haemorrhagic stroke rate (0.2%/year), was lower than expected.
3. The procedure of LAAO is relatively safe in Belgium, with a 4.1% rate of periprocedural MAE.
4. The outcome of patients undergoing LAAO in real life was similar regardless of the type of device used.
EFFICACY IN STROKE REDUCTION
Two randomised trials3,6 showed the non-inferiority of LAAO using the WATCHMAN device as compared with warfarin. The ACP multicentre registry9 showed a thromboembolism rate after LAAO of 2.3% among patients with a CHA2DS2-VASc score of 4.5. The thromboembolism rate after WATCHMAN implantation in the ASAP registry including patients contraindicated for OAC22 was 2.3% (mean CHA2DS2-VASc score of 4.4). The effectiveness in terms of cardioembolic event reduction was similar in our study: the observed stroke rate was 1.2%, and the thromboembolism rate was 2.2%, which translates into a 45% risk reduction for patients with a mean CHA2DS2-VASc score of 4.0.
IMPACT ON BLEEDING
The bleeding rate observed after LAAO in Belgium was 2%, while the expected bleeding rate was 3.7%. Only two haemorrhagic strokes were observed during the follow-up period (haemorrhagic stroke rate 0.2%/year), despite the fact that 29% of our population experienced an intracranial bleeding before the procedure. The rate of major bleeding in the new oral anticoagulants (NOACs) trials was 4.5% in the ROCKET23, 2.7% in the RELY24 and 2.1% in the ARISTOTLE trial25, while the rates of haemorrhagic stroke were 0.3, 0.1 and 0.2%/year, respectively. In the Belgian LAAO registry, 90% of patients were left untreated by any form of OAC at last follow-up, potentially explaining the lower rate of bleeding as compared with NOAC trials, despite the fact that the latter trials included patients with a lower risk profile than in our real-life study.
PROCEDURAL SAFETY
The rate of MAE (4.1%) in our study is lower than in the initial ACP European registry4 or the PROTECT AF study3 (7.3 and 7.7% MAE, respectively), and is similar to those in the more recent publications6,7,9, such as the CAP (3.7%), multicentre ACP registry (4.9%) and PREVAIL study (4.2%). The Belgian LAAO registry included all the procedures performed in all the centres (the first implants were not excluded). There was a wide range of number of implants per centre (three had more than 50 procedures while 10 others had less than 10). In our study, all the first cases in each institution were performed with an on-site proctor, explaining better results, compared to the initial experience3,4. Periprocedural mortality was similarly low at 0.6% and 0.7% in the Belgian and in the EWOLUTION registries, respectively.
As an elective and preventive procedure, these upfront risks must be taken into account for LAAO indication and must be weighed against serious bleeding issues when using (N)OAC.
DEVICE COMPARISON
To the best of our knowledge, the Belgian LAAO registry is the national registry with the highest number of patients, comparing three devices, namely ACP, Amulet and WATCHMAN. Betts et al16 reported the experience in the United Kingdom among 371 patients treated with the WATCHMAN, ACP, LARIAT (SentreHEART, Redwood City, CA, USA) or Coherex WaveCrest® (Biosense Webster, Inc., Irvine, CA, USA) devices, followed during a mean period of 24 months: they stated that the procedure is safe and successful regardless of the technology used but without side-by-side comparison of the devices. Our study compared baseline, procedural and follow-up data on 457 patients treated with ACP, Amulet or WATCHMAN, followed during a mean period of 20 months. Except for major bleedings during the periprocedural period, we showed that the outcome was similar after LAAO, using either the WATCHMAN or the ACP/Amulet device.
Limitations
This registry has an observational design limited by several factors:
– the centres included their data by themselves with no core lab, especially for the neurological evaluation in case of stroke and TIA
– the follow-up is limited to the clinical evaluation without non-invasive imaging information.
Conclusions
The Belgian LAAO registry showed that the procedure is relatively safe, and reduces the thromboembolism and the bleeding rates as compared with the expected risks calculated by the CHA2DS2-VASc and HAS-BLED score, respectively. It is important to emphasise that the observed rate of cerebral haemorrhage was very low at 0.2%/year in our registry, despite one third of this real-life population of patients having experienced a prior intracranial bleeding before the procedure. The overall and event-free survival was similar after LAAO in Belgium, regardless of the type of device used (ACP, Amulet or WATCHMAN). These data may support further studies, using one of these prostheses, evaluating NOAC versus LAAO to compare the impact on the clinical outcome and the cost-effectiveness of these two strategies among AF patients at high risk for stroke.
| Impact on daily practice The data of the Belgian left atrial appendage occlusion registry suggest that the procedure is safe and effective in a real-world setting, for atrial fibrillation-related thromboembolism prevention using either the WATCHMAN or the ACP/Amulet device. |
Conflict of interest statement
J. Kefer, W. Budts and A. Aminian are proctors for St. Jude Medical. A. Aminian is proctor for Boston Scientific. The other authors have no conflicts of interest to declare.




