Using Trace Metals, Peroxide, Acid and Iodine Values to Characterize Oils Bleached Using Clays from Central and Eastern Uganda ()
1. Introduction
There are four basic steps are used to refine oil. These include; neutralization and separation, bleaching and deodorizing [2] . When oil is neutralized, sodium hydroxide, also known as caustic soda, is added to lower the acidity. This neutralizes the bitter taste of the crude oil by combining with the oil to create a sodium salt, which is then separated out from the oil and used for soap stock. The neutralized oil then is ready for bleaching.
Diatomaceous earth, clays, peroxides or carbon is added to bleach and adsorb the dark colored impurities in the oil in order to give it a clear color. Some of oils may have a color that is objectionable to a consumer. Thus, the oil needs to be bleached to improve its color quality. To this end, a great many oils are commonly treated with bleaching clays to reduce oil color values by adsorptive purification.
Bleaching clays generally improve oil color quality by selectively adsorbing color impurities that are present. Color impurities typically present in oils include, for example, carotenoids, xanthophylls, xanthophyll esters, chlorophyll, tocopherols, as well as oxidized fatty acids and fatty acid polymers [3] [4] .
Any off smell that the oil may have is removed through a process known as deodorization. The oil is heated to very high temperatures in a 12-metre tall deodorizer. Vacuum and high heat removes any smell. The result is a clear, odorless, refined vegetable oil.
Clays are collectively called alumino-silicates as they contain aluminium oxide and silicon dioxide as universal minerals. Clays were classified into phyllosilicates and layers silicates [5] and have for long been used as clarifying agents for vegetable oils for human consumption [6] - [10] , filling white paper, drilling fluids, geophagy as well as making bricks, tiles, sanitary wares, cups and plates.
Bleaching of vegetable oils is a very important component of oils production and sales [11] [12] . The bleaching of oils for human consumption is efficiently carried out using commercial bleaching earths. Unbleached oils contain pesticide residues, oxidation products and heavy metals which can become harmful when consumed. So it is essential to bleach the oils [13] [14] .
This study has aimed at determining the effects of bleaching sunflower-seed and cotton-seed oils on trace elements, acid, iodine and peroxide values.
Mukwano, BIDCO and Gulu nanak oil factories bleach more than 200 metric tones of oil. The bleaching oils reduce chances of Ugandans contracting diseases resulting from consumption of heavy metals and pesticide residues in oils.
Whereas kaolinites are rarely used to bleach, acid-leached smectites are widely used to clarify oils. The bleaching of edible oils was accomplished using adsorptive bentonite clays [10] [14] . As early as 1933, the designing and testing procedures for bleaching clays had been published. The designed and tested procedures of bleaching and rating bleaching capacity of acid-leached clay samples were developed using petroleum [15] . Later workers decolorized cotton-seed oil by adsorption of color pigments on acid leached clays of Kajansi and Koki [14] [16] [17] . Cotton-seed oil was bleached by selective adsorption of impurities on sulphuric acid- leached clays from Ghana [18] [19] . The adsorption of components of oils like carotenoids and peroxides during bleaching was reported [20] .
It has been reported that degumming and bleaching removes gums, trace metals, vitamins, pigments and peroxides [21] - [27] . Oxidation products in the crude vegetable oils are removed by adsorption on the active surface of the bleaching earth to improve color and stability of the final oil [28] . The effects of filtering through bleaching media on decrease of peroxide value of autoxidized soybean oil were investigated and shown to improve its quality [29] - [31] .
Degumming and bleaching, however, remove minor constituents like carotenes, tocols, phytosterols and squalenes which have nutritional aspects [23] [32] [33] . b-carotenes play a role in the prevention of cancer, cataracts and degenerative diseases [34] [35] . Similarly, tocopherol and tocotrienols have vitamin E activity in the human body [36] [37] . Major phytosterols in palm oil include campesterol, stigmasterol and b-sitesterol, have pharmaceutical effects but are removed by bleaching [22] [38] - [40] .
It was shown that acid activated clay adsorbed carotenes on its active sites by formation of hydrogen bonding with the Broensted sites or coordination bonds with Lewis acid sites forming stable carbonium ions [3] [41] . Protonated carotenes have been found to degrade readily [42] - [44] .
Bleaching removes phospholipids, inorganic phosphates and gums from oils and the reduction in the total phosphorous present was proportional to the quantity of clay used [45] . They suggested mechanism for phosphorous reduction by adsorption of phosphorous ions on lattice structure of the clay. A review of the industrial bleaching of vegetable oils using clays has been documented by different authors [46] - [48] .
Whereas vegetable oil bearing seeds are grown widely in Uganda, establishing the composition of fully- processed oils sold in Uganda has never been fully studied. It is the aim of this study to establish the effect of bleaching oil on composition of vegetable oils.
2. Materials and Methods
2.1. Location and Geological Settings of Sampled Clays
As shown on the map, clays were mined from Central and Eastern Uganda. Nakawa, Lwanda and Seeta clay deposits are located along Jinja-Kampala Highway. Kajansi clay deposits are on Entebbe road in the Swamp of Kajansi River, Kawuku is located on the shores of Lake Victoria off the Gabba road.
Kumi district is located in Eastern Uganda close to the shores of Lakes Kyoga and Bisina. The clays in Central Uganda and Kumi districts are located in regions which are not associated with volcanic margins. The clay deposits in these areas are mainly sedimentary deposits found along river valleys and swamps.
2.2. Preparation of Clays
Raw samples of clays were separately soaked in distilled water, sieved to pass through a mesh 5.3 × 10−4 m diameter, dried at 105˚C and ground to powder using a rolling mill. The clay powders were stored for future use in desiccators.
2.3. Leaching of Clays
Clay powder (100 g, 0.25 mol) was mixed with acid (500 mL) at different concentrations (0, 5%, 10%, 20% v/v) in a flask. The mixture was heated at 105˚C for 4 hours; then cooled and filtered. The residue was washed to neutrality with distilled water; then dried at 105˚C in the thermo-stated oven. The dried leached powders were labeled and stored for future use [49] [50] .
2.4. Degumming of Vegetable Oils
Crude oil (100 g, 0.43 mol) was placed in a flask, 85% phosphoric acid (1 g, 0.1 mmol) was added, the mixture heated at 90˚C while stirring at 900 revolutions per minute, for 10 minutes under nitrogen blanket. The oil was filtered under nitrogen [51] .
2.5. Bleaching of Vegetable Oils
Degummed neutral oil (200 g, 0.85 mol) under nitrogen blanket was passed through columns containing appropriate neutral, acid-leached clay powders (5 g, 1.24 mmol) separately which had been heat-activated at various temperatures ranging from 40˚C to 130˚C for two hours and left to elute bleached oils [7] .
2.6. Analysis of Bleached Oils
The bleached oils were analysed for iron and copper content, peroxide value, free fatty acids value, iodine number, refractive index, transmittance and turbidity. The methods used are outlined below.
The bleached oil (0.2 g, 0.86 mmol) was digested in the perchloric-nitric-hydrofluoric acid mixture (3.0 mL, 0.13 mol) then made up to 25.0 mL with distilled water. The absorbance of copper was determined at 580 nm using the atomic absorption spectrophotometer Shimadzu-AA-6200. Similarly iron was determined at 478 nm when ammonium thiocyanate solution had been added to complex the ions [21] [24] . An average of three readings was taken.
Bleached oil (2.0 g, 8.6 mmol) was placed in a 250 mL flask. Ethanoic acid (30 mL, 0.43 mol) added together with chloroform (15 mL, 0.24 mol) to give 9.1:5.0 ratio. The mixture was stirred, water (50 mL, 2.7 mol) added and standard potassium iodide (0.5 mL, 0.001 mmol), followed by starch indicator (2 drops) and water (100 mL, 5.4 mol). The mixture was titrated with 0.01 M sodium thiosulphate to discharge the blue colour [52] .
Bleached oil (2 g, 8.6 mmol) was placed in flask; butan-1-ol (10 mL, 0.12 mol) added. The mixture was titrated with 0.5 M ethanolic potassium hydroxide solution to the faint pink phenolphthalein end point [52] . The experiment was repeated thrice.
Resublimed iodine (1.3 g, 5.1 mmol) was dissolved in ethanoic acid (100 mL, 1.3 mol) and slight excess chlorine bubbled through it. Oil (0.1 g, 0.43 mmol) was placed in the 250 mL volumetric flask containing chloroform (10 mL, 3.1 mmol) excess 0.1 M iodine (15 mL, 1.5 mmol) was added, stirred and mixture boiled, then cooled. The amount of unreacted iodine was determined by back titration with standard thiosulphate solution [53] .
The test portion (0.1 g, 0.43 mmol) was placed in a reaction flask and dissolved in 1:1 (v/v) mixture of 95% ethanol (15 ml, 0.26 mmol) and ethoxyethane (15 mL, 0.16 mmol). The solution was titrated with standard 0.1 M ethanolic potassium hydroxide solution, using phenolphthalein indicator. The determinations were duplicated for each batch of bleached and unbleached oils. The acid value, AV was calculated using the formula;
 (1)
(1)
V is volume of standardized potassium hydroxide solution used/mL.
T is the molarity of the standardised potassium hydroxide solution used.
M is mass in grams of the oil portion taken [52] . The experiment was repeated to get comparing results used to calculate mean acid values.
3. Results and Discussion
3.1. Effect of Bleaching on Metal Content of Oils
Since vegetable oils are bleached to remove coloring matter, traces of heavy elements, phospholipids, poly-aro- matics and oxidation products [54] , so as to improve the quality and oxidation stability of oils [23] . It was found necessary to determine the amount of iron and copper present in the bleached oils as test on quality of oil produced. The data in Table 1 and Table 2 was obtained. The data in Table 1 and Table 2 have been presented in Figure 1 and Figure 2 as bar charts.
The quantity of trace elements in oils has been shown to progressively decrease as the concentration of acid used to leach the clays increased as in Figure 1 and Figure 2, the content of iron and copper in the bleached oils decreased with increase in concentration of the acid used per fixed mass of clay [55] . While the decrease in iron content was highest for all oils bleached, content of copper showed the smallest change. The decrease in copper content for palm oils decreased from 0.2 ppm to 0.1 ppm when bleached with Kajansi clay which had be leached in 20% acid and a similar change occurred when Chelel clay leached in 20% acid was used. The decrease in copper content of cotton oils has been observed to change from 0.5 ppm to 0.15 ppm using Kajansi clay leached in 20% acid yet when Chelel clay leached under similar conditions was used decrease was from 0.5 to 0.1 ppm
![]()
Table 1. The content of iron and copper in oils bleached using Kajansi clays.
![]()
Table 2. The content of iron and copper in oils bleached using Chelel clays.
![]()
Figure 1. Content of trace metals in bleached oils using Chelel clay activated at 90˚C.
![]()
Figure 2. Percentages of metals in bleached oils using Chelel clays.
[24] . It showed that both clays were effective in removing copper. The decrease in copper content in sunflower bleached using Kajansi clay leached in 20% acid was from 0.3 to 0.15 and a similar decrease was observed when Chelel clay leached in 20% acid was used. So clays from volcanic sediments and acid-granitoids can effectively eliminate copper from oils.
The content of iron in palm oils bleached using Kajansi clay leached in 20% acid decreased from 3.0 to 0.2 ppm yet that bleached under the same conditions using Chelel clay decreased to 0.1 ppm. This showed the Chelel clay was better than Kajansi clay. Similarly content of iron in cotton oils bleached using Kajansi clay decreased from 2.5 to 0.2 yet that bleached using Chelel clay decreased to 0.1 ppm. The content of iron in sunflower oils bleached using Kajansi clay leached in 20% acid decreased from 1.6 to 0.2 ppm yet that bleached with Chelel clay under similar conditions decreased to 0.1 ppm.
Degumming and bleaching removed trace metals like copper and iron. Presence of these elements is known to increase ease of oxidation of oils, so their removal must increase the shelf life of the bleached oils [56] . Clays are known to adsorb metal ions like Cd2+, Pb2+, Ni2+,  [57] [58] . Similarly, acid-activated sepiolite adsorbed phosphorus from rapeseed oil [19] [59] .
[57] [58] . Similarly, acid-activated sepiolite adsorbed phosphorus from rapeseed oil [19] [59] .
The results in Figure 1 and Figure 2 depicted that for a fixed mass of clay, both iron and copper were increasingly removed with progressive increase in the mass percent of the acid used. The decrease in content of iron and copper in the bleached oils was greatest for the acid leached clays from Chelel possibly because it had the highest smectite content, so the clay acquired high capacity to adsorb metal ions from oils during the bleaching process. Unleached raw clays studied showed reduced adsorptive capacity for metal ions due to availability of very few adsorption sites in the matrix and would be adsorption sites are occupied by exchangeable ions in the interlayers. So the silicon skeleton left after acid-leaching of a clay is very important in the adsorption of metals. The fact that raw clays had a significant effect on the content iron and copper in the bleached oils underscored the importance of clays in removing heavy metals from the environment.
3.2. Effect of Bleaching on Acid Value and Free Fatty Acids Content
Bleaching vegetable oils with acid activated clays may lead to increased acidity of the oils as solid acids may catalyze hydrolysis of fats by water present in oils or clays [54] . So it has been found necessary to determine the amount acid present in the bleached oils. The data obtained is presented in Table 3.
The bleached oils were subjected to testing for acid value by titrating portions of the bleached oil (0.4 g) which were dissolved in ethanol-ether mixture to make 150 cm3 of solution with 0.1 M ethanolic potassium hydroxide solution using phenolphthalein indicator. The titres obtained were used to calculate the acid values in Table 3 for the clays used.
The calculated acid values showed that the acidity in sunflower oils is largely due to oleic acid as the average value for acids is in the range close to the molar mass of 282 for oleic acid. The calculated acid values for cotton-seed oil correspond to average value for linoleic acid, molar mass 279. The acid values for the raw and bleached oils are almost constant showing that bleaching did not cause the triglycerides to hydrolyze and this is not a new phenomenon because the bleached vegetable oils’ acid values were not affected by the bleaching procedure although a slight shift in the absorption maximum of the bleached cottonseed oil was observed [60] [61] . Efficient bleaching required determination of peroxide value, free fatty acid content, iron concentration, and conjugation values [54] .
Bleaching of vegetable oils at elevated temperatures and in presence of water normally leads to significant rise in levels of free fatty acids because the triglycerides are hydrolyzed by the water present in clays or oils or significantly dissociate when heated at temperatures as high as 200˚C [54] . Basing on this, it often necessary to determine the free fatty acid content of vegetable oils bleached with acid-leached clays. The data obtained in experiments to deduce the level of acidity of bleached clays has been presented in tabular form in Table 4.
The bleached oils were subjected to free fatty acids (FFAs) content of oils to investigate the effect of bleaching on fatty acid levels in oils. The data tabulated in Table 4; showed that bleaching sunflower, cotton and palm oils had no significant changes on the free fatty acid content of bleached oils as compared to unbleached oils.
The levels of free fatty acid did not rise significantly because most of the clay matrices are themselves acidic enough to disfavor dissociation of the triglycerides. It is likely that the surface acidity of the clay matrices greatly retarded dissociation of the triglycerides to give free fatty acids. The slight increase in level of free fatty acids has been attributed acid activated hydrolysis of the triglycerides as the clays used were acidic. As the oils feebly ionized in the acidic clay matrices, free fatty acid levels increased insignificantly in the bleached oils be-
![]()
Table 4. Percentages of FFA in bleached vegetable oils.
cause the oils lacked water or the clays lacked water to cause the triglycerides in vegetable oils to hydrolyze and the temperature at which bleaching was performed was low so the triglycerides could not dissociate to give free carboxylic acids [54] . The rise in acidity was automatically checked by the exchange capacity of the clays so it was not significant. The slight rise in free fatty acids with increase in mass percent of acid used to leach the clay is in agreement with earlier results [61] - [63] .
Vegetable oils always contain oxidation products that impart unpleasant odors. Bleaching with activated clays eliminates oxidation products and other impurities from oils; thus improving the quality and shelf life of the oils [23] [54] [64] . It is therefore necessary to determine the quantities of peroxides that may be still present to find out if bleaching effectively eliminated them. The data obtained on these experiments has been summarized in Table 3.
The titres obtained during the titration of the iodine liberated when the peroxides in the bleached oil (0.1 gm) reacted with potassium iodide in presence of acid were summarily used to calculate peroxide values given in Table 3 for clays used to bleach cotton oils and sunflower oils. The values of the peroxide values were computed using the formula:
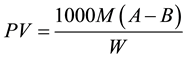 (2)
(2)
where A is titre obtained with sample of sodium thiosulphate/cm3; B is blank titre of sodium thiosulphate/cm3; M is molarity of sodium thiosulphate; W is mass in g of test portion.
3.3. Effect of Bleaching of Oils on Peroxide Value
The resulting ranges of peroxide values have been summarized in Table 5.
As shown in Table 5, the values are negligibly small showing that freshly bleached sunflower and cottonseed oils are fit for human consumption. The reductions found in peroxide values of the cotton and sunflower seed oils after their bleaching with the different kinds of bleaching earths developed in this study agree with those presented by earlier workers, [4] [54] [61] who showed that oxidation levels are reduced by the breakdown of hydroperoxide primary oxidation product on the adsorbent surfaces such as bleaching earth. Earlier workers suggested that a decrease in peroxide value is due to the decomposition of peroxides by the strongest acid on the surface of bleaching earth [4] . However, it is expected that peroxide values would be higher if the oils were stored for long to encourage rancidity. The primary oxidation products got removed significantly to lower levels as the concentration of the leaching medium increased. The efficiency of adsorption of oxidation products is increased by increase in mass percent of the acid used to leach the clays for all the studied bleached vegetable oil samples investigated [39] [40] [55] [56] . As the acid used to leach the clays was low, satisfactory removal of vegetable oil oxidation products was achieved in a manner similar to results adduced when medium activation of
![]()
Table 5. Peroxide values for oils bleached at 90˚C.
the clay (treatment of Ca-montmorillonite with 4・NH2SO4) and was found the most effective in bleaching the cottonseed oil, resulting in the best color index and the lowest peroxide value [60] . To evaluate efficiency of bleaching required measurements peroxide value, free fatty acid content, iron concentration, and conjugation values [54] .
Vegetable oils are unsaturated containing double C=C bonds. The level of unsaturation of the oils may be expressed by the iodine numbers. Iodine numbers of bleached oils have been presented in Table 6 and used to identify the dominant acid in the oils. To test the efficiency of bleaching, determination of iodine value or number for conjugation values of the bleached oils was essential [54] .
The titre values for the blank and iodine solutions generated when iodine solution (25 cm3) was added to bleached oil sample (1 g) which had been dissolved in trichloromethane (15 cm3) and the resulting mixtures separately titrated with 0.1 M sodium thiosulphate solution after adding water and potassium iodide solution and labeled basing on oil bleached. Using the average titres obtained from titration and applying the formula below, the iodine values (Iv) were calculated and summarily tabulated in Table 6 as shown below.
Iodine value is given by:
 (3)
(3)
T is exact molarity of sodium thiosulphate = 0.1 M.
V3 is number of cm3 of Na2S2O3, used on blank test.
V4 is number of cm3 of Na2S2O3, used on test portion.
M is mass of test portion in grams.
The iodine values for the vegetable oils bleached with raw and acid leached clays are low and are in same range showing that the structures of oils change negligibly as a result of bleaching.
The data adduced in this study compares very well with the internationally published data on iodine values of oils by journal of American oil Chemists in which the iodine value for cotton-seed oils lies in the range 108 - 110 and for sunflower oils it is in 109 - 135 range. This data has been deduced to indicate that the acid of formula CH3(CH2)7CH=CH(CH2)7COOH, oleic acid is dominant in cotton oils yet the acid of formula CH3CH2(CH=CHCH2)3(CH2) 6COOH, linolenic acid is dominant in sunflower oil [65] [66] . The data in Table 6 lacks molecular masses 232 and 270 and so it gives similar inferences those reported by many authors [61] [63] [64] [67] who confirmed that bleaching increases the level of conjugated trienes and reduces the content of conjugated dienes as these have molar masses lying in range of 232 and 270.
4. Conclusions
The decrease in content of iron in the bleached oils was highest for all oils bleached. The content of copper showed the smallest change. The decrease in copper content for palm oils decreased from 0.2 ppm to 0.1 ppm when bleached with either Kajansi or Chelel clay which had been leached in 20% acid. This showed the clays effectively removed iron and copper from oils bleached under different conditions.
The decrease in copper content of cotton oils was from 0.5 ppm to 0.15 ppm using Kajansi clay leached in 20% acid yet when Chelel clay leached under similar conditions was used decrease was from 0.5 to 0.1 ppm. The content of iron in palm oils bleached using Kajansi clay leached in 20% acid decreased from 3.0 to 0.2 ppm yet that bleached under the same conditions using Chelel clay decreased to 0.1 ppm. Similarly, the content of iron in cotton oils bleached using Kajansi clay decreased from 2.5 to 0.2 yet that bleached using Chelel clay decreased to 0.1 ppm. The content of iron in sunflower oils bleached using Kajansi clay leached in 20% acid decreased from 1.6 to 0.2 ppm yet that bleached with Chelel clay under similar conditions decreased to 0.1 ppm. So Chelel clay was better at removing trace elements from oils than Kajansi clay [25] .
The acid values showed that the acidity in sunflower oils is largely due to oleic acid as the average value for acids is in the range close to oleic acid; cotton-seed oil corresponded to linoleic acid. So the bleached oils contain different free fatty acids.
![]()
Table 6. Iodine values for oils bleached at 90˚C.
The levels of free fatty acid were found to lie in range from 3.8 - 32 for all clays used showing no significant rise in acidity resulted from bleaching.
The peroxide values of bleached oils lay between 1.2 and 0.8 showing that freshly bleached oils are fit for human consumption.
Acknowledgements
We thank Mr. Edward Ssekubunga for heating the clay samples studied in the furnace, Mr. Moses Nkolongo (rest in peace) for availing us chance to use the spectrophotometers in the analytical laboratories. Dr. M. Ntale for availing us space in Laboratories with reagents and encouraging remarks which enabled the compilation of the manuscript.
NOTES
*Corresponding author.