Research Article Open Access
Bioethanol Production by an Ethanol-Tolerant Bacillus cereus Strain GBPS9 Using Sugarcane Bagasse and Cassava Peels as Feedstocks
Victor Ezebuiro1*, Chimezie Jason Ogugbue1, Boma Oruwari2 and Francis Sopuruchukwu Ire11Department of Microbiology, Faculty of Science, School of Graduate Studies, University of Port Harcourt, P.M.B 5323, Choba, Nigeria
2Research and Development Division, Nigerian National Petroleum Corporation (NNPC), Life Camp, Eleme Port Harcourt, Nigeria
- Corresponding Author:
- Victor Ezebuiro
Department of Microbiology, Faculty of Science
School of Graduate Studies
University of Port Harcourt, Choba, Nigeria
Tel: +2348064944045
E-mail: ezebuirovictor@gmail.com
Received date: October 18, 2015; Accepted date: November 30, 2015; Published date: December 07, 2015
Citation: Ezebuiro V, Ogugbue CJ, Oruwari B, Ire FS (2015) Bioethanol Production by an Ethanol-Tolerant Bacillus cereus Strain GBPS9 Using Sugarcane Bagasse and Cassava Peels as Feedstocks. J Biotechnol Biomater 5:213. doi:10.4172/2155-952X.1000213
Copyright: © 2015 Ezebuiro V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Bioethanol production potential of ethanol-tolerant Bacillus cereus strain GBPS9 using sugarcane bagasse and cassava peels as feedstocks was investigated. The Bacillus cereus GBPS9 used in this study was isolated from agro-wastes impacted soil and classified based on phylogenetic analysis of its 16S rRNA gene. The sequence of the isolate has been deposited in GenBank under the accession number KT318371.1. The isolate was selected based on its cellulolytic ability, tolerance to ethanol concentration of 6% (v/v) and ability to ferment sugar to ethanol. The substrates employed in the study were cassava peels and sugarcane bagasse. Chemical composition analysis showed total carbohydrate and lignin contents (% dry weight) of 69.6 ± 1.2 and 13.9 ± 0.4 for cassava peels and 70.3 ± 1.9 and 16.2 ± 1.2 for sugarcane bagasse, respectively. The feedstocks were subjected to acid, alkali and steam explosion pretreatments to increase cellulose content and therefore, reduce lignin content. The best pretreatment methods (steam explosion for sugarcane bagasse and acid for cassava peels) increased total carbohydrate contents to 85.4 ± 2.33 and 80.4 ± 2.5 for sugarcane bagasse and cassava peels, respectively. The respective lignin contents after pretreatment were 4.2 ± 0.44 and 4.8 ± 0.8 for sugarcane bagasse and cassava peels. Cultural conditions (pH, temperature, nitrogen source, inoculum size and substrate concentration) of the bacterium were optimized to enhance cellulase production. The laboratory scale fermentation of the feedstocks to ethanol was carried out in 250 mL Erlenmeyer flasks. Gas Chromatography – Mass spectrometry (GC-MS) analysis of the fermentation broth of sugarcane bagasse and cassava peels substrates revealed ethanol contents of 18.40 and 17.80 g/L, respectively. The study has demonstrated efficient bioethanol production by Bacillus cereus GBPS9 using sugarcane bagasse and cassava peels as feedstocks.
Keywords
Bacillus cereus; Bioethanol, Cassava peels; Simultaneous saccharification and fermentation (SSF); Sugarcane bagasse; GC-MS
Introduction
The last two decades have seen increased interest in bioethanol as alternative source of energy to fossil fuel [1]. This trend follows the adoption of the Kyoto protocol in Kyoto, Japan on the 11th of December 1997 and its subsequent entry into force on 16th of February, 2005. Fossil fuel sources such as coal, oil, natural gas etc. have contributed to the drastic increase in the level of greenhouse gases (GHG) in the Earth’s atmosphere resulting in the need for alternative energy sources that are environmentally friendly, renewable and sustainable [2], a fit, bioethanol from lignocellulosics promises to achieve. However, the process has several challenges and limitations such as biomass transport, biomass handling, efficient pre-treatment methods for total delignification of lignocellulosics and appropriate fermentative organism [1].
Conventionally, ethanol is produced from the processing of starchand sucrose-based feedstocks, utilizing enzymatic liquefaction and saccharification; leading to the production of a relatively clean glucose pool [3]. However the food and feed crops for energy production crisis has prompted the need for bioethanol production from sources other than feedstocks with direct food and feed values. This reason has informed the interest in the use of lignocellulosics for the production of ethanol.
Agricultural wastes such as sugarcane bagasse and cassava are examples of lignocellulosics and have been employed in bioethanol production [4,5]. Sugarcane (Saccharum officinarum) is used worldwide as a feedstock for ethanol and sugar production [6] and Nigeria is one of the most important producers of the crop with a land potential of over 500,000 hectares of suitable cane field capable of producing over 3.0 million metric tonnes of sugarcane [7]. After sugarcane is milled for juice extraction, bagasse is obtained as a residue and corresponds to about 25% of the total weight; containing 60% to 80% of carbohydrates [6]. Cassava is particularly an interesting feedstock for bioethanol production especially in Nigeria, considering that it is produced in large quantity and that the country remains the largest producer of cassava in the world since 2005 [8]. Besides, cassava can be grown in arid, marginal soil where other crops, such as, corn, sugarcane and sugar beet fail [4,9]. The plant cell wall of agricultural wastes is formed by two carbohydrate fractions (cellulose and hemicellulose) embedded in a lignin matrix. Lignin is a phenolic macromolecule, resistant to enzyme attack and degradation, and thus its content and distribution are recognized as the most important factors determining cell wall recalcitrance to hydrolysis [10-12].
Processing of agricultural wastes to ethanol follows a general procedure for the conversion of lignocellulosics to ethanol. The procedure involves three major operations: pre-treatment for delignification, which is necessary to liberate cellulose and hemicellulose before hydrolysis; hydrolysis of cellulose and hemicellulose to produce fermentable sugars (glucose, xylose, arabinose, galactose, and mannose) and fermentation of sugars to ethanol. The non-carbohydrate components of lignin also have value-added applications [13].
This study was aimed at investigating bioethanol production by an ethanol-tolerant Bacillus cereus strain GBPS9 using sugarcane bagasse and cassava peels as feedstocks
Materials and Methods
Sugarcane bagasse and cassava collection, processing and comminution
The sugarcane bagasse (SB) and cassava peels (CP) used in the study were obtained from local sugarcane sellers in Port Harcourt and cassava farmers in Nonwa, Tai LGA, Rivers State, Nigeria, respectively. The biomass was washed and dried at atmospheric temperature for 3 days. The dry biomass was further grinded with an electric blender (Philips blender HR2001, Japan), filtered with a 60 Mesh (0.250 mm) sieve and stored under dry conditions until use.
Chemical analysis of the feedstocks
The method described by Milne et al. [14] was used to determine the dry matter, acid detergent fibre (ADF) and neutral detergent fibre (NDF) contents of the sugarcane bagasse and cassava peels. Crude protein was determined by Kjeldahl method and total carbohydrate by Clegg Anthone method as described by Sluiter et al. [15]. The method described by Sluiter et al. [16] was used to determine crude fibre and total ash.
Cellulose: The acid detergent fibre (ADF) content was used for the estimation of cellulose, employing the standard method described by Sluiter et al. [15]. The content of the crucible was covered with cooled (15oc) 75% (24 N) H2SO4 and stirred with glass rod to a smooth paste, breaking all lumps. Then the crucible was half-filled with acid. After 1 h when acid was drained off, the crucible was refilled with 72% acid. Three hours later, acid was filtered off as much as possible with vacuum; the content dried at 100oc overnight and weighed. The loss in weight was taken as cellulose and it was calculated using the following formula:
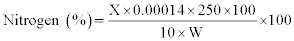
Wr.=Weight of dried residue; trt.=Treatment
Hemicellulose: Hemicellulose was determined by the difference between neutral detergent fibre NDF (%) and ADF (%).
Hemicellulose (%) = NDF (%)-ADF (%)
Lignin: The residue that remained after determination of cellulose was treated with 25 ml KMnO4 buffer for 90 min at 20-25°C. Lignin was dissolved leaving cutin and silica as insoluble materials. The contents were then filtered through tarred sintered crucible using gentle suction and residue was washed with distilled water, then with acetone. Crucible and residue were dried in an oven at 100°C.
Crude protein (CP): Crude protein was determined by Kjeldahl method (15). One gram of the processed sample (W) was digested with concentrated H2SO4 in the presence of a catalyst mixture containing HgSO4 and K2SO4 (1:9). The digested sample was diluted with water to a volume of 250 ml; 10 ml of aliquot of diluted sample was mixed with 10 ml of NaOH solution (40%). Excess alkaline reaction and mixture was distilled with steam in the presence of 50 mg zinc dust in the micro-Kjeldahl distillation apparatus. The ammonia so liberated was collected in 2% boric acid solution containing few drops of mixed indicator (methyl red and methylene blue). The distillate thus obtained was titrated against 0.01 N H2SO4. A blank was also run under the same conditions. From the actual volume of 0.01 N H2SO4 used, the % nitrogen was calculated by equating 1 ml of 0.01 (NH3)2SO4 to 0.00014 g of nitrogen. To obtain percentage of crude protein, % nitrogen was multiplied by 6.25.
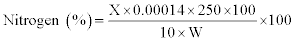
CP (%) =Nit % × 6.25
X=ml of 0.01 N H2SO4 used
1 ml of 0.01 N H2SO4=0.00014 g of NH3 nitrogen
W=Weight of the sample in grams
250=Dilution Factor; 6.25=N to protein conversion factor
Feedstocks pre-treatment
The feedstocks were each subjected to steam explosion, acid and alkali pre-treatment methods.
Steam explosion (SE) pretreatment of the feedstocks: SE pretreatment method described by Sharma et al. [17] was employed for the pre-treatment of the biomasses used in the study with slight modification. Ten (10) grams of each biomass was suspended in 90 ml of distilled water in a conical flask (PYREX Erlenmeyer Flask, USA) and placed in an autoclave for 45 min at 121°C. After 45 min, the autoclave was forcefully depressurized by spontaneously removing the lid. The solid residue was collected and extensively washed with tap water until neutral pH was reached. The solid residue was then dried at 60oc overnight with a laboratory grade electric oven (Zhengzhou Nanbei Instrument Co. Ltd., China) using the method described by Fan et al. [18]. The dry hydrolysate was analysed for cellulose, hemicellulose and lignin content and stored in sterile polypropylene bags for further use prior to simultaneous saccharification and fermentation (SSF).
Acid (H2SO4) pretreatment of feedstocks: Acid pretreatment method described by Olanbiwoninu and Odunfa [19] was employed for the pretreatment of the sugarcane bagasse and cassava peels. The acid used was H2SO4 (Sigma-Aldrich, Germany).
Alkali (NaOH) pretreatment of sugarcane bagasse: Alkali pretreatment method described by Olanbiwoninu and Odunfa [19]) was employed for the pretreatment of the sugarcane bagasse used in the study. The alkali used was NaOH (Oxoid, UK).
Isolation and screening of cellulolytic bacteria
To isolate cellulolytic bacteria, aliquots from various dilutions (10-3-10-6) were plated in duplicate on carboxylmethyl cellulose (CMC) agar [20]. The CMC agar comprising (g/L) CMC (Sigma- Aldrich, Germany), 5; NaNO3 (Lab M, India), 1; K2HPO4 (Applichem, Germany), 1; KCl (Lab M, UK), 1; MgSO4 (Sigma-Aldrich, Germany), 0.5; yeast extract (Sigma-Aldrich, Germany), 0.5; glucose (Oxoid, UK), 1 and Agar (Sigma-Aldrich, Germany), 17) was prepared by dissolving the ingredients in 1 L distilled water. The mixture was heated to a boil to homogenize the sample and sterilized in an autoclave at 121°C for 15 min at 15 psi. The sterile molten CMC agar was thereafter maintained at 45oc in a water bath. Fifteen to twenty (15-20) millilitres of the molten agar was dispensed into sterile petri dish and allowed to solidify. The inoculated CMC agar plates were incubated at40°C for 48 h [20,21].
After 48 h of incubation, each of the duplicate plates was screened for cellulase activity by flooding the plates with 0.1% Congo red (Sigma- Aldrich, Germany) solution and left undisturbed for 15-20 min and then destained with 1 M NaCl (Oxoid, UK) [21]. Halo zones around the growing cellulolytic bacteria confirmed positive isolates. The ratio of the clear zone diameter to colony diameter was measured and the highest cellulase and xylanase producers were selected. The largest ratio was assumed to contain the highest activity. The selected isolates were transferred into minimal CMC agar slants and the slants maintained at 4°C for further analysis.
Ethanol tolerance test for the cellulolytic bacteria
The isolates that showed high cellulolytic activity were subjected to ethanol tolerance test. CMC broths amended with varying concentrations of ethanol (Sigma-Aldrich, Germany) ranging from 0 (the control) to 10% (v/v) were used in the screening procedure. Ten microlitre (10 μl) of inoculum from a 24 h broth culture of each isolate was used to inoculate the test tubes containing the sterile CMC broths with various ethanol concentrations. The inoculated test tubes were incubated for 48 h. After 48 h of incubation, the absorbance reading at 600 nm and cellulase activity was determined. Isolates with the highest OD reading as well as cellulase activity at elevated ethanol concentrations were taken for further analysis.
Estimation of enzyme activity
Cellulase activity was assayed using dinitrosalisilic acid (DNS) reagent (Lab M, India) by estimation of reducing sugars released from CMC solubilized in 0.05 M phosphate buffer at pH 8 [22]. Culture broths were filtered using Whatman™ Qualitative Filter Paper (Whatman, UK) and the clear supernatant served as crude enzyme source. Crude enzyme was added to 0.5 ml of 1% CMC in 0.05 M phosphate buffer and incubated at 50oc for 30 min. After incubation, reaction was stopped by the addition of 3 ml of DNS reagent and boiled at 100°C in water bath for 5 min. Development of colour was observed after boiling and sugars liberated were determined by measuring absorbance at 540 nm. Cellulase production was estimated by using glucose calibration curves. One unit (U) of cellulase activity was expressed as the quantity of enzyme, required to release 1 μmole of glucose per min per ml under standard assay conditions [21].
Selection of the fermentation bacterial candidates
The best cellulase-producing bacterium (VCE-19) was selected based on the cellulase activity, its ability to ferment sugar to ethanol and tolerance to ethanol concentration of up to 6% v/v. Pure cultures of VCE-19 in triplicate were maintained on CMC supplemented minimal agar slant in a refrigerator (Haeir Thermocool, China) for further use.
Inoculum development
Pure cultures of VCE-19 were inoculated in CMC broth medium containing in 1 L of distilled water: 7 g K2HPO4; 0.1 g MgSO4; 2 g KHPO4 (Applichem, Darmstadt, Germany); 1 g yeast extract; 0.5 g Sodium citrate (Lab M, India); 10 g glucose (Sigma-Aldrich, Germany) (pH 7) and incubated for 24 h in a rotary shaker incubator (Zhengzhou Nanbei Instrument Co. Ltd., China). After 24 h of fermentation period the vegetative cells were used as inoculum source.
Optimization of cultural conditions for cellulase and reducing sugar production
The effect of temperature on the production of cellulase and reducing sugar: The effect of different incubation temperatures (25, 30, 35, 40, 45, 50 and 60oc) on the production of cellulase by the isolate was studied while other parameters were kept constant.
The effect of pH on the production of cellulase and reducing sugars: The effect of different pH (5, 6, 7, 8, 9, 10 and 11) on the production of cellulase by the isolate was studied by adjusting the pH of the culture medium containing the pre-treated bagasse and cassava peels with 0.1 M HCl and 0.1 M NaOH.
The effect of nitrogen sources on the production of cellulase and reducing sugars: To determine the effect of different nitrogen sources on the production of cellulase by the isolate, each culture medium containing the pre-treated bagasse and cassava peels was supplemented with 1% (w/v) of each of the following nitrogen sources: NaNO3, casein, NH4NO3, peptone, urea and yeast extract.
Phenotypic and biochemical characterisation of selected bacteria
The selected bacterial isolate was subjected to several biochemical tests as described by Holts et al. [23]; MacFaddin [24] and Madigan et al. [25].
Molecular identification of isolates
DNA extraction, PCR amplification of the bacterial 16S rRNA gene and gel electrophoresis of the isolate was carried out at the Molecular Biology Laboratory of National Institute for Medical Research (NIMR) Yaba, Lagos, Nigeria. The PCR product was sent to GATC Biotech AG (European Genome and Diagnostics Centre - Jakob-Stadier-Platz 7, 78467 Constance, Germany) where the Sanger Sequencing was carried out.
Chromosomal DNA extraction: DNA extraction was carried out directly from the sample using a Qiagen QiaAMP DNA extraction kit according to manufacturer’s instruction.
PCR amplification of bacterial 16S rRNA gene: The PCR amplification of the 16S rRNA gene was carried out using the primer set 27F- 5'- AGA GTT TGA TYM TGG CTC AG -3', and 515R 5'- TTA CCG CGG CKG CTG GCA C-3'. The reaction was carried out according to the method described by Yamada et al. [26] and Katsura et al. [27]. Twenty microlitres (20 μl) reaction mixture containing 1X PCR buffer (Solis Biodyne, Estonia), 1.5 mM Magnesium chloride (Solis Biodyne, Estonia), 0.2 mM of each dNTP (Solis Biodyne, Estonia), 2 U Taq DNA Polymerase (Solis Biodyne, Estonia), 20 pMol of each primer and sterile water was used to make up the reaction mixture. PCR was carried out in an Eppendorf Nexus thermal cycler with the following cycling parameters: an initial denaturation step at 95°C for 5 min, followed by 30 consecutive cycles of denaturation at 95°C for 30 sec., annealing at 55°C for 45 sec. and extension at 72°C for 1 min. After this, a final extension at 72°C for 10 min was carried out.
Agarose gel electrophoresis: After the PCR reaction, the PCR product was separated on a 1.5% agarose gel (Solis Biodyne, Estonia). One hundred base pair (100 bp) DNA ladder (Solis Biodyne, Estonia) was used as DNA molecular weight marker. Electrophoresis was done at 80 V for 1 h 30 min and the gel was viewed under UV light after staining with ethidium bromide (Solis Biodyne, Estonia).
Sequence analysis: The sequence generated by the sequencer was visualized using Chromaslite for base calling. BioEdit was used for sequence editing, before performing a Basic Local Alignment Search Tool (BLAST) using NCBI (National Centre for Biotechnology Information) database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Similar sequences were downloaded and aligned with ClustalW and phylogenetic tree drawn with MEGA 6 software [28].
Bioethanol production through SSF of pre-treated sugarcane bagasse and cassava peels using the VCE-19: SSF was carried out on pretreated bagasse and cassava peels by VCE-19 according to the method described by Kamble and Jadhav [29]. The optimized medium was used for the fermentation. Four percent (4% w/v) of pre-treated bagasse and cassava peels in 250 ml Erlenmeyer flask containing 200 ml of the fermentation medium was used. The medium was autoclaved at 121°C for 20 min at 15 psi. After cooling to room temperature, 4% v/v of the inoculum from a 24 h broth culture of VCE-19 was added to the suspension of the biomass and incubated at 40°C. The fermentation broth was monitored for seven days.
Estimation of fermentation products using gas chromatography-mass spectrometry
Chemicals and reagents: HPLC-grade acetone, ethanol, n-propanol, isobutanol, acetic acid and ethylacetate (Sigma-Aldrich, Germany) were used. Stock solutions of acetone, ethanol, n-propanol, isobutanol, acetic acid and ethylacetate were prepared at concentrations of 0, 20, 40, 60 and 80 g/L, respectively in distilled water. A series of solutions of each analyte was prepared with isobutanol as internal standard (IS; 6 g/L) for the construction of calibration curves.
Preparation of sample for analysis: The fermentation broth samples were centrifuged (Centrifuge 5804R, Eppendorf, Hamburg, Germany) at 13,000 g at 4°C for 3 min to separate sediments and the clear liquid was analysed for the presence of fermentation products. Before injection into the GC instrument, clarified samples and standards were filtered through 0.45 mm Whatmann nylon filter (Whatmann, UK) to remove insoluble materials that could 4°Ck the column. All clear filtrate samples were kept frozen in sealed vials to maintain the stability of volatile components until they would be analysed. Chromatographic samples were prepared with Isobutanol as the IS (6 g/L) in 2 mL screwcap septum vials, which were then loaded into the autosampler.
Chromatographic conditions: The experiment was performed as described by Lin et al. [30] using a GC system (Agilent 7890, Santa Clara, CA) equipped with an MS.
Statistical analysis
The results were compared by one-way analysis of variance (oneway ANOVA) and multiple range tests to find the differences between the measurement means at 5% (0.05) significance level using IBM® SPSS® Statistics Version 20.0 (Gailly and Adler, US).
Results and Discussion
Chemical analysis of sugarcane bagasse and cassava peels
The proximate composition analysis before and after pre-treatment of the biomass used in this study is given in Tables 1 and 2. The results indicated that the total available carbohydrate of the bagasse and cassava peels before pretreatment were 70.3 ± 1.9 and 69.6 ± 1.2 respectively. The result revealed that the total available carbohydrate for bagasse and cassava peels increased to 85.6 ± 2.33and 81.5 ± 5.8 respectively. While the lignin content reduced from 19.2 ± 1.2 to 4.2 ± 0.44 for sugarcane bagasse and 13.9 ± 0.4 to 4.8 ± 0.8 for cassava peels. The results obtained showed that steam explosion and acid pre-treatment methods were the best methods for bagasse and cassava peels.
| Component | Cassava peels% dry weight | Sugarcane bagasse% dry weight |
|---|---|---|
| Total carbohydrate | 69.6 ± 1.2 | 70.3 ± 1.9 |
| Cellulose | 38.2 ± 1.3 | 42.1 ± 2.4 |
| Hemicellulose | 31.4 ± 1.1 | 28.2 ± 2.2 |
| Total lignin | 13.9 ± 0.4 | 19.2 ± 1.2 |
| Ash | 9.5 ± 0.7 | 4.3 ± 0.5 |
| Crude protein | 4.8 ± 0.3 | 3.8 ± 0.1 |
| Fat | 0.9 ± 0.02 | 0.6 ± 0.04 |
Table 1: Baseline chemical composition of the biomass used in the study.
| Treatment | Total carbohydrate (% dry weight) | Lignin (% dry weight) |
|---|---|---|
| Sugarcane Bagasse | ||
| • Alkali pretreatment | 79.8 ± 4.6 | 6.9 ± 1.6 |
| • Alkali pretreatment |
80.21 ± 3.0 | 6.82 ± 0.83 |
| • SE pretreatment |
*85.4 ± 2.33 | 4.2 ± 0.44 |
| Cassava Peels | ||
| • Acid pretreatment |
*80.4 ± 2.5 | 4.8 ± 0.8 |
| • Alkali pretreatment |
78.1 ± 3.18 | 5.2 ± 1.1 |
| • SE |
80.1 ± 2.56 | 5.4 ± 0.9 |
Legend: SE=Steam Explosion
Table 2: Total carbohydrate and lignin content of biomass after pretreatment.
Screening and ethanol tolerance test
Out of the 45 bacterial isolates from agricultural waste soils screened for cellulase production, 27 showed varying zones of clearance. One cellulase-producing isolate (VCE-19) with the capacity to ferment glucose to ethanol and grow at 6% ethanol concentration was selected. The zones of clearance obtained for VCE-19 was 2.75 ± 0.02 while optical density readings of 0.4729 ± 0.03 for the ethanol tolerance tests. The result showed that the selected isolate was able to grow at ethanol concentration of 6% (v/v).
Optimization of cultural conditions for the production of cellulase
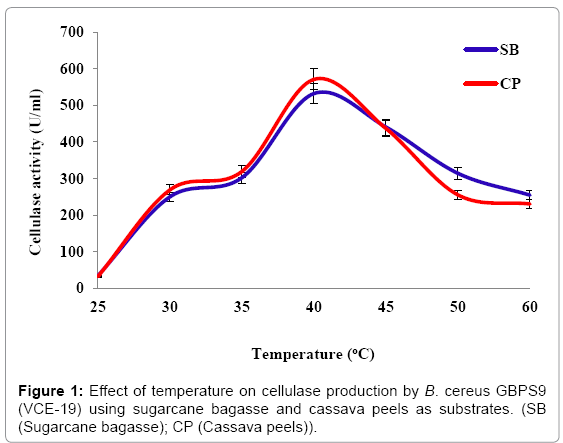
Effect of temperature: The effect of different incubation temperatures on the production of cellulase by VCE-19 using different agricultural wastes is presented in Figure 1. Using bagasse as substrate, the maximum cellulase production of 532.18 ± 5.17 U/ml was obtained at 40°C. When cassava peels was used as substrate the maximum cellulase production was 570.84 ± 4.64 U/ml obtained at 40°C. These results showed that VCE-19 produced the highest cellulase at incubation temperature of 40°C while utilizing both bagasse and cassava peels.
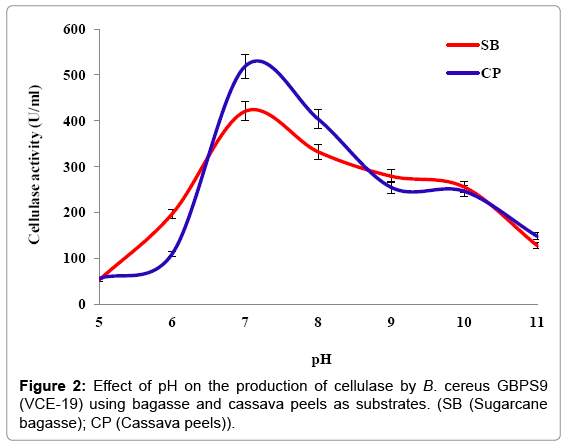
Effect of pH: Optimum cellulase production by isolate VCE-19 incubated at pH of 5, 6, 7, 8, 9, 10 and 11 were 53.15 ± 0.76, 197.31 ± 3.21, 420.77 ± 5.55, 332.31 ± 3.66, 279.23 ± 5.22, 255.64 ± 1 and 127.85 ± 2.18 U/ml respectively, when bagasse was used as the substrates. Meanwhile using cassava peels as a substrate, the incubation pH that yielded the highest cellulase enzyme was 7, with a cellulase production of 399.15 ± 4.15 U/ml. The results showed that the optimal pH for the production of cellulase by isolate VCE-19 using the different substrates was 7 (Figure 2).
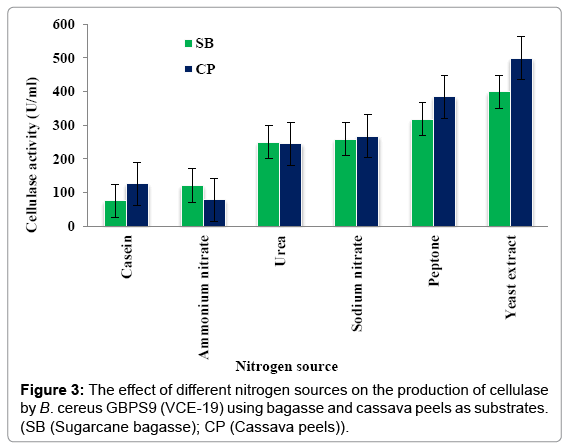
Effect of nitrogen source: Figure 3 shows the effect of different nitrogen sources (casein, NH4NO3, Urea, NaNO3, peptone and yeast extract) on the production of cellulase by VCE-19 using sugarcane bagasse and cassava peels as substrates. Maximum cellulase production of 398.49 ± 4.28 and 498.1 ± 6.29 U/ml were obtained with bagasse and cassava peels as the substrates when yeast extract was used as the nitrogen source. The results showed that the optimal nitrogen source for the different substrates.
Identification of isolate VCE-19
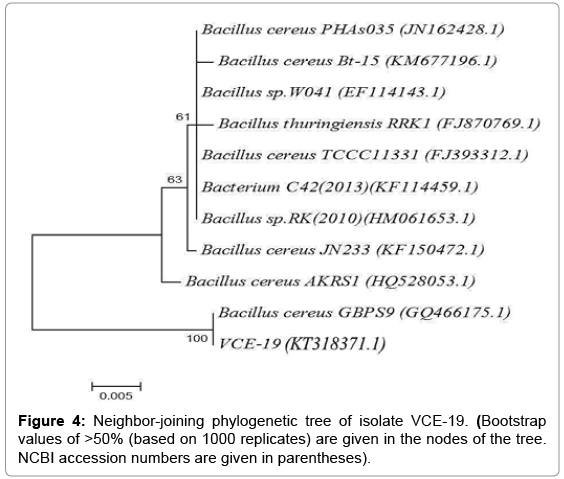
Biochemical characteristics of the isolate (VCE-19) are presented in Table 3. Phylogenetic analysis based on 16S rRNA gene classified VCE- 19 as Bacillus cereus GBPS9. The sequence has been deposited at the GenBank under the accession number KT318371.1. Figure 4 shows the phylogenetic tree analysis of the isolate.
| Isolate code | VCE-19 |
|---|---|
| Gram’s Stain | + (rods) |
| Endospore | + |
| Citrate | + |
| Motility | + |
| Oxidase | - |
| Catalase | + |
| Indole | - |
| Urease | - |
| MR | - |
| VP | + |
| TSI Slant Butt H2S |
K A - |
| Starch hydrolysis | + |
| Gelatin hydrolysis | + |
| Sugar Fermentation Maltose Glucose Lactose Mannitol Sucrose |
+/A +/A - - +/A |
| Probable genus | Bacillus |
Legend: +=positive; -=negative; K=alkaline; A=acid; MR=Methyl Red; VP=Vogues Proskauer; TSI=Triple Sugar Iron.
Table 3: Biochemical characteristics of the isolate used in the study.
Bioethanol production through SSF of pre-treated sugarcane bagasse and cassava peels
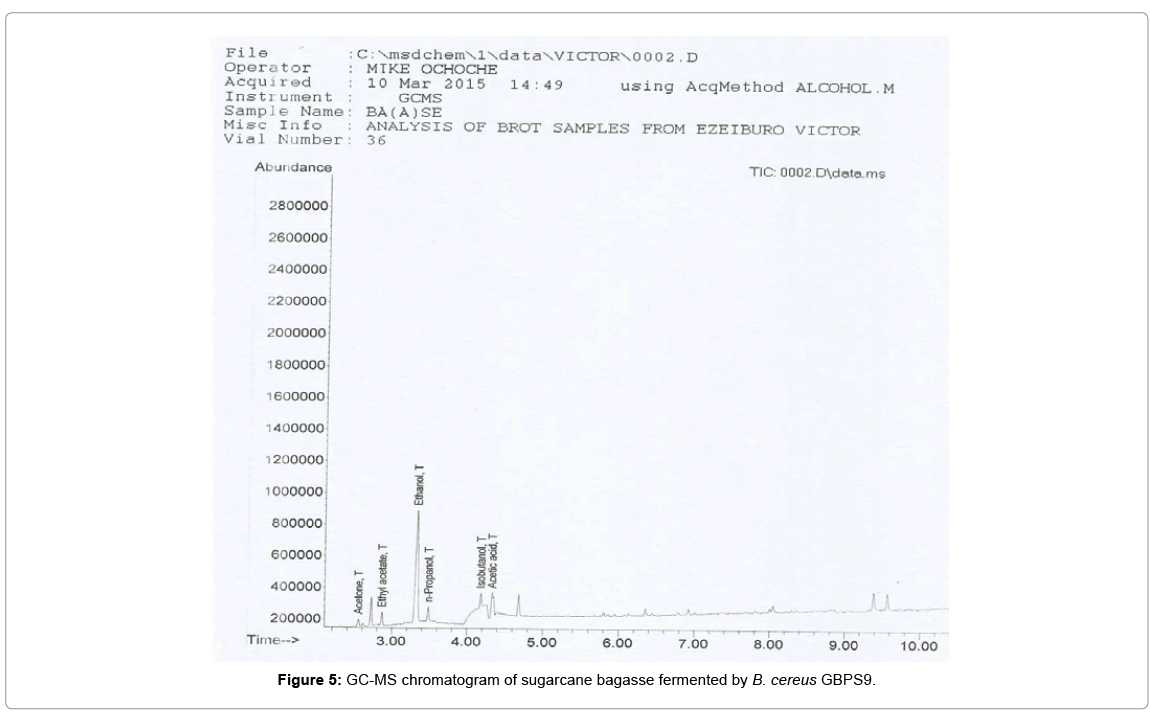
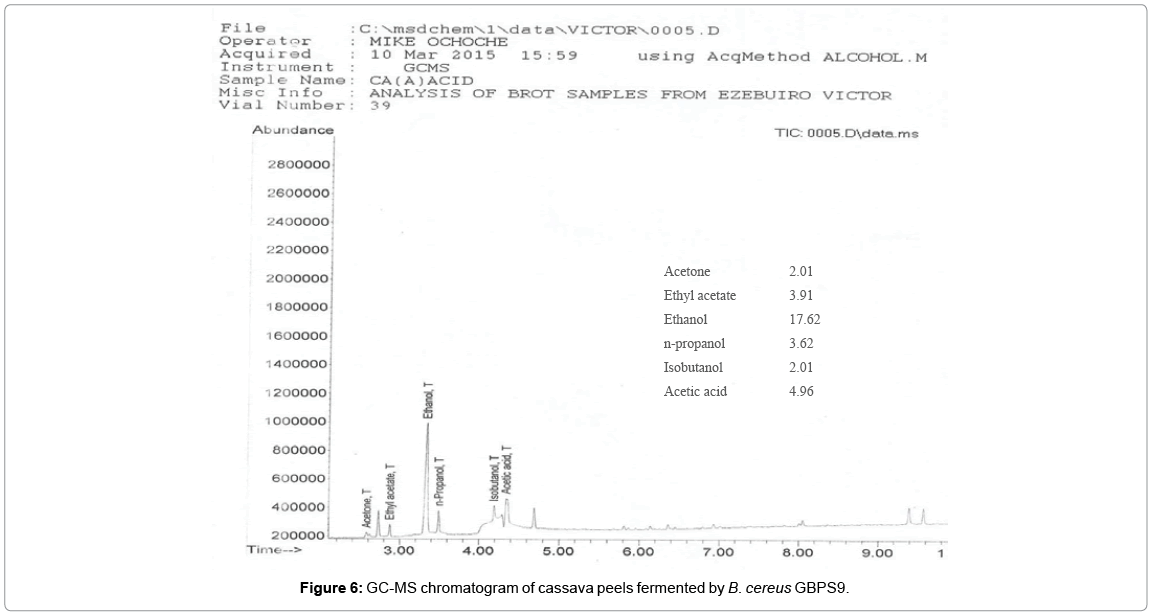
The chromatograms of the products obtained from the GC-MS analysis of the fermentation broths containing sugarcane bagasse and cassava peels substrate fermented by B. cereus GBPS9 is presented in Figures 5 and 6.
Discussion
This study was carried out to produce bioethanol from sugarcane bagasse and cassava peels using B. cereus strain GBPS9. This bacterium is a unique candidate for bioethanol production because it was able to tolerate ethanol concentration of 6% v/v, carry out hydrolysis and fermentation of the hydrolysate. The qualities have the advantage of making the ethanol production process more economical. Sugarcane bagasse and cassava peels are examples of agricultural wastes and can be converted to bioethanol [1,31]. Chemical analysis before and after comminution of the sugarcane bagasse and cassava peels showed the feedstock’s capacity for ethanol production. Moreover, it has been reported that minor differences in the chemical composition of bagasse between the different varieties of sugarcane exist [32]. Amores et al. [5] reported a total carbohydrate of 65% dry weight for sugarcane bagasse used in ethanol production. El-Tayeb et al. [33] reported total carbohydrate composition of 86.9% w/w for bagasse. The result of the compositional analysis for the raw cassava peels used in the study is similar to that obtained by Marx and Nquma [34]. A total carbohydrate percentage dry weight of 67% was reported by Marx and Nquma [34] for cassava peels used in bioethanol production. The choice of cassava as a feedstock for bioethanol production is particularly interesting especially in Nigeria. This is because cassava is produced in large quantity and Nigeria remains the largest producer of cassava in the world since 2005 [8]. In addition, arid, marginal soil where other crops, such as, sugarcane and sugar beet fail can easily support cassava growth [4,9]. The high cellulose contents of the feedstock made them suitable for ethanol production.
Pre-treatment is a necessary step in the use of lignocellulosics for bioethanol production. Joshi et al. [35] described pre-treatment as the most important rate limiting step in the overall bioethanol production process. Pre-treatment was carried to break the lignin-hemicellulosepectin complex, disrupt/loosen-up the crystalline structure of cellulose and increase the porosity of the biomass used in the study. When these changes are achieved, enzymatic saccharification becomes easier, resulting in higher fermentable sugar levels [36-38]. The pre-treatment methods employed achieved high delignification of the different agricultural biomass. The pretreatment method was also necessary to reduce the cyanide content of the cassava peels. This is necessary because cyanide is a toxic chemical to most bacteria and is a constituent of cassava peels. The presence of cyanide can significantly reduce the efficiency of the fermenting bacterium to produce bioethanol [11].
Researchers [5,39] have reported different pre-treatment methods for sugarcane bagasse. In this study the selected pre-treatment method for bagasse was steam explosion. The use of steam explosion pre-treated bagasse is supported by several studies. Ferreira-Leitão et al. [39] and Amores et al. [5] have reported steam explosion pre-treatment for bagasse used as feedstock for ethanol production. Martin et al. [40] reported bagasse pre-treatment by steam explosion using different impregnating agents. Acid pre-treatment was the choice pre-treatment method used on cassava peels in this study. The dilute H2SO4 pretreatment employed achieved up to 65% delignification of the cassava peels. This is similar to the work done by Olanbiwoninu and Odunfa [19]; they studied the enhancement of reducing sugars production from cassava peels by different pre-treatment studies and obtained the highest reducing sugar with acid (H2SO4) pre-treatment. Similar results had been reported by Kongkiattikajorn and Yoonan [41].
The isolate used in this study was identified as Bacillus cereus strain GBPS9 based of the phylogenetic analysis of the 16S rRNA gene. The sequence generated have been deposited in the GenBank under the accession number KT318371.1. There are reports on the production of cellulase by Bacillus cereus strains and consequently their potential in the production of bioethanol. This organism showed high potentials for cellulase and bioethanol production at the stationary phase of its growth with all the biomass used. The production of cellulase by Bacillus cereus strains is supported by many researchers [21,42]. In order to achieve maximum cellulase production the cultural conditions of the incubation medium of the bacterium was optimized. The effects of pH, temperature and nitrogen on the production of cellulase by B. cereus were studied. The results obtained showed significant difference (p<0.05) in effect of all these parameters on cellulase production. Immanuel et al. [43] reported that cellulose quality, temperature, aeration, carbon sources, incubation period, medium additives, pH of the medium and presence of inducers are important parameters for the optimized production of cellulase enzymes. Other researchers [21,44-46] have also reported cellulase production enhancement by the optimization of cultural conditions.
The optimum temperature, pH and nitrogen source for cellulase production were40°C, 7 and yeast extract for both substrates. This finding is similar to the result obtained in other studies. Fagade and Bamigboye [47] reported optimum cellulase activity for three Bacillus species when incubated at temperature of40°C. Incubation temperature is a critical factor in enzymatic productivity [48]. Maximum enzyme production is achieved at optimum temperature and the decrease in enzyme production at lower or higher temperatures may be due to the facts that at these temperatures, growth of the organisms was inhibited, causing a decrease in the synthesis of the enzymes [49]. In addition, production of more activity at optimum temperature may be due to the faster metabolic activity and increase in protein content and extracellular enzyme production in culture supernatant. At very low temperatures, membranes solidify and high temperatures damage microorganisms by denaturing enzymes, transport carriers and other proteins thus lowering enzyme activity [50]. Fagade and Bamigboye [42] reported optimum cellulase activity at pH of 7 for Pseudomonas putida, Bacillus subtilis and B. lichenformis I grown on corn cob. High cellulolytic activity is essential for optimal bioethanol production [1].
The ethanol yield obtain from this study is higher than the result (7.5 g/L) obtained from the fermentation of sugarcane bagasse hydrolysate using Pichia stipitis DSM 3651 as reported by Canilha et al. [51] and 17.1 g/L as reported by Ingale et al. [52] from banana pseudo stem. However, the yield is lower than the reported [53] ethanol maximum yield of 58.6 g/L from soybean molasses by Saccharomyces cerevisiae. This yield obtained can by compared with the yield by other wild type bacteria. Svetlitchnyi et al. [54] reported maximum ethanol yield of 3.5 g/l from the wild type bacterium Caldicellulosiruptor DIB 004C. Sato et al. [55] reported ethanol production of 4 g/l by wild type Clostridum thermocellum strain I-1-B and an improved 23.6 g/l ethanol by the same strain when grown in optimized medium. The fermenting bacterium B. cereus GBPS9 used in this study was able to also produce other important fermentation products (acetone, ethyl acetate, n-propanol, isobutanol and acetic acid). These products could have reduced the ethanol production quality of the isolate. For commercial production of ethanol from the strain, the production of these fermentation products that accompanied ethanol production, should be regulated for enhanced ethanol yield.
Conclusion
The study has demonstrated efficient bioethanol production by Bacillus cereus GBPS9 using sugarcane bagasse and cassava peels as feedstocks. It was also observed that cultural conditions affected the ability of the isolate to produce cellulase.
References
- Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energ 37: 19-27.
- Ballesteros I, Negro MJ, Oliva JM, Cabañas A, Manzanares P, et al. (2006) Ethanol production from steam-explosion pretreated wheat straw.ApplBiochemBiotechnol 129-132: 496-508.
- Champagne P (2008) Bioethanol from agricultural waste residues. Environ Prog 27: 51-57.
- Zhang C, Han WJ, Pu GQ, Wang CT (2003) Life cycle economic analysis of fuel ethanol derived from cassava in south west China. Renew Sus Energ 7: 353-366.
- Amores I, Ballesteros I, Manzanares P, Sáez P, Michelena G, et al. (2013) Ethanol production from sugarcane bagasse pretreated by steam explosion. Electron J Energ Environ 1: 25-36.
- Rezende CA, de Lima MA, Maziero P, Garcia DRE, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54.
- National Sugar Development Council, NSDC (2003)Information Brochure towards Self Sufficiency in Sugar. Abuja.
- FAOSTAT (2012) Searchable online database from Food and Agriculture Division of the United Nations. Rome, Italy.
- Sriroth K, Piyachomkwan K, Wanlapatit S, Nivitchanyong S (2010) The promise of a technology revolution in cassava bioethanol: from Thai practice to the world practice. Fuel 89: 1333-1338.
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. BioresourTechnol 96: 673-686.
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, et al. (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315: 804-807.
- Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J MolSci 9: 1621-1651.
- Balat M, Balat H, Oz C (2008) Progress in bioethanol processing. Energy Comb Sci 34: 551-573.
- Milne TA, Chum HL, Agblevor FA, Johnson DK (1992) Standardized Analytical Methods. Biomass and Bioenergy 2: 341-366.
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J et al. (2011) Determination of Structural Carbohydrates and Lignin in Biomass. Technical Report NREL/TP–510-42618. National Renewable Energy Laboratory, Golden, Colorado.
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, et al. (2008) Determination of ash in biomass. Technical Report NREL/TP–510-42622. National Renewable Energy Laboratory, Golden, Colorado.
- Sharma N, Kalra KL, Oberoi HS, Bansal S (2007) Optimization of fermentation parameters for production of ethanol from kinnow waste and banana peels by simultaneous saccharification and fermentation. Indian J Microbiol 47: 310-316.
- Fan LT, Lee YH, Beard DH (1980) Mechanism of the enzymatic hydrolysis of cellulose: effects of major structural features of cellulose on enzymatic hydrolysis. BiotechnolBioeng 22: 177-179.
- Olanbiwoninu AA, Odunfa SA (2012) Enhancing the production of reducing sugars from cassava peels by pre-treatment methods. Int J SciTechnol 2: 650-657.
- Apun K, Jong BC, Salleh MA (2000) Screening and isolation of a cellulolytic and amylolytic Bacillus from sago pith waste. J Gen ApplMicrobiol 46: 263-267.
- Behera BC, Parida S, Dutta SK, Thatoi HN (2014) Isolation and identification of cellulose degrading bacteria from mangrove soil of Mahanadi River Delta and their cellulase production ability. Am J Microbiol Res2: 41-46.
- Bailey MJ, Biely P, Poutanen K (1992) Inter-laboratory testing of methods for assay of xylanase activity. J Biotechnol 23: 257-270.
- Holt JG, Krieg NR, Sneath PHA (1994)Bergey’s Manual of Determinative Bacteriology. Lippincott Williams & Wilkins, USA.
- MacFaddin JF (2000) Biochemical Tests for Identification of Medical Bacteria. Lippincott Williams and Wilkins, Philadelphia.
- Madigan MT, Martinko JM, Stahl DA, Clark DP (2012) Brock Biology of Microorganisms. Benjamin Cummings California, USA.
- Yamada R, Tanaka T, Ohnishi Y, Suematsu K, Minami M, et al. (2000) Identification of 142 single nucleotide polymorphisms in 41 candidate genes for rheumatoid arthritis in the Japanese population. HumGenet 106: 293-297.
- Katsura K, Kawasaki H, Potacharoen W, Saono S, Seki T, et al. (2001) Asaiasiamensissp. nov., an acetic acid bacterium in the α-proteobacteria. Int J SystEvolMicr51: 559-563.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. MolBiolEvol 30: 2725-2729.
- Kamble RD, Jadhav AR (2012) Isolation, purification, and characterization of xylanase produced by a new species of bacillus in solid state fermentation. Int J Microbiol 2012: 683193.
- Lin YH, Knipping EM, Edgerton ES, Shaw SL, Surratt JD (2013) Investigating the influences of SO2 and NH3 levels on isoprene-derived secondary organic aerosol formation using conditional sampling approaches. AtmosChemPhys 13: 8457-8470.
- Ibeto CN, Okoye COB, Ofoefule AU (2014) Bioethanol production from thermally pre-treated corn chaff and cassava waste water. Int Res J Pure ApplChem 4: 227-233.
- Gastón C, Bambanaste R, Correa JL, Alfonso G, Herryman M (2000) Bagazo. In: Manual de los Derivados de la Caña de azúcar.Galvéz LO (edr.), Grupo de PaísesLatinoamericanos y del Caribe.
- El-Tayeb TS, Abdelhafez AA, Ali SH,Ramadan EM (2012) Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Braz J Microbiol 43: 523-1535.
- Marx S, Nquma TY (2013) Cassava as feedstock for ethanol production in South Africa. Afr J Biotechnol 12: 4975-4983.
- Joshi B, Bhatt MR, Sharma D, Joshi J, Malla R (2011) Lignocellulosic ethanol production: current practices and recent developments. BiotechnolMolBiol Rev 6: 172-182.
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. BioresourTechnol 96: 673-686.
- Sun FB, Cheng HZ (2007) Evaluation of enzymatic hydrolysis of wheat straw pretreated by atmospheric glycerol autocatalysis. J Chem Tech Biotechnol 82: 1039-1044.
- Yang B, Wyma CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuel BioprodBiorefin 2: 26-40.
- Ferreira-Leitão V, Perrone CC, Rodrigues J, Franke APM, Macrelli S, et al. (2010) An approach to the utilisation of CO2 as impregnating agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol Biofuels 3: 1-8.
- Martín C, Galbe M, Nilvebrant NO, Jonsson LJ (2002) Comparison of the fermentability of enzymatic hydrolysates of sugarcane bagasse pretreated by wet oxidation and steam explosion. J ChemTechnolBiotechnol 81: 1669-1677.
- Kongkiattikajorn J, Yoonan K (2006) Conversion of cassava industry waste to fermentable sugars. The 2nd Joint International Conference on “Sustainable Energy and Environment (SEE)” E-031: 21-23.
- Yan H, Dai I, Zhang Y, Yan L, Liu D (2011)Purification and characterization of an endo-1, 4-β-glucanase from Bacillus cereus.Afr J Biotechnol 10: 16277-16285.
- Immanuel G, DhanushaR, Prema P, Palavesam A (2006) Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment.Int J Environ SciTechnol 3: 25-34.
- Ray AK, Bairagi A, Ghosh KS, Sen SK (2007) Optimization of fermentation conditions for cellulase production by Bacillus subtilisCY5 and Bacillus circulansTP3 isolated from fish gut. ActaIchthyolPiscat37: 47-53.
- Abou-Taleb AAK, Mashhoor WA, Nasr AS, Sharaf MS, Abdel-Azeem HMH (2009) Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic Bacilli.Aust J Basic ApplSci 3: 2429-2436.
- Ladeira SA, Cruz E, Delatorre AB, Barbosa JB, Martins MLL (2015) Cellulase production by thermophilicBacillus sp. SMIA-2 and its detergent compatibility. ElectronJBiotechn 18: 110-115.
- Fagade OE, Bamigboye OO (2012) Effect of cultural conditions on the cellulase activity of bacteria species isolated from degrading corn cob. Arch ApplSci Res 4: 2540-2545.
- Seyis I, Aksoz N (2003) Determination of some physiological factors affecting xylanase production from Trichodermaharzianum1073-d3. New Microbiol 26: 75-81.
- Simões MLG, Tauk-Tornisielo MS, Tapia DMT (2009) Screening of culture condition for xylanase production by filamentous fungi. Afr J Biotechnol 8: 6317-6326.
- Willey JM, Sherwood LM, Woolverton CJ (2008) Prescott, Harley and Kleins Microbiology. McGraw Hill Co. Inc., Boston.
- Canilha L, Carvalho W, Felipe MGA, Almeida JB, Giulietti ME (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichiastipitis. ApplBiochemBiotechnol 161: 84-92.
- Ingale S, Joshi JS, Gupte A (2014) Production of bioethanol using agricultural waste: Banana pseudo stem. Braz J Microbiol 45: 885-892.
- Sequeira CAC, Brito PSD, Mota AF, Carvalho JL, Rodrigues LF, et al. (2007) Fermentation gasification and pyrolysis of carbonaceous residues towards usage in fuel cells. Energ Convers Manage 48: 2203-2220.
- Svetlitchnyi V, Kensch O, Falkenhan AD, Korseska GS, Lippert N, et al. (2013) Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol Biofuels 6: 31.
- Sato K, Tomita M, Yonemura S, Goto S, Sekine K, et al. (1993) Characterization of and ethanol hyper-production by Clostridium thermocellum I-1-B. Biosci Biotech Biochem 57: 2116-2121.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13091
- [From(publication date):
December-2015 - May 25, 2024] - Breakdown by view type
- HTML page views : 12051
- PDF downloads : 1040