- Department of Experimental Biomedicine and Clinical Neuroscience, Neurosurgical Unit, University Hospital, Paolo Giaccone, Palermo, Italy
Correspondence Address:
Domenico Gerardo Iacopino
Department of Experimental Biomedicine and Clinical Neuroscience, Neurosurgical Unit, University Hospital, Paolo Giaccone, Palermo, Italy
DOI:10.4103/2152-7806.174894
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Graziano F, Maugeri R, Basile L, Meccio F, Iacopino DG. Aulogous fibrin sealant (Vivostat®) in the neurosurgical practice: Part II: Vertebro-spinal procedures. Surg Neurol Int 25-Jan-2016;7:
How to cite this URL: Graziano F, Maugeri R, Basile L, Meccio F, Iacopino DG. Aulogous fibrin sealant (Vivostat®) in the neurosurgical practice: Part II: Vertebro-spinal procedures. Surg Neurol Int 25-Jan-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/aulogous-fibrin-sealant-vivostat-in-the-neurosurgical-practice-part-ii-vertebro-spinal-procedures/
Abstract
Background:Epidural hematomas, cerebrospinal fluid fistula, and spinal infections are challenging postoperative complications following vertebro-spinal procedures. We report our preliminary results using autologous fibrin sealant as both fibrin glue and a hemostatic during these operations.
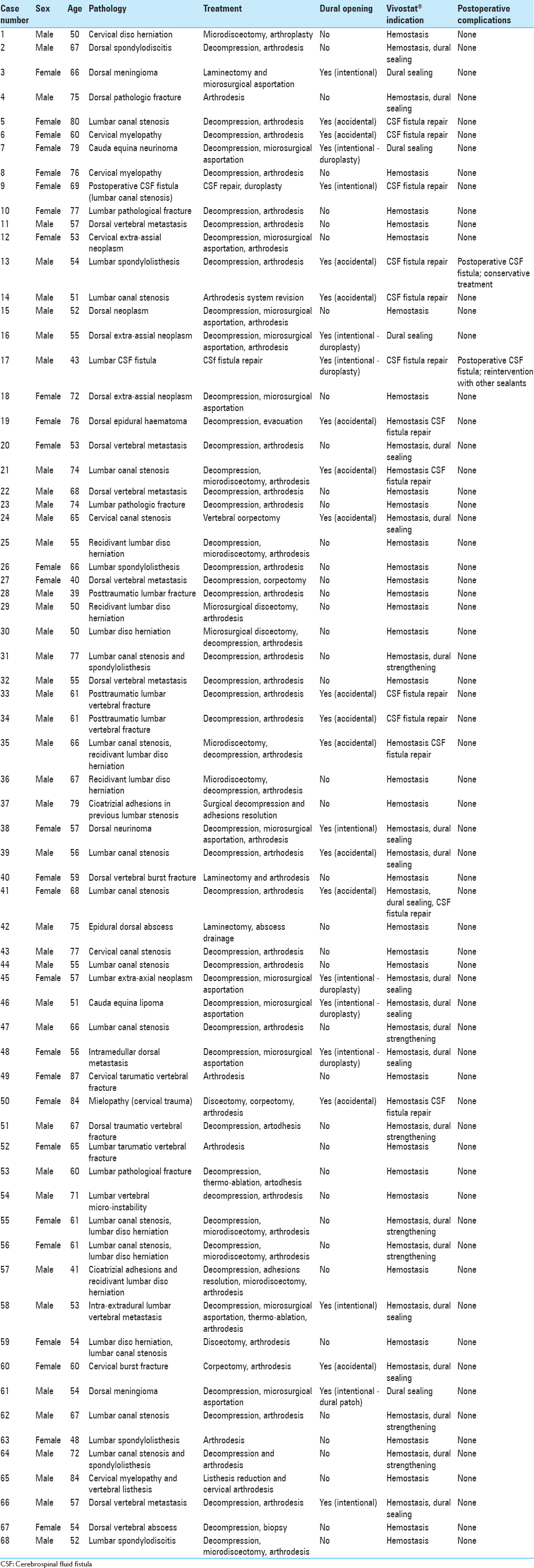
Methods:Prospectively, between January 2013 and March 2015, 68 patients received an autologous fibrin sealant prepared with the Vivostat® system applied epidurally to provide hemostasis and to seal the dura. The surgical technique, time to bleeding control, and associated complications were recorded.
Results:Spinal procedures were performed in 68 patients utilizing autologous fibrin glue/Vivostat® to provide rapid hemostasis and/or to seal the dura. Only 2 patients developed postoperative dural fistulas while none exhibited hemorrhages, allergic reactions, systemic complications, or infections.
Conclusions:In this preliminary study, the application of autologous fibrin sealant with Vivostat® resulted in rapid hemostasis and/or acted as an effective dural sealant. Although this product appears to be safe and effective, further investigations are warranted.
Keywords: Autologous fibrin glue, cerebrospinal fluid fistula, dural repair, dural sealant, hemorrhage, hemostasis
INTRODUCTION
Cerebrospinal fluid (CSF) fistulas and postoperative hematomas constitute two of the major complications of spinal surgery.[
MATERIALS AND METHODS
From January 2013 to March 2015, 68 patients undergoing spinal surgery received autologous fibrin sealant prepared with the Vivostat® system and applied epidurally, over the resection bed.
Patients population
Upon approval of the local Institutional Review Board, between January 2013 and March 2015 we performed 68 neurosurgical spinal procedures utilizing autologous fibrin sealant/Vivostat® to achieve hemostasis and/or to seal the dura.
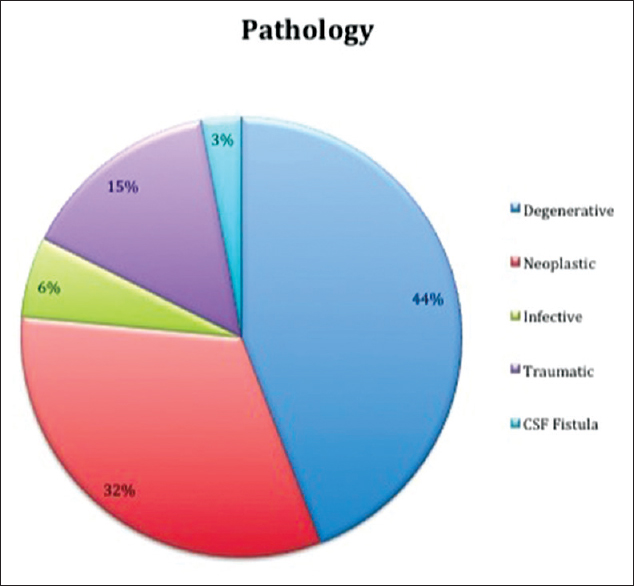
In 47% of cases (32 cases), the autologous fibrin glue was used only as an hemostatic agent; in 34% of cases it was used both as an hemostatic and dural sealant agent for strengthening atretic dura (without frank CSF fistula); in 19% of cases the autologous fibrin glue was used to achieve both hemostasis and CSF fistula repair; [
RESULTS
Technical and economic considerations
This system was effective in all three circumstances; as a hemostatic alone, as a hemostatic and to strengthen atretic dura, and for hemostasis and dural repair. The Notably, Vivostat® formed an extremely thin white coat and did not compress the neural structures; additionally, it was physiologically eliminated within 24–36 h [
Figure 2
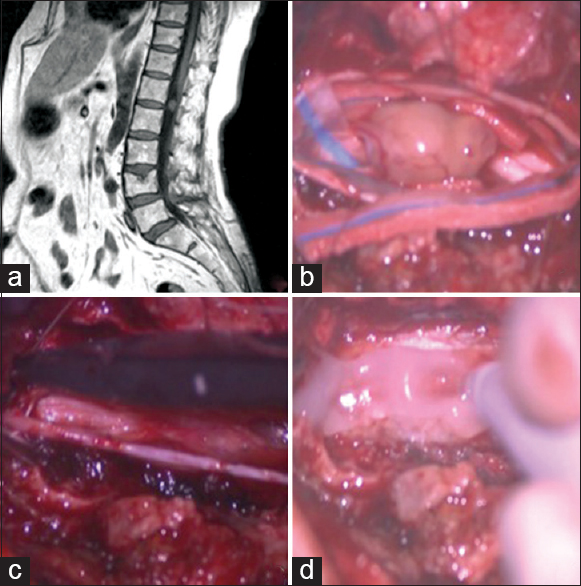
Cauda equina neurinoma. (a) Preoperative Magnetic resonance (MR), T1-weighted sagittal view: An iperintense homogeneous enhancing circular lesion is visible posterior to the disc space L1–L2. (b) Intraoperative picture of the intradural lesion. (c) After the lesion removal, the dura mater is closed in watertight fashion with single stitches. (d) The autologous fibrin glue is applied on the reconstructed dural layer in order to achieve a satisfactory dural sealing
DISCUSSION
Application for durotomies and hemostasis
In spinal surgery, the major intraoperative complications are typically due to accidental durotomies or postoperative hematomas. Cammisa et al. found 66 (3.1%) durotomies occurring during 2144 spinal operations; they were immediately treated with dural suturing and fibrin glue.[
Do dural sealants inhibit fusion
Some are concerned whether these sealants on the vertebral fusion rate.[
Arguments favoring utilization of Vivostat system
The Vivostat® system is successfully used in several specialties.[
CONCLUSION
Vivostat® system appears to be a safe/effective fully autologous hemostatic and dural sealant agent. Its composition and mechanism of action makes it able to adhere immediately to tissues and its rapid degradation time avoids any potential long-term mass effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Belboul A, Dernevik L, Aljassim O, Skrbic B, Rådberg G, Roberts D. The effect of autologous fibrin sealant (Vivostat) on morbidity after pulmonary lobectomy: A prospective randomised, blinded study. Eur J Cardiothorac Surg. 2004. 26: 1187-91
2. Black P. Cerebrospinal fluid leaks following spinal or posterior fossa surgery: Use of fat grafts for prevention and repair. Neurosurg Focus. 2000. 9: e4-
3. Buchta C, Hedrich HC, Macher M, Höcker P, Redl H. Biochemical characterization of autologous fibrin sealants produced by CryoSeal and Vivostat in comparison to the homologous fibrin sealant product Tissucol/Tisseel. Biomaterials. 2005. 26: 6233-41
4. Cammisa JT, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine (Phila Pa 1976). 2000. 25: 2663-7
5. Epstein NE. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg Neurol Int. 2014. 5: S304-14
6. Giugno A, Maugeri R, D’Arpa S, Visocchi M, Iacopino DG. Complex reconstructive surgery following removal of extra-intracranial meningiomas, including the use of autologous fibrin glue and a pedicled muscle flap. Interdiscip Neurosurg. 2014. 1: 84-7
7. Giugno A, Maugeri R, Graziano F, Iacopino DG. Intraoperative reparation of superior sagittal sinus (SSS) rupture with autologous fibrin glue: Management of a complication. Our experience and technical note. J Neurol Disord. 2015. 3: 2-
8. Graziano F, Certo F, Basile L, Maugeri R, Grasso G, Meccio F. Autologous fibrin sealant (Vivostat(®)) in the neurosurgical practice: Part I: Intracranial surgical procedure. Surg Neurol Int. 2015. 6: 77-
9. Hanks JB, Kjaergard HK, Hollingsbee DA. A comparison of the haemostatic effect of Vivostat patient-derived fibrin sealant with oxidised cellulose (Surgicel) in multiple surgical procedures. Eur Surg Res. 2003. 35: 439-44
10. Kjaergard HK, Trumbull HR. Bleeding from the sternal marrow can be stopped using Vivostat patient-derived fibrin sealant. Ann Thorac Surg. 2000. 69: 1173-5
11. Kjaergard HK, Trumbull HR. Vivostat system autologous fibrin sealant: Preliminary study in elective coronary bypass grafting. Ann Thorac Surg. 1998. 66: 482-6
12. Landi A, Tarantino R, Marotta N, Ruggeri AG, Domenicucci M, Giudice L. The use of platelet gel in postero-lateral fusion: Preliminary results in a series of 14 cases. Eur Spine J. 2011. 20: S61-7
13. Landriel Ibañez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M. A new classification of complications in neurosurgery. World Neurosurg. 2011. 75: 709-15
14. Lardinois D, Jung FJ, Opitz I, Rentsch K, Latkoczy C, Vuong V. Intrapleural topical application of cisplatin with the surgical carrier Vivostat increases the local drug concentration in an immune-competent rat model with malignant pleuromesothelioma. J Thorac Cardiovasc Surg. 2006. 131: 697-703
15. Lassen MR, Solgaard S, Kjersgaard AG, Olsen C, Lind B, Mittet K. A pilot study of the effects of Vivostat patient-derived fibrin sealant in reducing blood loss in primary hip arthroplasty. Clin Appl Thromb Hemost. 2006. 12: 352-7
16. Ochsner MG. Fibrin solutions to control hemorrhage in the trauma patient. J Long Term Eff Med Implants. 1998. 8: 161-73
17. Ruban D, O’Toole JE. Management of incidental durotomy in minimally invasive spine surgery. Neurosurg Focus. 2011. 31: E15-
18. Schexneider KI. Fibrin sealants in surgical or traumatic hemorrhage. Curr Opin Hematol. 2004. 11: 323-6
19. Schips L, Dalpiaz O, Cestari A, Lipsky K, Gidaro S, Zigeuner R. Autologous fibrin glue using the Vivostat system for hemostasis in laparoscopic partial nephrectomy. Eur Urol. 2006. 50: 801-5
20. Schmidt SC, Langrehr JM. Autologous fibrin sealant (Vivostat) for mesh fixation in laparoscopic transabdominal preperitoneal hernia repair. Endoscopy. 2006. 38: 841-4
21. Theodosopoulos PV, Ringer AJ, McPherson CM, Warnick RE, Kuntz C, Zuccarello M. Measuring surgical outcomes in neurosurgery: Implementation, analysis, and auditing a prospective series of more than 5000 procedures. J Neurosurg. 2012. 117: 947-54
22. Turgut M, Erkus M, Tavus N. The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: An experimental study with cats. Acta Neurochir (Wien). 1999. 141: 273-8
23. Velada JL, Hollingsbee DA. Physical characteristics of Vivostat patient-derived sealant. Implications for clinical use. Eur Surg Res. 2001. 33: 399-404