- Department of Neurosurgery, Kanazawa University, Kanazawa, Ishikawa, Japan - 13-1, Takara-machi, Kanazawa, 920-8641, Japan
Correspondence Address:
Yasuhiko Hayashi
Department of Neurosurgery, Kanazawa University, Kanazawa, Ishikawa, Japan - 13-1, Takara-machi, Kanazawa, 920-8641, Japan
DOI:10.4103/2152-7806.158898
Copyright: © 2015 Hayashi Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Hayashi Y, Iwato M, Kita D, Fukui I. Spontaneous cerebrospinal fluid leakage through fistulas at the clivus repaired with endoscopic endonasal approach. Surg Neurol Int 16-Jun-2015;6:106
How to cite this URL: Hayashi Y, Iwato M, Kita D, Fukui I. Spontaneous cerebrospinal fluid leakage through fistulas at the clivus repaired with endoscopic endonasal approach. Surg Neurol Int 16-Jun-2015;6:106. Available from: http://surgicalneurologyint.com/surgicalint_articles/spontaneous-cerebrospinal-fluid-leakage-fistulas/
Abstract
Background:Causes of cerebrospinal fluid (CSF) leakage are primarily traumatic or iatrogenic in origin. In contrast, spontaneous CSF leakage is somewhat rare, and detection of the fistula can be challenging. Meningitis associated with CSF leakage can be life threatening. It is therefore critical to surgically repair the fistula once the underlying cause has been accurately identified. Spontaneous CSF leakage located at the clivus is an extremely rare condition.
Case Description:We present the case of a 38-year-old male with sudden-onset headache and subsequent disturbances of consciousness. The patient was diagnosed with severe meningitis caused by CSF leakage through fistulas at the clivus, which were clearly identified on dynamic imaging using high-resolution computed tomography (CT) with intrathecal injection of contrast medium. After the meningitis was resolved, successful endoscopic repair of the CSF fistula with autologous materials was performed. There has been no recurrence of meningitis for 5 years.
Conclusion:Spontaneous CSF leakage at the clivus is an extremely rare condition. High-resolution CT cisternogram could accurately detect CSF leakage through the clivus. A transnasal endoscopic approach was a useful and reliable method of repairing the fistula at the clivus.
Keywords: Cerebrospinal fluid, clivus, endoscope, fistula
INTRODUCTION
The cause of cerebrospinal fluid (CSF) leakage is predominantly traumatic, iatrogenic, or neoplastic, and can occasionally be spontaneous or congenital.[
Recent neuroradiological advancements enable physicians to detect the precise localization of the CSF fistula, even in cases with subtle spontaneous CSF leakage.[
CASE REPORT
A 38-year-old male presented with progressing high fever, headache, and vomiting for 2 days. Owing to gradual deterioration of his level of consciousness into a stupor, he was transported by ambulance to our hospital. Neurological examination revealed severe nuchal stiffness with no focal deficits. He never suffered any rhinorrhea before admission. The result from serological examination showed that the white blood cell count was 21,200 and C-reactive protein level was 24.0 mg/dL, indicating a remarkable infectious state.
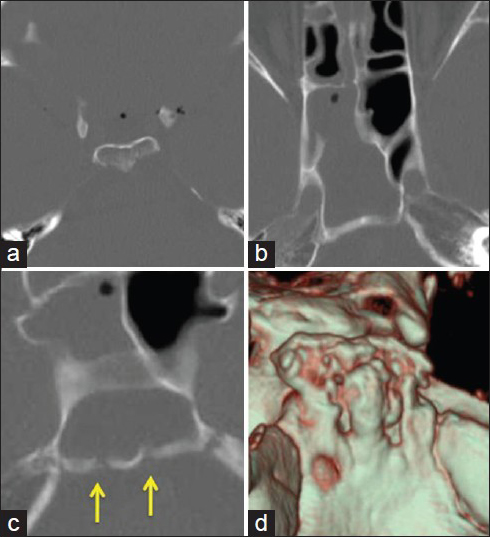
A CT study was performed using 64-slice multidetector computed tomography (MDCT; Toshiba Aquilion, Tokyo, Japan). Three-dimensional images with surface rendered and multiplanar reformatted (MPR) images were reconstructed on the workstation. The axial CT scan images revealed multiple intracranial air bubbles with pneumatization of the dorsum sellae [
Figure 1
A CT scan of the axial images reveals (a) multiple intracranial air bubbles and pneumatization of the dorsum sellae, (b) fluid accumulation in the sphenoid sinus, and (c) bony defects at the clivus (arrows). (d) Reconstructed three-dimensional CT image clearly demonstrates multiple bony erosive defects at the clivus
According to previous medical records, the patient had experienced severe meningitis once 3 years prior. However, he never recognized CSF rhinorrhea and a CT scan was not performed at that time. Therefore, fluid collection in the sphenoid sinus or intracranial air had never been detected. This had been treated successfully with antibiotics, although the cause of the meningitis remained unknown.
The cause of the high-grade fever was investigated with CSF sampling, which revealed evidence of severe meningitis; sugar, 1 mg/dL, protein, 576 mg/dL, and cell count, 27920/mm3. CSF culture showed presence of Streptococcus pneumoniae. The meningitis was treated appropriately with the antibiotic meropenem, which resulted in a good recovery of general symptoms and resolution of high-grade fever.
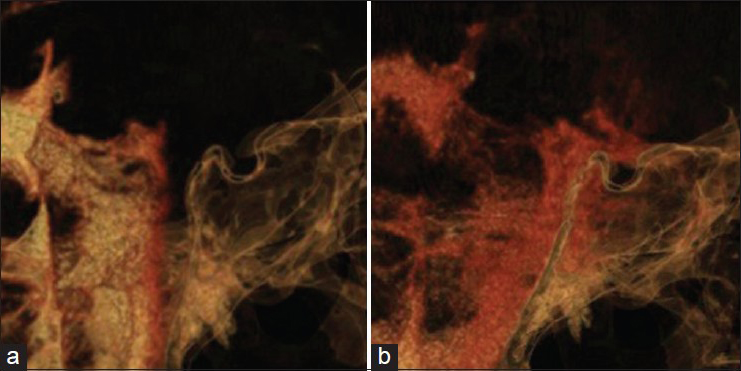
The multiple bone defects at the clivus were considered the cause of the CSF leakage. We therefore aimed to determine the dynamics of CSF through the defects. High-resolution CT cisternography was performed with intrathecal infusion of 5 mL contrast medium. The reconstructed MPR images confirmed the passage of contrast medium through the bone defects at the clivus into the sphenoid sinus 1 h after injection of the contrast medium [
Figure 2
Sagittal reconstruction of MPR images obtained by high-resolution CT with intrathecal injection of contrast medium. (a) Thirty minutes after injection, contrast medium advances into the interpeduncular and prepontine cistern, but not into the sphenoid sinus. (b) One hour after injection, contrast medium is clearly shown entering into the sphenoid sinus through the bone defects at the clivus
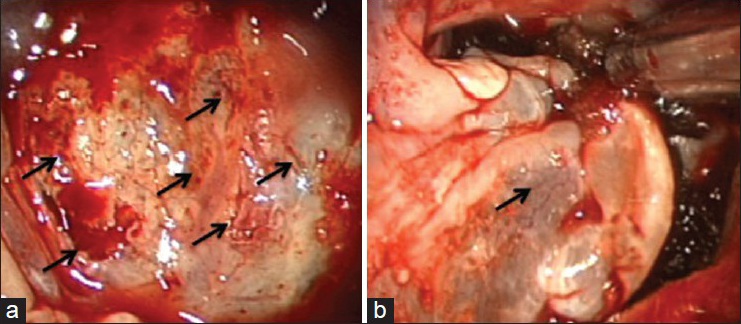
The mucosa over the posterior wall of the sphenoid sinus was completely removed in order to visualize the clival bone accurately. The intraoperative endoscopic observation revealed CSF leakage through bone dehiscences at the clivus detected on the preoperative CT images. The fistulas were successfully repaired in a multilayered fashion with fibrin glue, absorbable polyglycolic acid felt (Neoveil, Gunze, Japan), autologous fat, and fascia grafts from the abdomen followed by a pedicled nasoseptal mucosal flap [
DISCUSSION
The sphenoid sinus is a primary location for CSF leakage related to not only head trauma and postoperative causes, but also spontaneous leaks that can be safely and effectively managed using endoscopy.[
Although CSF leakage through defects in the middle fossa floor into the sphenoid sinus extending laterally have been frequently reported, fistulas located at the clivus are extremely rare.[
Defects in the sphenoid bone might also arise as the sinuses develop. Bony defects are always located at the upper part of the clivus, above this spheno-occipital synchondrosis, which makes clival dysgenesis as a cause of spontaneous CSF leak less likely.[
It is considered that spontaneous CSF transclival leakage is caused by a combination of anatomical and functional factors. From the point of anatomical explanation, excessive pneumatization of the sphenoid bone leads to weakening of the bone posteriorly[
It might be impossible to draw conclusions from one case, but workup for patients with meningitis of unknown etiology is described below. First, it is important to check a CT scan for points of fluid collection in the sphenoid sinus and intracranial air at the sellar, suprasellar, and parasellar regions. Subsequent thin slice CT bone images of the sphenoid sinus were needed to detect the fistula in the current case. Second, it is important to check the medical history for repetitive meningitis, but it is not a requirement. Third, CT cisternography is useful to evaluate fistulas through which CSF can pass.
Wielgosz has reported the usefulness of high-resolution CT for bone defect localization and management of spontaneous CSF rhinorrhea from leakages in the posterior wall of the sphenoid sinus.[
A satisfactory surgical outcome requires exact preoperative diagnosis, an appropriate operative approach, and a surgeon's skill and experience. Transnasal endoscopic repair has now been established as a reliable method for CSF leakage repair in the ethmoid and sphenoid sinuses.[
Recently, materials to repair bone defects have been presented by many surgeons. Many cases with CSF fistulas in the sphenoid sinus are repaired using a procedure of packing with autologous muscle or fat. The reported success rate of endoscopic fistula repair is considerably high, depending mainly on the accurate detection of the fistula. Cassano addressed the effectiveness of the overlay apposition of a lower turbinate mucoperiosteal graft fixed with fibrin glue and oxidized cellulose polymer (Surgicell, Ethicon Inc., USA).[
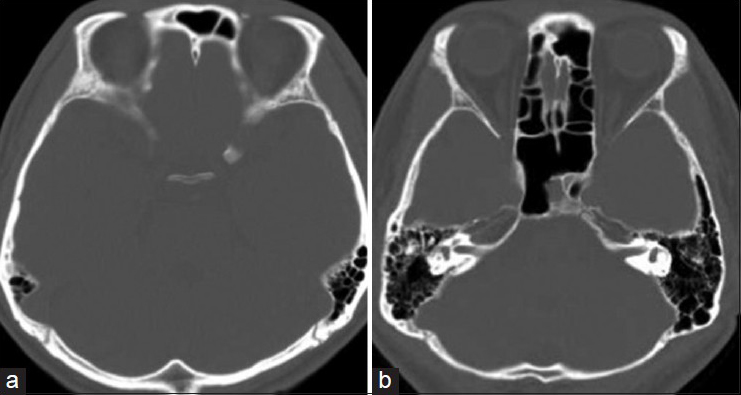
How to follow up with patients after surgical repair of CSF fistula is a very important problem that has not been discussed before in the literature. It is difficult to confirm surgical success leading to the reliable resolution. In our case, CSF lumbar drainage was placed for 4 days. CT cisternography was performed again 2 weeks later, which showed no evidence of CSF leakage into the sphenoid sinus. After discharge, CT scan was performed to rule out findings of CSF accumulation in the sphenoid sinus or intracranial air. If CSF rhinorrhea appeared again and CT scan showed fluid accumulation in the sphenoid sinus, I would consider CT-cisternography should be performed immediately. Van Zele et al. insisted on the importance of follow up with patients after surgical repair of CSF fistulas and their follow up was longer (10.3 years, 3–15) than other reports (1 month to 2 years).[
In conclusion, spontaneous CSF leakage through fistulas at the clivus was clearly identified in a dynamic study using MDCT with intrathecal injection of contrast medium. The repair was successfully performed with an endoscopic endonasal approach without further recurrence.
References
1. Ahmad FU, Sharma BS, Carg A, Chandra PS. Primary spontaneous CSF rhinorrhea through the clivus: Possible etiopathology. J Clin Neurosci. 2008. 15: 1304-8
2. Akyuz M, Arslan G, Gurkanlar D, Tuncer R. CSF rhinorrhea from a transclival meningocele. J Neuroimaging. 2008. 18: 191-3
3. Algin O, Hakyemez B, Gokalp G, Ozcan T, Korfali E, Parlak M. The contribution of 3D-CISS and contrast-enhanced MR cisternography in detecting cerebrospinal fluid leak in patients with rhinorrhoea. Br J Radiol. 2010. 83: 225-32
4. Ammori MB, King AT, Siripurapu R, Herwadkar AV, Rutherford SA. Factors influencing decision-making and outcome in the surgical management of trigeminal neuralgia. J Neurol Surg B Skull Base. 2013. 74: 82-90
5. Aquilina K, Clarke DF, Wheless JW, Boop FA. Microencephaloceles: Another dural pathology of intractable temporal lobe epilepsy in childhood. J Neurosurg Pediatr. 2010. 5: 360-4
6. Araujo Filho BC, Butugan O, Pádua FG, Voegels RL. Endoscopic repair of CSF rhinorrhea: Experience of 44 cases. Braz J Otorhinolaryngol. 2005. 71: 472-6
7. Calcaterra TC. Extracranial surgical repair of cerebrospinal rhinorrhea. Ann Otol Rhinol Laryngol. 1980. 89: 108-16
8. Cassano M, Felippu A. Endoscopic treatment of cerebrospinal fluid leaks with the use of lower turbinate grafts: A retrospective review of 125 cases. Rhinology. 2009. 47: 362-8
9. Coiteiro D, Tavora L, Antunes JL. Spontaneous cerebrospinal fluid fistula through the clivus: Report of two cases. Neurosurgery. 1995. 37: 826-8
10. Costantino PD, Hiltzik DH, Sen C, Friedman CD, Kveton JF, Snyderman CF. Sphenoethmoid cerebrospinal fluid leak repair with hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 2001. 127: 588-93
11. Cui JY, Wu AH, Zhang SG, Qin XF, Wang YJ. Microsurgical treatment of cerebrospinal fluid rhinorrhea in sphenoidal sinus. Chin J Traumatol. 2010. 13: 178-81
12. Cui S, Han D, Zhou B, Zhang L, Li Y, Ge W. Endoscopic endonasal surgery for recurrent cerebrospinal fluid rhinorrhea. Acta Otolaryngol. 2010. 130: 1169-77
13. Forer B, Sethi DS. Endoscopic repair of cerebrospinal fluid leaks in the lateral sphenoid sinus recess. J Neurosurg. 2010. 112: 444-8
14. Fu Y, Komiyama M, Nagata Y, Tamura K, Yagura H, Yasui T. MR findings in traumatic cerebrospinal fluid leakage with special reference to indications of the need for dural repair. No Shinkei Geka. 1983. 21: 319-23
15. Ko AL, Gabikian P, Perkins JA, Gruber DP, Avellino AM. Endoscopic repair of a rare basioccipital meningocele associated recurrence meningitis. J Neurosurg Pediatrics. 2010. 6: 188-92
16. Komisar A, Weitz S, Ruben RJ. Cerebrospinal fluid dynamics and rhinorrhea: The role of shunting in repair. Otolaryngol Head Neck Surg. 1983. 91: 399-403
17. Leblanc R, Tampieri D, Robitallie Y. Developmental anterobasal temporal encephalocele and temporal lobe epilepsy. J Neurosurg. 1991. 74: 933-9
18. Liu P, Wu S, Li Z, Wang B. Surgical strategy for cerebrospinal fluid rhinorrhea repair. Neurosurgery. 2011. 66 (6 Suppl Operative): 281-5
19. Loew F, Pertuiset B, Chaumier EE, Jaksche H. Traumatic, spontaneous and postoperative CSF rhinorrhea. Adv Tech Stand Neurosurg. 1984. 11: 169-207
20. Lopatin AS, Kapitanov DN, Potapov AA. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2010. 138: 715-20
21. Manelfe C, Cellerier P, Sobel D, Prevost C, Bonafé A. Cerebrospinal fluid rhinorrhea: Evaluation with metrizamide cisternography. Am J Roentgenol. 1982. 138: 471-6
22. Saeki N, Yamaura A, Numata T. Transsphenoidal reoperations for removal of pituitary adenomas: Rhinological management and timing of reoperation. J Clin Neurosci. 1999. 6: 385-8
23. Sano H, Matsuwaki Y, Kaito N, Joki T, Okushi T, Moriyama H. A case of sphenoid sinus meningoencephalocele repaired by an image-guided endoscopic endonasal approach. Auris Nasus Larynx. 2011. 38: 632-7
24. Selcuk H, Albayram S, Ozer H, Ulus S, Sanus GZ, Kaynar MY. Intrathecal gadolinium-enhanced MR cisternography in the evaluation of CSF leakage. Am J Neuroradiol. 2010. 31: 71-5
25. Shetty PG, Shroff MM, Fatterpekar GM, Sahani DV, Kirtane MV. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. Am J Neuroradiol. 2000. 21: 337-42
26. Stamm AC, Pignatari SS, Vellutini E. Transnasal endoscopic surgical approaches to the clivus. Otolaryngol Clin North Am. 2006. 39: 639-56
27. Tolley NS. A clinical study of spontaneous CSF rhinorrhoea. Rhinology. 1991. 29: 223-30
28. van Zele T, Kitice A, Vellutini E, Balsalbre L, Stamm A. Primary spontaneous cerebrospinal fluid leaks located at the clivus. Allergy Rhinol. 2013. 4: e100-4
29. Tomovic S, Esmaeilli A, Chan NJ, Shukla PA, Choudhry OJ, Liu JK. High-resolution computed tomography analysis of variations of the sphenoid sinus. J Neurol Surg B. 2013. 74: 82-90
30. Wielgosz R, Mroczkowski E. Intranasal microsurgery in treatment of cerebrospinal rhinorrhea. Otolaryngol Pol. 1995. 49: 525-31