Abstract
Background: Diffusion tensor imaging (DTI) is based upon the phenomenon of water diffusion known as “Brownian motion.” DTI can detect changes in compressed spinal cord earlier than magnetic resonance imaging and is more sensitive to subtle pathological changes of the spinal cord. DTI observation in compressed and noncompressed spinal cord in tuberculosis (TB) spine is not described. This study presents observations in Pott’s spine patients with or without neural deficit.
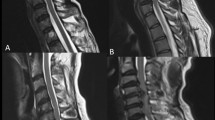
Materials and Methods: Thirty consecutive cases ofTB spine with mean age of 32.1 years of either sexes with paradiscal lesion, with/without paraplegia divided into two groups: Group A: (n = 15) without paraplegia and group B: (n = 15) with paraplegia were evaluated by DTI. The average fractional anisotropy (FA) and mean diffusivity (MD) values were calculated at 3 different sites, above the lesion (SOL)/normal, at the lesion and below SOL for both groups and mean was compared. Visual impression of tractography was done to document changes in spinal tracts.
Results: The mean canal encroachment in group Awas 39.60% and group B 44.4% (insignificant). Group Amean FA values above SOL, at the lesion and below SOL were 0.608 ± 0.09, 0.554 ± 0.14, and 0.501 ± 0.16 respectively. For group B mean FA values above SOL, at the lesion and below SOL were 0.628 ± 0.09, 0.614 ± 0.12 and 0.487 ± 0.15 respectively. There was a significant difference in mean FA above the SOL as compared to the mean FA at and below SOL. P value above versus below the SOL was statistically significant for both groups (0.04), but P value for at versus below the SOL (0.01) was statistically significant only in group B. On tractography, disruption of fibertract at SOL was found in 14/15 (93.3%) cases of group Aand 14/15 cases (93.3%) of group B (6/6 grade 4, 3/3 grade 3 and 5/6 grade 2 paraplegic cases).
Conclusion: The FA and MD above the lesion were same as reported for healthy volunteer hence can be taken as control. FA increases, and MD decreases at SOL in severe grade of paraplegia because of epidural collection while in milder grade, both decrease. In group A (without neurological deficit), mean FA and MD in patients with and without canal encroachment was similar. On tractography, both groups A and B (with or without neurological deficit) showed disruption of fiber tract at SOL and thickness of distally traced spinal cord was appreciably less than the upper cord. FA and MD could not differentiate between various grades of paraplegia. Although the number of patients in each group are small.
Similar content being viewed by others
References
Le Bihan D, Breton E. Imagerie de diffusion in vivo par resonance magnetique nucleaire. C R Acad Sci Paris 1985;301:1109–12.
Merboldt KD, Hanicke W, Frahm J. Self-diffusion NMR imaging using stimulated echoes. J Magn Reson 1985;64:479–86.
Taylor DG, Bushell MC. The spatial mapping of translational diffusion coefficients by the NMR imaging technique. Phys Med Biol 1985;30:345–9.
Ito R, Mori S, Melhem ER. Diffusion tensor brain imaging and tractography. Neuroimaging Clin N Am 2002;12:1–19.
Ries M, Jones RA, Dousset V, Moonen CT. Diffusion tensor MR1 of the spinal cord. Magn Reson Med 2000;44:884–92.
Kerkovsky M, Bednarfk J, Dusek L, Sprlakova-Pukova A, Urbanek I, Mechl M, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: Correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 2012;37:48–56.
Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br 2010;92:905–13.
Jain AK, Sinha S. Evaluation of systems of grading of neurological deficit in tuberculosis of spine. Spinal Cord 2005;43:375–80.
Rajasekaran S, Kanna RM, Shetty AP. Diffusion tensor imaging of the spinal cord and its clinical applications. J Bone Joint Surg Br 2012;94:1024–31.
Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J Magn Reson Imaging 2005;22:38–43.
Avadhani A, Ilayaraja V, Shetty AP, Rajasekaran S. Diffusion tensor imaging in horizontal gaze palsy with progressive scoliosis. Magn Reson Imaging 2010;28:212–6.
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 2005;26:1587–94.
Renoux J, Facon D, Fillard P, Huynh I, Lasjaunias P, Ducreux D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am J Neuroradiol 2006;27:1947–51.
Ducreux D, Lepeintalre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol 2006;27:214–6.
Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol 2008;29:655–9.
Rajasekaran S, Kanna RM, Karunanithi R, Shetty AP. Diffusion tensor tractography demonstration of partially injured spinal cord tracts in a patient with posttraumatic Brown Sequard syndrome. J Magn Reson Imaging 2010;32:978–81.
Maier SE, Mamata H. Diffusion tensor imaging of the spinal cord. Ann N Y Acad Sci 2005;1064:50–60.
Mamata H, De Girolami U, Hoge WS, Jolesz FA, Maier SE. Collateral nerve fibers in human spinal cord: Visualization with magnetic resonance diffusion tensor imaging. Neuroimage 2006;31:24–30.
Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol 2008;29:1976–82.
Chang Y, Jung TD, Yoo DS, Hyun JK. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 2010;27:2033–40.
Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J 2011;20:422–8.
Mohamed FB, Hunter LN, Barakat N, Liu CS, Sair H, Samdani AF, et al. Diffusion tensor imaging of the pediatric spinal cord at 1.5T: Preliminary results. AJNR Am J Neuroradiol 2011;32:339–45.
Kamble RB, Venkataramana NK, Naik AL, Rao SV. Diffusion tensor imaging in spinal cord injury. Indian J Radiol Imaging 2011;21:221–4.
Lee JW, Kim JH, Park JB, Park KW, Yeom JS, Lee GY, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: Preliminary results. Skeletal Radiol 2011;40:1543–51.
Uda T, Takami T, Sakamoto S, Tsuyuguchi N, Yamagata T, Ohata K. Normal variation of diffusion tensor parameters of the spinal cord in healthy subjects at 3.0-Tesla. J Craniovertebr Junction Spine 2011;2:77–81.
Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 2012;29:1556–66.
Gupta RK, Srivastava S, Saksena S, Rathore RK, Awasthi R, Prasad KN, et al. Correlation of DTI metrics in the wall and cavity of brain abscess with histology and immunohistochemistry. NMR Biomed 2010;23:262–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbas, S., Jain, A.K., Saini, N.S. et al. Diffusion tensor imaging observation in Pott’s spine with or without neurological deficit. IJOO 49, 289–299 (2015). https://doi.org/10.4103/0019-5413.156195
Published:
Issue Date:
DOI: https://doi.org/10.4103/0019-5413.156195
Key words
- Diffusion tensor imaging
- fractional anisotropy
- mean diffusivity
- tractography
- tuberculosis spine
- paraplegia
- tractography