- Community Health Sciences Department, Ziauddin University, Karachi, Pakistan
- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
- Department of Pathology and Laboratory Medicine, Aga Khan University Hospital, Karachi, Pakistan
- Khyber Medical College Undergraduate Program, Peshawar, Pakistan
Correspondence Address:
Naveed Z. Akhunzada
Khyber Medical College Undergraduate Program, Peshawar, Pakistan
DOI:10.4103/sni.sni_468_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sidra Sattar, Naveed Z. Akhunzada, Gohar Javed, Zeeshan Uddin, Yasir A. Khan. Pilocytic astrocytoma: A rare presentation as intraventricular tumor. 13-Jun-2017;8:116
How to cite this URL: Sidra Sattar, Naveed Z. Akhunzada, Gohar Javed, Zeeshan Uddin, Yasir A. Khan. Pilocytic astrocytoma: A rare presentation as intraventricular tumor. 13-Jun-2017;8:116. Available from: http://surgicalneurologyint.com/surgicalint-articles/pilocytic-astrocytoma-a-rare-presentation-as-intraventricular-tumor/
Abstract
Background:Pilocytic astrocytoma (PA) is the most prevalent central nervous system (CNS) tumor in pediatric population and accounts for an approximate of 5–6% of all gliomas. This neoplasm can occur at all levels of the neuraxis, with majority (67%) arising in the cerebellum and optic pathway. PAs are World Health Organization Grade I tumors and are the most benign of all astrocytomas characterized by an excellent prognosis. Other differentials include subependymal giant cell astrocytoma (SEGA), ependymoma, meningioma, and low-grade gliomas such as pilocytic or diffuse astrocytoma; calcification is more commonly regarded as a feature of benign or slow-growing tumors.
Case Description:We present a case of a 17-year-old female presenting with an unusual cause of hydrocephalus, a rare case of a calcified pilocytic astrocytoma as an intraventricular tumor.
Conclusion:PA rarely presents as an intraventricular tumor and should be included in the differential diagnosis of a large mass with massive intratumoral calcification.
Keywords: Intraventricular tumor, pilocytic astrocytoma, slow-growing tumors
INTRODUCTION
The central nervous system (CNS) is the most common site of primary solid tumors in pediatric population. Among pediatric patients (age 0–19 years), pilocytic astrocytoma (PA) is the most prevalent CNS tumor, representing 19.7% of all cases and accounting for an approximate of 5–6% of all gliomas.[
Calcification is a subtle and an infrequent finding, seldom seen in the optic nerve or hypothalamic/thalamic tumors or in superficially located cerebral tumors.[
We present a case of a 17-year-old female presenting with an unusual cause of hydrocephalus, a rare case of a calcified PA as an intraventricular tumor.
CASE REPORT
A 17-year-old old female, with no known comorbidity, presented with complaints of headache off and on for 1 month, which increased over a period of few days, associated with nonbilious, nonprojectile vomiting for 2 weeks. She presented to the emergency department and was referred to the neurosurgery service for further management upon evaluation of symptomatology. On examination, the patient was alert, awake, and oriented in time place and person. She denied any history of seizures, focal deficit, numbness, or any episode of unconsciousness.
General physical examination was unremarkable with normal blood pressure and pulse rate. Motor examination was unremarkable, sensory examination was normal, no cerebellar signs could be elicited, and no gait abnormality was identified. Direct ophthalmoscopy was suggestive of mild papilledema with no evidence of disc cupping or atrophic changes and no blurring of vision. Visual acuity was within normal limits with normal color vision.
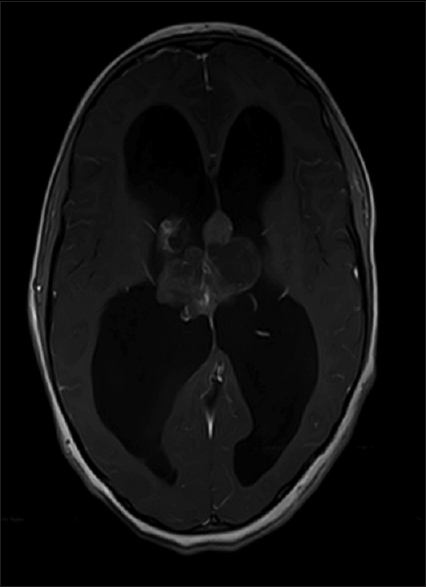
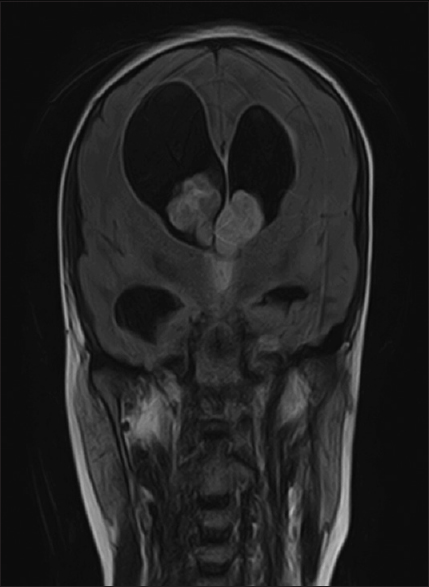
On investigation, blood profile was normal. Radiological imaging consisting of magnetic resonance imaging (MRI) of the brain with and without contrast; on T1-weighted postcontrast images, a heterogeneously enhancing mass lesion identified within the lateral ventricles in the proximity of the foramina of Monro [
Anesthesia evaluation was done, and after a written and informed consent, the patient underwent neuronavigation-guided (NNG) right-sided frontal craniotomy with excision of space occupying lesion (SOL) and left-sided frontal short tunnel extraventrivular drain (EVD) placement.
A linear incision was made. Intraoperative findings were of a grossly large intraventricular tumor which was moderately vascular containing cystic and necrotic components. The tumor was found to be occupying the septum pellucidum and extending into the lateral ventricles bilaterally. It was obliterating the foramen of Monro and third ventricle and causing biventricular hydrocephalus.
Postoperatively, the patient was uneventfully extubated; however, later in the recovery room patient had two episodes of generalized tonic–clonic seizure which were managed with intravenous antiepileptic medication.
Postoperative CT scan was done which was suggestive of pneumocephalus and postsurgical changes. Bilateral subdural collections were noted. Scan also showed diffuse cerebral edema with hydrocephalus and small intraventricular hemorrhage.
Patient was stepped down while EVD was removed on the fifth postoperative day after a successful challenge. Patient had an uneventful course during the hospital stay and was discharged with an early follow-up.
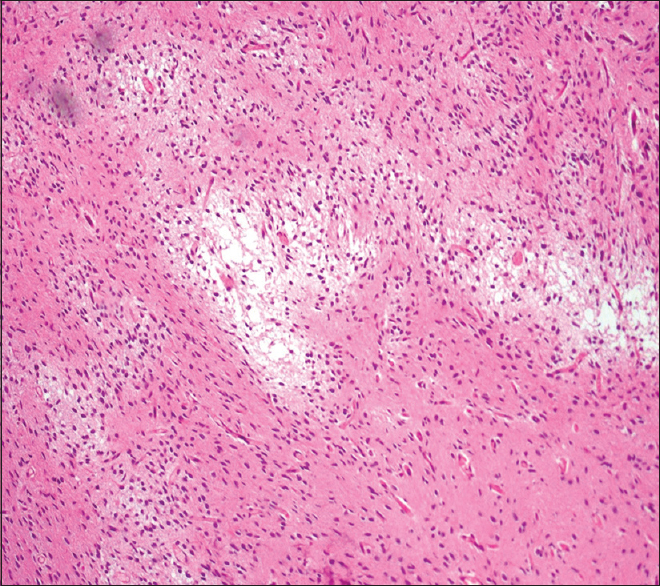
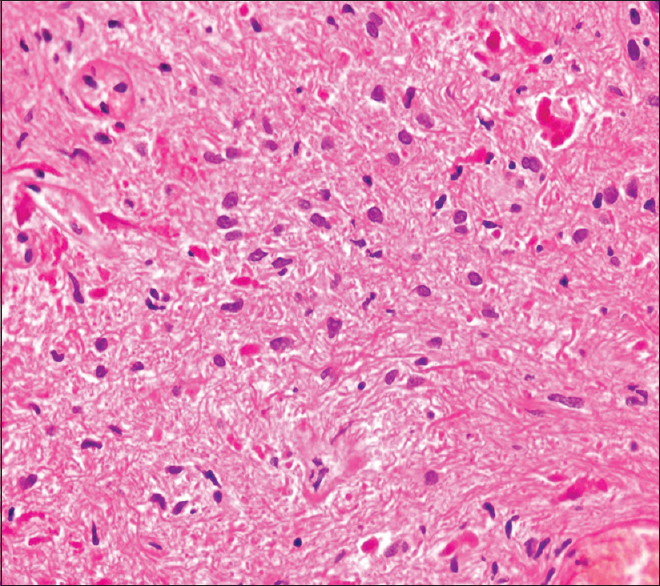
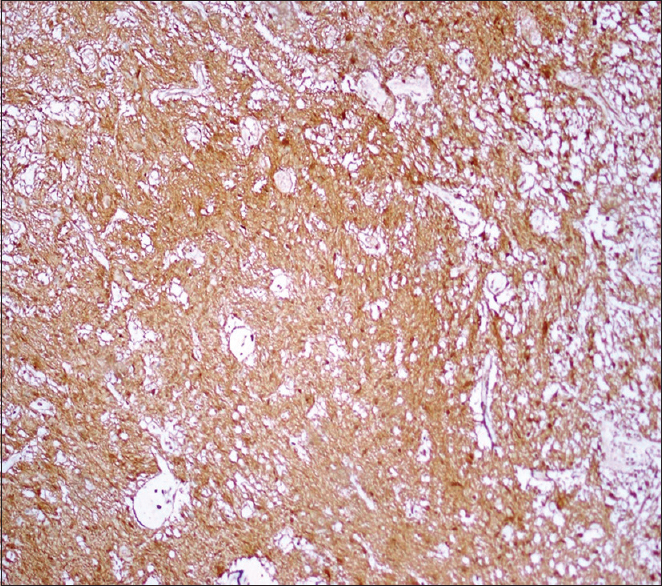
Histopathology showed multiple fragments of a neoplastic lesion comprising spindle-to-stellate-shaped cells arranged in alternating compact and loose areas [
On follow-up in the clinic, the patient presented with left-sided hemiparesis with power of 4/5, backache, and generalized lower limb tenderness. Follow-up CT scan showed interval decrease in hydrocephalus with the bifrontal diameter measuring 42 mm which was previously 55 mm in size. There was an interval reduction in the intraventricular hemorrhage; however, a slight interval increase in bilateral subdural collections was noted. Interval resolution of pneumocephalus with interval decrease in cerebral edema was also noted. Patient was followed up with serial MRI brain scans which showed progressive betterment supported by clinical condition.
DISCUSSION
This is a rare presentation of PA as an intraventricular tumor. Intraventricular neoplasms originate from cells forming the ependymal lining or the subependymal plate of the ventricular wall, choroid plexus, and glial lined structures such as septum pellucidum.[
Tumors localized in the lateral ventricles of the cerebral hemispheres account for less than 1% of all intracranial tumors,[
Pilocytic and diffuse astrocytomas were considered as our main differential diagnosis. Kim in his case report elucidated the histology of the viable tumor tissue that showed the presence of Rosenthal fibers and biphasic, compact, spongy pattern of PA, which is in lieu with our findings. Furthermore, degenerative atypia and regressive changes of PA were reported in a study by Tibbetts, similar to our findings;[
As illustrated by the WHO, PA is classified as grade I neoplasms and typically have an excellent prognosis. In this regard, tumors amenable to gross total resection (GTR) are considered “cured,” with low risk of tumor recurrence following resection.[
A GTR was achieved in our case with subsequent regression of symptoms and interval decrease in intraventricular hemorrhage. Patient was followed with serial MRI brain for recurrence and long term management.
CONCLUSION
PA rarely presents as intraventricular tumor and should be included in the differential diagnosis of a large mass with massive intratumoral calcification.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Budka H. Partially resected and irradiated astrocytoma of childhood: Malignant evolution after 28 years. Acta Neurochir (Wien). 1975. 32: 139-46
2. Last accessed on 2016 Nov 01. Available from: http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf.
3. Das DK. Psammoma body: A product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor?. Diagn Cytopathol. 2009. 37: 534-41
4. Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A. Pilocytic astrocytomas in children: Prognostic factors—A retrospective study of 80 cases. Neurosurgery. 2003. 53: 544-55
5. Filippidis AS, Tsonidis CA. Intraventricular brain tumors in children. Pediatr Neurosurg. 1989. 5: 230-3
6. Fisher BJ, Naumova E, Leighton CC, Naumov GN, Kerklviet N, Fortin D. Ki-67: A prognostic factor for low-grade glioma?. Int J Radiat Oncol Biol Phys. 2002. 52: 996-1001
7. Gajjar A, Sanford R, Heideman R, Jenkins JJ, Walter A, Li Y. Low-grade astrocytoma: A decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997. 15: 2792-9
8. Garcia DM, LatiW HR, Simpson JR, Picker S. Astrocytomas of the cerebellum in children. J Neurosurg. 1989. 71: 661-4
9. Geisssinger JD, Bucy PC. Astrocytomas of the cerebellum in children. Arch Neurol. 1971. 24: 125-35
10. Kim YE, Shin HJ, Suh YL. Pilocytic astrocytoma with extensive psammomatous calcification in the lateral ventricle: A case report. Childs Nerv Syst. 2012. 28: 649-52
11. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
12. Makariou E, Patsalides AD. Intracranial calcifications. Appl Radiol. 2009. 38: 48-60
13. Nishio S, Takashita I, Fujii K, Fukui M. Supratentorial astrocytic tumors of the childhood: A clinicopathologic study of 41 cases. Acta Neurochir (Wien). 1989. 101: 3-8
14. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005. 64: 479-89
15. Okuchi K, Hiramatsu K, Morimoto T, Tsunoda S, Sakaki T, Iwasaki S. Astrocytoma with widespread calcification along axonal fibres. Neuroradiology. 1992. 34: 328-30
16. Piepmeier JM. Tumors and approaches to the lateral ventricles: Introduction and overview. J Neurooncol. 1996. 30: 267-74
17. Scheithauer BW, Hawkins C, Tihan T, VandenBerg SR, Burger PC, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. Astrocytic tumor. WHO classification of tumours of the central nervous system. Lyon: World Health Organization; 2007. p. 16-
18. Spencer D, Collins W, Sass K, Schmidek H, Sweet W.editors. Surgical management of lateral ventricular tumors. Operative neurosurgical techniques. Philadelphia: Saunders; 1998. p. 583-607
19. Tibbetts KM, Emnett RJ, Gao F, Perry A, Gutmann DH, Leonard JR. Histopathologic predictors of pilocytic astrocytoma event-free survival. Acta Neuropathol. 2009. 117: 657-65
20. Tomita T, Larsen MB. Calcified metastases to the brain in a child: Case report. Neurosurgery. 1983. 13: 435-7
21. Zuccaro G, Sosa F, Cuccia V, Lubieniecky F, Monges J. Lateral ventricle tumors in children: A series of 54 cases. Childs Nerv Syst. 1999. 15: 774-85